Abstract
Intracerebral hemorrhage (ICH) is an important public health problem in neurology, which is not only associated with high mortality but also leading to disability. Yet no satisfactory treatment has been developed. The secondary injury that resulted from a number of self-destructive processes such as neuroinflammation, apoptosis and oxidative stress, is the key factor contributing to ICH-induced brain damage. Baicalein has been proved to improve neuronal functional recovery in rat model of subarachnoid hemorrhage and ischemic brain damage. To investigate the effect of baicalein on ICH and its underlying mechanism, a collagenase-induced ICH rat model was performed. Baicalein treatment significantly decreased neurological severity score at day 1 and 3 after ICH injury. Our results showed that the lesion volume, the brain water content, the expression levels of four pro-inflammatory cytokines (IL-1β, IL-4 and IL-6 and TNF-α) and the numbers of apoptotic cells were reduced significantly in ICH rats receiving baicalein treatment, especially in 50 mg/kg baicalein-treated group. Moreover, baicalein increased SOD and GSH-Px activities and down-regulated MDA level of brain tissues in rats. These results suggested that the therapeutic efficacy of baicalein on repairing brain damage is probably caused by suppressing apoptosis, oxidative stress and neuroinflammation. Baicalein could be developed into a novel drug for clinical treatment of ICH and ICH-related brain injuries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) is a stroke subtype accounting for 15–20% of all strokes in the Caucasian population [1], and an even higher proportion, up to 20–30%, in Asian [2]. It is one of the most lethal and destructive types of stroke associated with poor prognosis, with 30–50% of ICH patients died within 1 month and less than one-third of survivors returning to functional independence [3]. Presently, no pharmacological or surgical therapy has demonstrated any significant benefit in ICH [4].
Studies have demonstrated that ICH-induced neurological damage can be divided into primary and secondary brain injury [5]. Primary injury evoked by the physical destruction due to rapid hematoma formation [6] and the subsequent developments of various parallel pathological processes such as neuroinflammation, oxidative stress, neuronal apoptosis and excitotoxicity altogether contribute to secondary injury [7, 8]. Activated inflammatory cells including resident microglia, infiltrating neutrophils and macrophages secrete different kinds of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-4 (IL-4) that induce cerebral edema, blood–brain barrier disruption and neuronal apoptosis [9, 10]. On the other hand, oxidative stress caused by the imbalance between free radicals and the anti-oxidant system is a prominent and early feature in the pathogenesis of neuronal damage [11]. Thus, targeting the mechanism underlying the secondary damage could serve as a promising strategy for the treatment of ICH.
Baicalein (5,6,7-trihydroxyflavone), a flavone subclass of flavonoid, is a major active constituent in the roots of medicinal herb Scutellaria baicalensis [12]. This compound has been shown to possess a variety of biological characteristics including anti-bacterial effect [13], anti-hypertension effect [14], tumor growth inhibition [15] and amelioration of ischemic/reperfusion injury [16]. Several studies have demonstrated that baicalein has a highly potent cytoprotective effect on preventing doxorubicin-induced cardiomyocyte apoptosis [17] and radiation-induced apoptosis in human keratinocyte cell [18]. It has also been reported that baicalein can significantly attenuate reactive oxygen species (ROS) generation [19] and effectively reduce inflammatory response by inhibiting the NF-κB [20], JAK/STATs [21], and MAPK [22] signaling pathways. In addition, as a small molecule, baicalein is able to penetrate the blood brain barrier [23]. Given this, we hypothesize that baicalein could offer beneficial effects on ICH-related brain damage by inhibiting cell apoptosis, inflammation and oxidative stress.
Materials and Methods
Experimental Design
Adult female Sprague–Dawley rats aged 8 weeks were purchased from the Slac Laboratory Animal (Slac, Shanghai, China). All procedures were approved by the Animal Care and Use Committee of Guangxi Medical University. Baicalein was obtained from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA).
In total, 192 rats were equally randomized into four groups: (1) Sham group, rats were injected with 1 μl saline into the striatum over 10 min. At 1 h after sham-ICH induction, the vehicle (1 ml sterile saline plus 1% DMSO) was administrated via intraperitoneal injection (i.p.); (2) ICH + vehicle group, 1 ml vehicle was administered i.p. to each animal 1 h after ICH induction; (3, 4) ICH + baicalein group, 10 mg/kg baicalein (group 3) or 50 mg/kg baicalein (group 4) in 1 ml vehicle was administered i.p. to each animal 1 h after ICH. Since baicalein is practically insoluble in water, we obtained the baicalein suspension through ultrasonic fragmentation, centrifugation and mixing with vortexer in order to dissolve as much baicalein as possible. The vehicle was preheated at 42 °C and the pH was adapted to 6–7 using sodium bicarbonate. The suspension was prepared fresh at the time of use.
Equivalent volume (1 ml) of the vehicle or baicalein was intraperitoneal injected at a 12 h interval for 3 days.
Establishment of the ICH Model
The ICH model was generated via the stereotaxic intrastriatal injection of collagenase type VII (Sigma-Aldrich, St. Louis, MO, USA) according to a published method [24]. After anesthetization, a burr hole was drilled at the injection site (3.0 mm left lateral to the midline, 0.2 mm posterior to bregma, 6 mm in depth below the skull) and bacterial collagenase type VII (0.23 U dissolved in 1 μl saline) was infused slowly (0.5 μl/min) into the central striatum. The needle was maintained in the injection site for an additional 10 min to prevent backflow. The craniotomies were sealed with bone wax.
Neurological Scoring
To assess neurological abnormalities of the animals, the modified neurological severity score (mNSS) method was performed at 1 h, 1 day and 3 days after ICH by two independent investigators blinded to the experimental treatment scheme. The mNSS test included motor tests, sensory tests, beam balance tests, and reflexes absent and abnormal movements [25]. Neurological function was graded on a 0–18 scale (normal score = 0; maximal deficit score = 18).
Morphometric Measurement of the Lesion Volume
In short, the rat brain on day 3 post-ICH (n = 8, each group) was cut coronally through the needle entry site (identifiable on the brain surface) to obtain serial slices (2 mm thickness) anterior and posterior to the needle entry site. Digital photography of the serial slices was taken and the lesion volume was computed using ImageJ software. The total lesion volume (mm3) was calculated by summing the blood clot area in each section by the distance between sections.
Assessment of Brain Edema
A total of 32 rats were randomly used in the assessment of brain water content, using a common wet/dry method according to a published protocol [26]. Three days after ICH or sham-operation, rats were anesthetized and decapitated. The brain was removed and separated into contralateral and ipsilateral hemispheres and cerebellum. The sample was weighed immediately to obtain the wet weight, and then dried at 160 °C for 24 h to obtain the dry weight. Water content was calculated as: [(wet weight−dry weight)/(wet weight)] × 100%.
TUNEL assay
TUNEL staining was performed to detect cell apoptosis [27] using an In Situ Cell Death Detection kit (Roche, Mannheim, Germany). In short, the sections were permeabilizated in 0.1% Triton X-100 at 4 °C for 2 min, followed by incubation with TdT enzyme in reaction buffer containing TMRred labeled dUTP at 37 °C for 1 h. Finally, the sections were visualized using a converter-POD with 0.03% 3,3′ diaminobenzidine.
Immunofluorescence Tissue Staining
The sections were fixed and blocked with 5% goat serum in PBST (0.1% TritonX-100 in phosphate buffered saline) for 1 h. Then, the sections were incubated with primary antibodies against activated caspase-3 (Sigma-Aldrich, St. Louis, MO, USA, 1:200) for 2 h at room temperature, followed by an Alexa Fluor 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA). Immunofluorescence signals were captured using a confocal microscope (LSM 710, META Laser Scanning Microscope, Zeiss).
Western Blot Analysis
Eight rats from each group were sacrificed at day 3 after ICH or sham-operation. For western blot analysis, tissues surrounding the hematoma or sham group were excised, carefully isolated and lysed using radioimmunoprecipitation assay (RIPA) lysis buffer. Protein concentrations were determined using the micro bicinchoninic acid (BCA) assay (Thermo Fisher Scientific). The lysate sample (40 μg) was separated using 10 or 12% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The primary antibodies used included the following: anti-caspase-3, anti-cleaved caspase-3 (Abcam, Cambridge, UK, 1:1000), anti-IL-1β, anti-IL-4, anti-IL-6, anti-TNF-α (Sigma-Aldrich, St. Louis, MO, USA, 1:1000), and anti-GAPDH (Sigma-Aldrich, St. Louis, MO, USA, 1:10,000). Immunoreactive bands were quantitatively analyzed with ImageJ software.
Determination of Oxidative Stress Factors
The level of MDA in the brain tissues was detected using the thiobarbituric acid (TBA) reaction method [28]. In brief, 100 μl of homogenization sample and 100 μl of SDS lysis buffer were added into micro centrifuge tubes, shaken thoroughly, and incubated for 5 min at room temperature. Then, 250 μl of TBA was added to each tube and the tubes were incubated at 95 °C for 60 min. After incubation, samples were centrifuged at 3000 rpm for 15 min and 300 μl of supernatant from each tube was transferred to another tube containing 300 μl of n-Butanol. After centrifugation (10,000g, 4 °C, 5 min), the absorbance values of each sample were read at 532 nm on a Microplate Reader (Tecan Group AG, Männedorf, Switzerland).
To measure superoxide dismutase (SOD) activity, tissues homogenate and 25 mM HEPES buffer (pH 7.4) containing 250 mM sucrose and 1 mM EDTA were mixed and centrifuged at 1500g for 5 min at 4 °C [29]. Supernatant were collected to assay SOD activity using a SOD assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
For the analysis of glutathione peroxidase (GSH-Px) activity, samples were homogenized in 25 mM HEPES buffer (pH 7.4) containing 250 mM sucrose, 5 mM EDTA and 1 mM DTT and centrifuged at 10,000g for 15 min at 4 °C [29]. The activity of GSH-Px was measured in supernatants using a GSH-Px assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) following the manufacturer’s instructions.
Sandwich ELISA Method
According to the manufacturer’s instructions, the expression levels of IL-1β, IL-4, IL-6 and TNF-α in the rat ipsilateral striatal tissue extract (n = 8, each group) were detected by ELISA kits from the R&D system (Minneapolis, MN, USA). Based on the color reaction of antibodies and cytoplasm extract, the absorbance values were determined at 450 nm on a Microplate Reader (Tecan Group AG, Männedorf, Switzerland).
Statistical Analysis
Data were statistically analyzed using statistical software package SPSS 20.0 (SPSS, Chicago, IL, USA) and are presented as mean ± standard deviation (SD). For the behavioral test, analysis of variance (ANOVA) with Bonferroni post hoc test was performed. For other analyses, data were analyzed by unpaired Student’s t test if they were normally distributed. A significant difference among groups was set at p < 0.05.
Results
Baicalein Reduced Neurological Damages
Firstly, we investigated the impact of baicalein on the collagenase-induced rat model of intracerebral hemorrhage, through analysis of the neurological severity score. At 1 h after ICH or sham-operation, all ICH rats showed varying degrees of neurological damage symptoms (Fig. 1a), confirming that the ICH model was successfully established. Furthermore, as shown in Fig. 1b, intraperitoneal injection of baicalein significantly improved the mNSS score in a dose- and time-dependent manner compared with the vehicle-treated group at day 1 and 3 after ICH injury. Additionally, the treatment of baicalein at the dose of 50 mg/kg offered more remarkably suppressive effects of neurological deficits compared with the dose of 10 mg/kg (Fig. 1b).
Baicalein promoted neurological functional recovery after ICH a The mNSS test was performed at 1 h after ICH or sham-operation. b Rats received vehicle or baicalein at a dose of 10 mg/kg or 50 mg/kg. The mNSS test was performed at day 1 and day 3 after ICH or sham-operation. Data were presented as mean ± SD, *p < 0.05; **p < 0.01; ***p < 0.001
Baicalein Reduced the Lesion Volume and Brain Edema
Brain lesion volume is highly associated with the degree of brain injury [30]. Therefore we investigated whether baicalein could reduce brain lesion volume using image analysis techniques on day 3 post-ICH. Our results demonstrated that brain sections of baicalein-treated rats exhibited a decreased lesion area compared with ICH + vehicle group (Fig. 2a). Quantification data showed, compared with the vehicle-injected group, that baicalein-injected rats presented a markedly reduced lesion volume on day 3 and the inhibitory effects of ICH + 50 mg/kg baicalein group was more obvious than ICH + 10 mg/kg baicalein group (Fig. 2b).
Baicalein reduced the lesion volume and brain water content after ICH a Images of coronal Sect. (2 mm thickness) of rats in four groups. b Quantitative analysis of the lesion volume on day 3 post-ICH. c Brain water content evaluated on day 3 post-ICH was expressed as percentage of the wet weight: [(wet weight−dry weight)/(wet weight)] × 100%. Data were presented as mean ± SD, *p < 0.05; **p < 0.01; ***p < 0.001
As the brain edema plays a crucial role in ICH-related brain damage [26], we further quantified brain water content using the wet/dry method [26]. In comparison with the sham group, the brain water content in the ICH + vehicle group was significantly increased 3 days after ICH (Fig. 2c). As the same trend as the lesion volume assay, when rats were treated with baicalein for 3 days after ICH, the brain water content was significantly decreased than that of rats treated with vehicle (Fig. 2c).
Baicalein Suppressed Neuronal Apoptosis
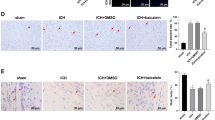
ICH-induced neuronal apoptosis appear to be a key factors contributing to secondary brain damage [31]. To investigate whether baicalein had any inhibitory effect on neuronal apoptosis, TUNEL staining, Western blot analysis and immunofluorescence tissue staining were performed. The TUNEL staining and quantification results showed that the number of TUNEL positive cells in the hemorrhagic boundary decreased dose-dependently in baicalein-treated rats compared with ICH + vehicle group (Fig. 3a, b). Next, it was found by Western blot that, when rats were intraperitoneal injected with baicalein for 3 days, the protein levels of cleaved caspase-3 were markedly repressed (Fig. 3c). Further immunofluorescence staining demonstrated a high expression of activated caspase-3 protein within the hemorrhage lesion and in the surrounding periphery. Baicalein treatment significantly attenuated the expression of activated caspase-3 than those treated with vehicle (Fig. 3d, e), which was in accordance with our Western blot results.
The anti-apoptotic effect of baicalein a Images of TUNEL staining at the perihematomal region in different experimental groups and b quantification of the percentage of TUNEL positive cells. Scale bars, 50 μm. c The protein expression of caspase-3 and cleaved caspase-3 at day 3 after baicalein treatment. d Immunofluorescence images of activated caspase-3 (green) staining at the perihematomal region in different experimental groups. Nucleus was stained with DAPI (blue). Scale bars, 50 μm. e Quantification of the expression of activated caspase-3. Data were presented as mean ± SD, *p < 0.05; **p < 0.01; ***p < 0.001. (Color figure online)
Baicalein Inhibited Oxidative Stress
Exaggerated oxidative stress contributes to ICH induced brain damages [32]. In light of this, we determined the levels of MDA, SOD and GSH-Px, three confirmed biomarkers of oxidative stress, in the brain tissues. The results show that the level of MDA was significantly higher and the SOD and GSH-Px activity were markedly decreased in the ICH + vehicle group than the sham group (Fig. 4a, b, c). We also found that baicalein (50 mg/kg) treatment significant increase of SOD and GSH-Px activities and down-regulated of the MDA level (Fig. 4a–c). The anti-oxidative effect was also observed to some extent in ICH + 10 mg/kg baicalein group and was not significant.
Baicalein Attenuated Pro-Inflammatory Cytokine Levels
At last, we studied the anti-inflammation effect of baicalein on ICH. In comparison with the sham group, the protein levels of IL-1β, IL-4 and IL-6 and TNF-α were increased remarkably in ICH + vehicle group according to the ELISA and Western blot analysis (Fig. 5a–c). In addition, the expressions of these four pro-inflammatory cytokines were notably inhibited in ICH + 50 mg/kg baicalein group compared with groups administrated with vehicle (Fig. 4a). However, only IL-1β and TNF-α were significantly down-regulated and IL-4 and IL-6 were not obviously changed between ICH + 10 mg/kg baicalein group and ICH + vehicle group (Fig. 5a–c).
The anti-inflammatory effect of baicalein a ELISA analysis for IL-1β, IL-4 and IL-6 and TNF-α protein expression in the ICH and sham-operation brain tissue. b The protein expression of IL-1β, IL-4 and IL-6 and TNF-α at day 3 after baicalein treatment. c The band intensities corresponding to IL-1β, IL-4, IL-6 and TNF-α were quantitated and normalized to GAPDH using ImageJ. Data were presented as mean ± SD, *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
Intracerebral hemorrhage is an important public health problem associated with high mortality and morbidity and yet no effective medical treatments has been found [3]. Although the pathophysiological mechanism of ICH are still unclear, the secondary injury, which was resulted from a number of self-destructive processes such as neuroinflammation, apoptosis and oxidative stress, is the key factor contributing to ICH-induced brain damage [30], hence approaches involving agents that had the ability of anti-inflammatory, anti-oxidative and neuronal apoptosis inhibition might lessen the consequence of hemorrhagic stroke.
In the present study, we identified baicalein as a novel compound that significantly promoted the recovery of nerve function in collagenase-induced rat model of ICH. Our results indicated that baicalein treatment, especially in dose of 50 mg/kg, significantly decreased neurological severity score at day 1 and 3 after ICH injury. This appeared to be consistent with the previous studies that baicalein exerted a protective effect on blood–brain barrier (BBB) disruption in the rat model of ICH via inhibition of MAPKs and NF-κB signaling pathways, leading to decreased formation of nitric oxide synthase (iNOS) and peroxynitrite [33]. Additionally, the therapeutic effect of baicalein also be confirmed in other brain injury models including subarachnoid hemorrhage [34] and ischemic brain injury [35].
Brain edema is one of the most important surrogate markers of perihematomal inflammation and tissue damage and peaks at day 3 after ICH [36]. Reducing brain edema can be seen as a aspect of rescuing the neurovascular unit. Our results demonstrated that baicalein markedly reduced the cerebral water content. This phenomenon might be due to the anti-inflammation effect of baicalein, which consequently decreased capillary permeability and exudation of tissue fluid. Besides, we also uncovered that baicalein could significantly attenuate the lesion volume on day 3 post-ICH. Inflammatory effect, oxidative stress and neuronal apoptosis, 3 crucial factors of secondary brain damage [37], were remarkably reduced by baicalein treatment. These results made us easy to understand the suppressive effects of baicalein on brain lesion volume. On the other hand, baicalein could significantly ameliorate angiotensin II-induced hypertension [38] and lower blood pressure, reducing absolute volume of hemorrhage. Therefore, these data indicated that the reduction of lesion volume observed in our study were probably not only due to the inhibition of secondary injury, but also owing to its anti-hypotensive effect.
Many bioactive small molecules derived from different natural resources have been identified as effective anti-inflammatory drugs for years [39]. Baicalein has been proved to serve as a promising agent of many inflammatory diseases such as atopic dermatitis [40], asthma [41] and rheumatoid arthritis [42]. Inflammation clearly plays a major role in ICH-induced brain injury. After ICH, the hemorrhage lesion and the surrounding tissues presents an exaggerated inflammatory response characterized by a larger number of inflammatory cells infiltration and pro-inflammatory cytokine secretion [24]. Our experiments uncovered that the protein levels of IL-1β, IL-4 and IL-6 and TNF-α were markedly decreased in rats treated with baicalein compared with rats in ICH + vehicle group, thus limiting inflammation and promoting behavioral recovery. In addition, inflammation leads to cell apotosis [43]. Neuronal apoptosis represents a prominent form of cell death in the perihematoma regions after ICH and results in blood brain barrier dysfunction and oxidative cascades, leading to brain damage [44]. We found that the number of TUNEL positive cells and the expression of activated caspase-3 were down-regulated after baicalein treatment, indicating that baicalein could promote neural functional recovery by suppressing neuronal apoptosis.
Previous studies demonstrated that the activity of enzymatic antioxidant systems decreased and the products of lipid peroxidation increased after ICH, and oxidative stress is highly associated with ICH-induced early brain injury [11]. It was reported that baicalein offered anti-oxidative effect in many kinds of disease models [45, 46], which was in accordance with our finding that baicalein intraperitoneal injection significantly increased SOD and GSH-Px activity and down-regulated MDA level.
Although these findings are noteworthy, some limitations in our research must be recognized. In future work, these findings should be replicated in other validated models of ICH like autologous blood injection. Moreover, the therapeutic effect of baicalein needs to be investigated in other nerve injury models including traumatic brain injury and spinal cord injury. Also, more investigations are still essential to elucidate the precise mechanisms of how baicalein affects neuron apoptosis, neuroinflammation and oxidative stress.
In conclusion, our work revealed that baicalein could effectively promote neuronal and behavioral recovery through suppressing many aspects of ICH-related brain damage. Baicalein is a promising anti-inflammatory, anti-oxidative and anti-apoptosis agent and has a potential to be developed into a novel drug for clinical treatment of ICH and ICH-related brain damage.
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344:1450–1460
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9:167–176
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11:720–731
Qureshi AI, Mendelow AD, Hanley DF (2009) Intracerebral haemorrhage. Lancet 373:1632–1644
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63
Boulouis G, van Etten ES, Charidimou A, Auriel E, Morotti A, Pasi M, Haley KE, Brouwers HB, Ayres AM, Vashkevich A, Jessel MJ, Schwab KM, Viswanathan A, Greenberg SM, Rosand J, Goldstein JN, Gurol ME (2016) Association of key magnetic resonance imaging markers of cerebral small vessel disease With hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA neurol 73(12):1440–1447
Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF (2001) Intracerebral hemorrhage-induced neuronal death. Neurosurgery 48:875–882 discussion 882–873
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92:463–477
Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, Kim M, Roh JK (2004) HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke 35:1744–1749
Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, Kim JM, Park DK, Kun Lee S, Kim M, Roh JK (2007) Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis 26:464–472
Duan X, Wen Z, Shen H, Shen M, Chen G (2016) Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid med cell longev 2016:1203285
Sahu BD, Kumar JM, Kuncha M, Borkar RM, Srinivas R, Sistla R (2016) Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci 144:8–18
Qian M, Tang S, Wu C, Wang Y, He T, Chen T, Xiao X (2015) Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int J Med Microbiol 305:501–504
El-Bassossy HM, Hassan NA, Mahmoud MF, Fahmy A (2014) Baicalein protects against hypertension associated with diabetes: effect on vascular reactivity and stiffness. Phytomedicine 21:1742–1745
Wang Y, Han E, Xing Q, Yan J, Arrington A, Wang C, Tully D, Kowolik CM, Lu DM, Frankel PH, Zhai J, Wen W, Horne D, Yip ML, Yim JH (2015) Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett 358:170–179
Wu K, Li H, Tian J, Lei W (2015) Protective effect of baicalein on renal ischemia/reperfusion injury in the rat. Ren Fail 37:285–291
Song L, Yang H, Wang HX, Tian C, Liu Y, Zeng XJ, Gao E, Kang YM, Du J, Li HH (2014) Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis 19:567–580
Oh MC, Piao MJ, Fernando PM, Han X, Madduma Hewage SR, Park JE, Ko MS, Jung U, Kim IG, Hyun JW (2016) Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol Ther 24(6):616–622
Chang WT, Li J, Haung HH, Liu H, Han M, Ramachandran S, Li CQ, Sharp WW, Hamann KJ, Yuan CS, Hoek TL, Shao ZH (2011) Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. J Cell Biochem 112:2873–2881
Patwardhan RS, Sharma D, Thoh M, Checker R, Sandur SK (2016) Baicalein exhibits anti-inflammatory effects via inhibition of NF-kappaB transactivation. Biochem Pharmacol 108:75–89
Qi Z, Yin F, Lu L, Shen L, Qi S, Lan L, Luo L, Yin Z (2013) Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm res 62:845–855
He X, Wei Z, Zhou E, Chen L, Kou J, Wang J, Yang Z (2015) Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-kappaB and MAPK signaling pathways in LPS-induced mastitis in mice. Int Immunopharmacol 28:470–476
Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF (2002) The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br J Pharmacol 137:1314–1320
Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK (2008) Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131:616–629
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005–1011
Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, Roh JK (2004) Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab 24:926–933
Zhang YF, Zhou SZ, Cheng XY, Yi B, Shan SZ, Wang J, Li QF (2016) Baicalein attenuates hypertrophic scar formation via inhibition of the transforming growth factor-beta/Smad2/3 signalling pathway. Br J Dermatol 174:120–130
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Skalska J, Dabrowska-Bouta B, Struzynska L (2016) Oxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silver. Food chem toxicol 97:307–315
Liew HK, Pang CY, Hsu CW, Wang MJ, Li TY, Peng HF, Kuo JS, Wang JY (2012) Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats. J Neuroinflamm 9:13
Salihu AT, Muthuraju S, Idris Z, Izaini Ghani AR, Abdullah JM (2016) Functional outcome after intracerebral haemorrhage—a review of the potential role of antiapoptotic agents. Rev Neurosci 27:317–327
Galho AR, Cordeiro MF, Ribeiro SA, Marques MS, Antunes MF, Luz DC, Hadrich G, Muccillo-Baisch AL, Barros DM, Lima JV, Dora CL, Horn AP (2016) Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebral haemorrhage in rats. Nanotechnology 27:175101
Chen M, Lai L, Li X, Zhang X, He X, Liu W, Li R, Ke X, Fu C, Huang Z, Duan C (2016) Baicalein attenuates neurological deficits and preserves blood-brain barrier integrity in a rat model of intracerebral hemorrhage. Neurochem Res 41:3095–3102
Kuo CP, Wen LL, Chen CM, Huh B, Cherng CH, Wong CS, Liaw WJ, Yeh CC, Lin BF, Wu CT (2013) Attenuation of neurological injury with early baicalein treatment following subarachnoid hemorrhage in rats. J Neurosurg 119:1028–1037
Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K (2008) Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 39:2538–2543
Zheng H, Chen C, Zhang J, Hu Z (2016) Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc dis 42:155–169
Nagatsuna T, Nomura S, Suehiro E, Fujisawa H, Koizumi H, Suzuki M (2005) Systemic administration of argatroban reduces secondary brain damage in a rat model of intracerebral hemorrhage: histopathological assessment. Cerebrovasc dis 19:192–200
Wang AW, Song L, Miao J, Wang HX, Tian C, Jiang X, Han QY, Yu L, Liu Y, Du J, Xia YL, Li HH (2015) Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERK1/2, NF-kappaB, and calcineurin signaling pathways in mice. Am J Hypertens 28:518–526
Gardi C, Bauerova K, Stringa B, Kuncirova V, Slovak L, Ponist S, Drafi F, Bezakova L, Tedesco I, Acquaviva A, Bilotto S, Russo GL (2015) Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch Biochem Biophys 583:150–157
Heratizadeh A, Werfel T (2016) Anti-inflammatory therapies in atopic dermatitis. Allergy
Mabalirajan U, Ahmad T, Rehman R, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B, Sharma SK (2013) Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PloS one 8:e62916
Chen S, Yang Y, Feng H, Wang H, Zhao R, Liu H (2014) Baicalein inhibits interleukin-1beta-induced proliferation of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 37:163–169
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44
Beurel E, Grieco SF, Jope RS (2015) Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol ther 148:114–131
Kumar M, Kasala ER, Bodduluru LN, Dahiya V, Lahkar M (2016) Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm res 65:613–622
Kim KC, Lee IK, Kang KA, Kim HS, Kang SS, Hyun JW (2012) Baicalein (5,6,7-trihydroxyflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol Toxicol 28:421–433
Acknowledgements
We are grateful to Dr. Jiayi Wang for his constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal studies have been approved by the Animal Care and Use Committee of Shanghai Jiao Tong University and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Wei, N., Wei, Y., Li, B. et al. Baicalein Promotes Neuronal and Behavioral Recovery After Intracerebral Hemorrhage Via Suppressing Apoptosis, Oxidative Stress and Neuroinflammation. Neurochem Res 42, 1345–1353 (2017). https://doi.org/10.1007/s11064-017-2179-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2179-y