Abstract
Cancer-induced bone pain (CIBP) is a challenging medical problem that considerably influences cancer patients’ quality of life. Currently, few treatments have been developed to conquer CIBP because of a poor understanding of the potential mechanisms. Our previous work has proved that spinal RANTES (a major ligand for CCR5) was involved in the maintenance of CIBP. In this study, we attempted to investigate whether spinal CCR5 and its downstream PKCγ pathway is involved in the maintenance of CIBP. Inoculation of Walker 256 cells into the tibia could induce a marked mechanical allodynia with concomitant upregulation of spinal CCR5 and p-PKCγ expression from day 6 to day 15 after inoculation. Spinal CCR5 was prominently expressed in microglia, and mechanical allodynia was attenuated by intrathecal injection of DAPTA (a specific antagonist of CCR5) with downregulation of spinal CCR5 and p-PKCγ expression levels at day 15 in inoculated rats. Pre-intrathecal injection of RANTES could reverse the anti-allodynia effects of DAPTA. Intrathecal administration of GF109203X (an inhibitor of PKC) could alleviate mechanical allodynia as well as decrease of spinal p-PKCγ expression level, but no influence on spinal CCR5 level. Our findings suggest that CCR5/PKCγ signaling pathway in microglia may contribute to the maintenance of CIBP in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of cancer diagnoses and treatments, the life expectancy of cancer patients has been markedly increased. However, cancer-induced bone pain (CIBP) is a challenging medical problem that considerably influences cancer patients’ quality of life [1–3]. It has been reported that more than one-third of patients with advanced-stage cancer will undergo skeletal metastases and experience severe CIBP [4, 5]. Currently, about 45% of patients with CIBP have inadequate pain control because current therapies are lack of efficacy or associate with dose-limiting side effects [6, 7]. Thus, understanding the potential molecular and cellular mechanisms underlying CIBP is necessary for the development more effective therapies to conquer CIBP.
Recent studies indicated that several chemokines and chemokine receptors were involved in modulating pain responses [8, 9]. CC chemokine receptor 5 (CCR5) is one of the chemokine receptors, which belongs to the family of seven-transmembrane G protein coupled receptor. The RANTES (regulated on activation normal T cell expressed and secreted; also named as CCL5) is a major ligand for CCR5. Our previous work has proved that the antinociceptive effects of triptolide correlated with inhibition of spinal RANTES expression in a rat model of CIBP [10]. The analgesic effects of triptolide also have been proved in inflammatory and neuropathic pain models [11, 12]. The established mechanical allodynia could be attenuated by intrathecal injection of anti-RANTES neutralizing antibody in CIBP rats [13]. These results indicated that spinal RANTES might participate in the CIBP processing. However, the potential mechanism of CCR5 in the maintenance of CIBP remains unknown.
In vitro study has shown that CCR5 can be coupled to Gαq, Gas, and Ga12/13 protein [14]. Gaq activates the phospholipase C-γ, leading to the activation of protein kinase C (PKC). Activation of Gas is able to activate PKA signaling pathway through increased adenylyl cyclase activity. Ga12/13 can activate RhoA/Rho kinase (ROCK) signaling pathway. A few researches have indicated that involvement of spinal PKC pathway in inflammatory and neuropathic pain [15–17]. If spinal CCR5 participates in the maintenance of CIBP, whether its downstream PKC signaling pathway is also involved in the CIBP still remains unknown. In the current study, we attempted to investigate whether spinal CCR5/PKCγ pathway is involved in the maintenance of CIBP.
Methods
Animals
All animal studies were approved by the Animal Care and Use Committee of the Jiangsu University, and complied with the National Institutes of Health guidelines for Care and Use of Laboratory Animals. Female Wistar rats, weighing 150–180 g, were used in this study. Rats were kept in a temperature- controlled room (22 ± 2 °C) with free access to food and water.
Drug Application
DAPTA (D-Ala-peptide T-amide), a specific antagonist of CCR5, and recombinant rat RANTES, an agonist of CCR5 were obtained from R&D Systems (Minneapolis, USA). GF109203X was purchased from Sigma-Aldrich (Shanghai, China), and dissolved in 1% DMSO. The dosage of drugs was determined by the results of pilot experiments.
A Rat Model of CIBP
A rat model of CIBP was conducted as described previously [18]. In brief, under anesthesia with sodium pentobarbital (50 mg/kg, i.p), the toe pinch test was performed to ensure an adequate state of anesthesia for animal. The rat was placed with the supine position, and the left tibia was prepared for inoculation. After iodine disinfection, a small incision was cut on the anterior-medial surface to expose the tibial plateau. The needle of 23-gauge was used to drill a hole in the tibial plateau, then, Walker 256 mammary gland carcinoma cells (10 μl, 1 × 105 cells, supplied by the Soochow University) or heat-killed cells (for sham group) was inoculated into the intramedullary space of the tibia. After inoculation, the medical glue was used to close the hole. Finally, the incision was dusted with penicillin powder and sutured using silk thread. After fully awake, the rats returned to their individual cages.
Intrathecal Cannula Implantation
Intrathecal cannula implantation was carried out 3 days after inoculation [19]. Under anesthesia, a PE-10 tube was inserted through the L5 and L6 space, and the tip of the catheter was put near the lumbar enlargement. The position of the catheter was identified by intrathecal administration of lidocaine (2%, 10 μl).
Pain Behavior
Mechanical allodynia was assessed by von Frey hairs [19]. In brief, animals were put in individual acrylic boxes (15 × 20 × 25 cm) with a metal mesh floor and permitted 30 min to acclimatize to the chamber. A series of von Frey hairs with logarithmically incremental force was tested on the plantar surface of left hind-paw to measure the paw withdrawal threshold (PWT). Paw flinching or quick withdrawal was defined as a positive response. The investigators for behavioral test were blinded to the experimental protocol.
Immunohistochemistry
Rats were transcardially perfused with 0.9% normal saline followed by 200 ml of 4% paraformaldehyde under deeply anesthesia with pentobarbital sodium. Spinal dorsal horns (L4 and L5) were quickly removed on ice and postfixed with the same fixative for 24 h. The tissues were sectioned at 30 μm and every fifth section was collected in PBS. The sections were incubated for 60 min in blocking buffer (0.3% Triton X-100 and 2% goat serum), then incubated with one of the following primary antibodies overnight at 4 °C: anti-CCR5 antibody (1:500; Abcam, USA), anti-GFAP antibody (a marker for astrocyte; 1:500; Abcam, USA), anti-OX42 antibody (a marker for microglia; 1:500; Abcam, USA), and anti-NeuN antibody (a marker for neuron; 1:500, Abcam, USA). The DyLight® 488 or 594 conjugated secondary antibodies (1:500, Abcam, USA) were used for detection. In double label immunofluorescence experiments, the sections were incubated with a cocktail of two primary antibodies and the corresponding secondary antibodies. Finally, images in laminae I and II were captured by a fluorescence microscope. The Image J software was used to measure the staining intensity. The average intensity from four rats was presented as group data.
Western Blot
The tissue samples (dorsal horn of L4-L5) were solubilized in ice-cold RIPA buffer plus phosphatase inhibitors, and centrifuged at 10,000g for 30 min at 4 °C. The Bradford method was used to determine the protein concentrations in the supernatant. Fifty micrograms of proteins were loaded for each lane, separated using 12% SDS–PAGE gel, and electrophoretically transferred onto PVDF membrane (Millipore, USA). Then, the blots were incubated with anti-CCR5 antibody (1:1000; Abcam, USA), anti-phospho PKCγ antibody (1:1000; Abcam, USA) or anti-PKCγ antibody (1:1000; Abcam, USA) overnight at 4 °C, and then for 1 h with HP-conjugated secondary antibody. The blots were visualized by the ECL detection system. Expression of CCR5 and p-PKCγ was normalized to the β-actin.
Statistical Analysis
All data are expressed as mean ±SEM. Repeated-measures (RM) ANOVA followed by Bonferroni test was used to analyze the behavioral data. One-way ANOVA followed by Bonferroni test was used to analyze western blot and immunofluorescence data. p < 0.05 was considered significant. Statistical analysis was carried out with the SPSS16.0 software.
Results
Pain Behavior Over Time
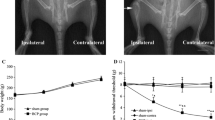
The baseline PWT values were not statistically different between sham and CIBP rats (n = 8, p > 0.05). Compared with sham rats, the PWT values of the ipsilateral hind paw were markedly reduced from day 6 to day 15 in inoculated rats (p < 0.05 or 0.01; Fig. 1), suggesting the progressive development of mechanical allodynia. However, within the 15-day observation time, the PWT values of contralateral hind paw kept relatively unaltered.
Mechanical allodynia was induced by Walker 256 cells intramedullary inoculation. Compared with sham rats, the paw withdrawal threshold (PWT) values of the ipsilateral hind paw were markedly reduced on days 6, 12 and 15 at the same time-point in inoculated rats. The baseline PWT values were not statistically different between sham and CIBP rats. Within the 15-day observation time, the PWT values of contralateral hind paw kept relatively unaltered. (▲ p < 0.05, ▲▲ p < 0.01 versus baseline, *p < 0.05, **p < 0.01 versus sham group. Data were expressed as the mean ± SEM, each group contained eight rats.)
Upregulation of Spinal CCR5 and p-PKCγ Expression in CIBP Rats
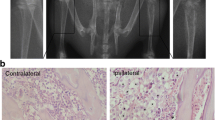
To examine whether CCR5 participates in the maintenance of CIBP, CCR5 expression levels were checked by immunohistochemistry and Western blot. The results of immunostaining showed that CCR5 expression in the ipsilateral spinal dorsal horn was markedly increased at days 6, 12, and 15 in inoculated rats (Fig. 2a–d). From day 6 to day 15 after inoculation, a statistically significant increase in the intensity of CCR5 was found (Fig. 2k). Moreover, progressively increased protein expression levels of CCR5 (Fig. 2m) and p-PKCγ (Fig. 2n) were found by Western blot analysis in inoculated rats.
Upregulation of CCR5 and p-PKCγ expression in CIBP rats. a–d The results of immunostaining showed that the CCR5 expression in the ipsilateral spinal dorsal horn was markedly increased at days 6, 12, and 15 in inoculated rats. The intensities of immunostaining were calculated in laminae I and II of the ipsilateral spinal dorsal horn. e Control tissues that were immunostained only with secondary antibody. Scale bar 100 μm. The images of double immunostaining revealed that CCR5 (green) was mainly colocalized with microglial marker OX-42 (h and i, yellow, white arrows), but not colocalized with the astrocytic marker GFAP (red, f) or neuronal marker NeuN (red, g). Scale bar 25 μm. j The white box indicates the location of panel I on the lower power image. Scale bar 200 μm. Compared with CIBP 6 d group, the number of CCR5/OX-42 double positive cells was markedly increased in CIBP 15 d group (i, h, and l). From day 6 to day 15 after inoculation, a statistically significant increase the intensity of CCR5 was found (k). Moreover, progressively increased protein expression levels of CCR5 (m) and p-PKCγ (n) were found by western blot analysis in inoculated rats. (*p < 0.05, **p < 0.01 versus sham rats. Data were presented as mean ± SEM, each group contained four rats.) (Color figure online)
To investigate the cellular distribution of CCR5 in spinal dorsal horn, double immunostaining was used. The images showed that CCR5 was mainly colocalized with OX-42 (microglial marker; Fig. 2h), but not with NeuN (neuronal marker; Fig. 2g) or GFAP (astrocytic marker; Fig. 2f), suggesting expression of CCR5 by microglia. Compared with CIBP 6 d group (Fig. 2i), the number of CCR5/OX-42 double positive cells was markedly increased in CIBP 15 d group (Fig. 2h, l). Taken together, these results suggested that the close relationship between the number of microglia and CCR5 expression level.
Intrathecal of DAPTA Attenuated Mechanical Allodynia and Decreased CCR5 and p-PKCγ Expression Levels in CIBP Rats
To further check the roles of CCR5 in the maintenance of CIBP, DAPTA (5 or 10 μg, a CCR5 antagonist) was injected intrathecally in CIBP rats at 15 days after inoculation. Intrathecal DAPTA could significantly decrease the PWT values at 1, 2, and 3 h in a time- and dose-dependent manner (p < 0.05 or 0.01). Intrathecal RANTES (0.2 μg, a CCR5 ligand) had no effects on the values of PWT. However, the anti-allodynia effects of DAPTA (10 μg) was reversed by pre-intrathecal injection of RANTES (0.2 μg) 15 min before intrathecal DAPTA (Fig. 3a). These data suggested that CCR5 might play a key role in the maintenance of CIBP.
Intrathecal administration of DAPTA attenuated mechanical allodynia and decreased CCR5 and p-PKCγ expression in CIBP rats. a Intrathecal DAPTA could significantly increase the PWT values at 1, 2, and 3 h in a time- and dose-dependent manner (p < 0.05 or 0.01). Intrathecal RANTES (0.2 μg, a CCR5 ligand) had no effects on the values of PWT. However, the anti-allodynia effects of DAPTA (10 μg) was reversed by pre-intrathecal injection of RANTES (0.2 μg) 15 min before intrathecal DAPTA.NS group indicates intrathecal administration of normal saline. (*p < 0.05, **p < 0.01 versus NS group. Data were presented as the mean ± SEM, each group contained eight rats.). b Compared with sham group, the CCR5 and p-PKCγ expression levels were higher in the NS group of inoculated rats. Intrathecal DAPTA (10 μg) could significantly attenuate spinal CCR5 and p-PKCγ levels in inoculated rats. The tissues of spinal dorsal horn were collected at 3 h after intrathecal injection. (▲▲ p < 0.01 versus NS group, **p < 0.01 versus sham rats. Data were presented as mean ±SEM, each group contained four rats.)
To examine the effects of intrathecal DAPTA on the spinal CCR5 and p-PKCγ expression levels, the Western blot analysis was used. The tissues of spinal dorsal horn were harvested at 3 h after intrathecal injection. Compared with sham group, the CCR5 and p-PKCγ expression levels were higher in the NS group of inoculated rats. Intrathecal DAPTA (10 μg) could significantly attenuate spinal CCR5 and p-PKCγ levels in inoculated rats (Fig. 3b).
Intrathecal of GF109203X Attenuated Mechanical Allodynia and Reduced p-PKCγ Expression in CIBP Rats
To further check whether PKCγ is a downstream molecule of CCR5 for maintenance of CIBP, GF109203X, an inhibitor of PKC, was chosen to verify this hypothesis. The results showed that intrathecal injection of GF109203 × (0.18 and 0.36 μg) could dose-dependently attenuate mechanical allodynia at 15 days in inoculated rats. The peak effect was at the 1.5 h after intrathecal administration (p < 0.01, Fig. 4a). Meanwhile, intrathecal GF109203 × (0.36 μg) could significantly attenuate spinal p-PKCγ expression (p < 0.01). However, intrathecal administration of GF109203X had no effects on the expression of spinal CCR5 (Fig. 4b). Taken together, these findings suggested that PKC was an important downstream molecule of CCR5 in the maintenance of CIBP in rats.
Intrathecal of GF109203X attenuated mechanical allodynia and blocked PKCγ activation in CIBP rats. a Intrathecal GF109203 × (0.18 and 0.36 μg) could dose-dependently attenuate mechanical allodynia at 15 days in inoculated rats. The peak effect was at the 1.5 h after intrathecal administration (p < 0.01, Fig. 4a). (**p < 0.01 versus NS group. Data were expressed as the mean ±SEM, each group contained 8 rats.) b Intrathecal of GF109203 × (0.36 μg) could significantly attenuate spinal p-PKCγ expression (p < 0.01). However, intrathecal administration of GF109203X had no effects on the expression of CCR5. Compared with sham rats, the expression of OX-42 was higher in NS group. Intrathecal DAPTA (10 μg) or GF109203 × (0.36 μg) could significantly attenuate spinal OX-42 expression level (p < 0.01). (▲▲ p < 0.01 versus NS group, **p < 0.01 versus sham rats. Data were presented as mean ± SEM, each group contained four rats.)
Compared with sham rats, the expression of OX-42 was higher in NS group. Intrathecal DAPTA (10 μg) or GF109203 × (0.36 μg) could significantly attenuate spinal OX-42 expression level (p < 0.01). The results indicated that a positive correlation between microglial activation and the expression level of CCR5 or p-PKCγ.
Discussion
More recent studies have indicated that a few chemokines and chemokine receptors were involved in central sensitization in CIBP models [20–22]. RANTES, which is the main ligand for CCR5, is a member of the CC chemokine subfamily. In addition to RANTES, other CC chemokines, such as CCL3 and CCL4, may also contribute to the CCR5 activation [23]. Recently, some studies have confirmed that CCL3/CCR5 or CCL4/CCR5 signaling pathway might participate in neuropathic pain [24–26]. Our previous study verified that RANTES was involved in the alleviation of nociceptive responses of triptolide in the CIBP rats [10]. The established mechanical allodynia could be abolished by intrathecal injection of anti-RANTES neutralizing antibody in inoculated rats [13]. In the present study, the results showed that inhibition of spinal CCR5 expression was associated with the attenuated mechanical allodynia in CIBP rats. These results suggested that RANTES/CCR5 signaling pathway might contribute to the maintenance of CIBP. Lee and colleagues found that CCR5 knockout mice to chemical and inflammation stimuli were significantly attenuated, and intracerebroventricular infusion of DAPTA could markedly reduce chemical and inflammatory pain responses in wild-type mice [27]. Peritoneal administration of Met-RANTES, a CCR5 antagonist, could reduce nociceptive responses in a partial sciatic nerve ligation (PSNL) model [28]. Similarly, oral administration of RAP-103, a CCR2 and CCR5 antagonist, could attenuate mechanical allodynia and thermal hyperalgesia in PSNL rats [29]. Taken together, CCR5 may play an important role in the modulation of nociceptive perception.
The peak effect of DAPTA was at 2 h after intrathecal administration. However, the effects of intrathecal administration of DAPTA on the expression levels of CCR5 and p-PKCγ were measured at 3 h after intrathecal, owing to the fact that variation of protein expression lags behind pain behavioral changes. In pharmacology, a receptor antagonist is defined as a molecule binding to the specific receptor can inhibit the function of the agonist or inverse agonist to its receptor, which has affinity but no efficacy for its cognate receptor. The efficacy of a receptor depends on its expression level [30]. DAPTA, a specific antagonist of CCR5, can reduce the efficacy of the CCR5. So, decrease of the CCR5 expression level was observed after intrathecal of DAPTA.
CCR5/PKC signaling pathway has been proved in some studies [14, 31]. PKC is a family of serine/threonine kinases, which comprises more than ten members. PKCγ is widely distributed in the central nervous system (CNS). Immunocytochemical studies showed PKCγ was mainly localized in the inner part of lamina II of spinal cord [32], suggesting PKCγ might be involved in the modulation of pain processing. A reduced neuropathic pain behavior was observed in the mice lack of PKCγ [33]. Inflammatory pain induced by complete Freund’s adjuvant (CFA) could be attenuated by intra-arcuate nucleus injection of a PKC inhibitor [34]. These findings indicated that PKCγ participated in neuropathic and inflammatory pain. However, CIBP is different from neuropathic and inflammatory pain, and has its distinguishing feature [35]. Interestingly, in the current study, we found that only p-PKCγ expression level was increased in inoculated rats, but not total PKCγ. Similarly, some studies found that there was no upregulation of spinal PKCγ in a mouse model of CIBP [35, 36]. These results indicated that PKCγ participated in CIBP processing through its phosphorylation. Intrathecal DAPTA could reduce mechanical allodynia with concomitant downregulation of spinal CCR5 and p-PKCγ expression at day 15 after inoculation. Moreover, intrathecal injection of GF109203X could alleviate mechanical allodynia as well as decrease of spinal p-PKCγ expression level, but no influence on spinal CCR5 level. These findings indicated that CCR5/PKCγ signaling pathway was involved in the maintenance of CIBP.
Recently, it is increasingly recognized that microglia plays a key role in the initiation and maintenance of CIBP [37, 38]. Inhibition of microglia activation by minocycline could alleviate CIBP in rats [39]. Furthermore, inhibition of microglia signaling pathway, such as CX3CR1/p38 and TLR4/p38 signaling, could also attenuate CIBP [40, 41]. In the present study, we found CCR5 was mainly expressed in microglia, and intrathecal DAPTA or GF109203X could significantly attenuate microglial activation. These studies supported that spinal microglia might play an important role in CIBP. It was reported that RANTES was secreted mainly by neurons in the CNS [42]. So, we speculate that the RANTES-CCR5- PKCγ pathway may contribute to the maintenance of CIBP via neuronal-microglial interaction in the spinal cord.
References
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA et al (2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 51(6):1070–1090
Hendriks LE, Hermans BC, van den Beuken-van Everdingen MH et al (2016) Effect of bisphosphonates, denosumab, and radioisotopes on bone pain and quality of life in patients with non-small cell lung cancer and bone metastases: a systematic review. J Thorac Oncol 11(2):155–173
Reis-Pina P, Lawlor PG, Barbosa A (2015) Cancer-related pain management and the optimal use of opioids. Acta Med Port 28(3):376–381
von Moos R, Body JJ, Egerdie B et al (2016) Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer 24(3):1327–1337
Patrick DL, Cleeland CS, von Moos R et al (2015) Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer 23(4):1157–1168
Schug SA, Chandrasena C (2015) Pain management of the cancer patient. Expert Opin Pharmacother 16(1):5–15
Muralidharan A, Smith MT (2013) Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology 21(5):339–363
Llorián-Salvador M, González- Rodríguez S, Lastra A (2016) Involvement of CC Chemokine Receptor 1 and CCL3 in Acute and Chronic Inflammatory Pain in Mice. Basic Clin Pharmacol Toxicol 119(1):32–40
Guo G, Gao F (2015) CXCR3: latest evidence for the involvement of chemokine signaling in bone cancer pain. Exp Neurol 265:176–179
Hang LH, Li SN, Shao DH et al (2014) Evidence for involvement of spinal RANTES in the antinociceptive effects of triptolide, a diterpene triepoxide, in a rat model of bone cancer pain. Basic Clin Pharmacol Toxicol 115(6):477–480
Tang J, Li ZH, Ge SN et al (2012) The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012:185167.
Xu F, Li Y, Li S et al (2014) Complete Freund’s adjuvant-induced acute inflammatory pain could be attenuated by triptolide via inhibiting spinal glia activation in rats. J Surg Res 188(1):174–182
Hang LH, Shao DH, Chen Z et al (2013) Involvement of spinal cc chemokine ligand 5 in the development of bone cancer pain in rats. Basic Clin Pharmacol Toxicol 113(5):325–328
Wu Y, Yoder A (2009) Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog 5(12):e1000520
Koda K, Hyakkoku K, Ogawa K et al (2016) Sensitization of TRPV1 by protein kinase C in rats with mono-iodoacetate-induced joint pain. Osteoarthritis Cartilage 24(7):1254–1262
de Souza Nunes JP, da Silva KA, da Silva GF et al (2014) The antihypersensitive and antiinflammatory activities of a benzofuranone derivative in different experimental models in mice: the importance of the protein kinase C pathway. Anesth Analg 119(4):836–846
Loram LC, Taylor FR, Strand KA et al (2013) Intrathecal injection of adenosine 2 A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun 33:112–122
Hang LH, Yang JP, Yin W et al (2012) Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur J Neurosci 36(1):2107–2117
Hang LH, Li SN, Luo H et al (2016) Connexin 43 mediates CXCL12 production from spinal dorsal horn to maintain bone cancer pain in rats. Neurochem Res 41(5):1200–1208
Guan XH, Fu QC, Shi D et al (2015) Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol 263:39–49
Hang LH, Luo H, Li SN et al (2015) Involvement of spinal Bv8/Prokineticin 2 in a rat model of cancer-induced bone pain. Basic Clin Pharmacol Toxicol 117(3):180–185
Zhou YQ, Gao HY, Guan XH et al (2015) Chemokines and their receptors: potential therapeutic targets for bone cancer pain. Curr Pharm Des 21(34):5029–5033
Alkhatib G (2009) The biology of CCR5 and CXCR4. Curr Opin HIV AIDS 4(2):96–103
Matsushita K, Tozaki-Saitoh H, Kojima C et al (2014) Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 120(6):1491–1503
Sun S, Chen D, Lin F et al (2016) Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol Immunol 77:184–192
Saika F, Kiguchi N, Kobayashi Y et al (2012) CC-chemokine ligand 4/macrophage inflammatory protein-1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 16(9):1271–1280
Lee YK, Choi DY, Jung YY et al (2013) Decreased pain responses of C-C chemokine receptor 5 knockout mice to chemical or inflammatory stimuli. Neuropharmacology 67:57–65
Liou JT, Mao CC, Ching-Wah Sum D et al (2013) Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain 14 (1): 24–35.
Padi SS, Shi XQ, Zhao YQ et al (2012) Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain 153(1):95–106
Páldy E, Bereczki E, Sántha M et al (2008) CB(2) cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: role of CB(2) receptor in pain. Neurochem Int 53(6–8):309–316
Hüttenrauch F, Pollok-Kopp B, Oppermann M (2005) G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem 280(45):37503–37515
Mori M, Kose A, Tsujino T et al (1990) Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol 299(2):167–177
Malmberg AB, Chen C, Tonegawa S, Basbaum AI (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278(5336):279–283
Bu F, Tian H, Gong S et al (2015) Phosphorylation of NR2B NMDA subunits by protein kinase C in arcuate nucleus contributes to inflammatory pain in rats. Sci Rep 5:15945
Honore P, Rogers SD, Schwei MJ et al (2000) Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98(3):585–598
Schwei MJ, Honore P, Rogers SD et al (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19(24):10886–10897
Zhang MY, Liu YP, Zhang LY et al (2015) Levo-tetrahydropalmatine attenuates bone cancer pain by inhibiting microglial cells activation. Mediators Inflamm 2015:752512
Yang Y, Li H, Li TT et al (2015) Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2 × 7 receptor and IL-18. J Neurosci 35(20):7950–7963
Wang LN, Yang JP, Zhan Y et al (2012) Minocycline-induced reduction of brain-derived neurotrophic factor expression in relation to cancer-induced bone pain in rats. J Neurosci Res 90(3):672–681
Hu JH, Yang JP, Liu L et al (2012) Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res 1465:1–9
Liu S, Yang J, Wang L et al (2010) Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res 1346:213–223
Liou JT, Lee CM, Day YJ (2013) The immune aspect in neuropathic pain: role of chemokines. Acta Anaesthesiol Taiwan 51(3):127–132
Acknowledgements
This work was financed by the Social Development Fund of Zhenjiang, Jiangsu Province (SH2015049) and the Natural Science Foundation of the PR China (No. 81600799).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hang, LH., Li, SN., Dan, X. et al. Involvement of Spinal CCR5/PKCγ Signaling Pathway in the Maintenance of Cancer-Induced Bone Pain. Neurochem Res 42, 563–571 (2017). https://doi.org/10.1007/s11064-016-2108-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2108-5