Abstract
Tumor metastasis to bone can subsequently lead to bone cancer pain (BCP). Currently, BCP is difficult to conquer due to a poor understanding of the potential mechanisms. Several studies have indicated that astrocyte-specific connexin 43 (Cx43) was involved in the neuropathic pain, and Cx43 induced the release of chemokine CXCL12 in bone marrow stromal cells. However, whether spinal Cx43 mediates the production of CXCL12 to participate in the maintenance of BCP is still unknown. Here we showed that Walker 256 tumor cells inoculation into the tibia induced a significant mechanical allodynia, which was accompanied by upregulation of spinal p-Cx43 and CXCL12 expression levels from day 6 to day 18 after inoculation. Spinal Cx43 was mainly expressed in astrocytes, and intrathecal 43Gap26 (a selective Cx43 blocker) markedly attenuated mechanical allodynia as well as reduced p-Cx43 and CXCL12 expression at day 18 after inoculation. Pre-intrathecal administration of CXCL12 almost abolished the attenuated mechanical allodynia by 43Gap26. Furthermore, intrathecal injection of anti-CXCL12 neutralizing antibody could ameliorate mechanical allodynia with concomitant inhibition of upregulation of CXCL12 expression, but not influence on p-Cx43 expression. Our results indicate that Cx43 mediates CXCL12 production from spinal dorsal horn in astrocytes to maintain bone cancer pain in rats. These findings may improve our understanding of the underlying mechanisms of BCP and provide a novel target for the treatment of BCP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metastasis is a common characteristic of many aggressive cancers, such as breast, kidney, lung, and prostate cancer [1, 2]. Tumor metastasis to bone can subsequently lead to bone cancer pain (BCP) [3, 4]. Clinically, BCP is difficult to conquer because response to current pharmacological treatment still remains poor. Therefore, understanding the potential molecular mechanisms of BCP may explore new targets for the therapeutic intervention.
Gap junctions allow cells to communicate directly with one another in the central nervous system (CNS). The main structural constituents of gap junctions are connexins, and the connexin 43 (Cx43) subunit is the most prominent connexin expressed by astrocytes [5]. Increasing evidence demonstrated that activation of astrocytes in the spinal dorsal horn might play a vital role in BCP [6, 7]. Several studies indicated that astrocyte-specific Cx43 was involved in the neuropathic pain in multiple animal models [8–11]. However, the characteristics of BCP are not the same as neuropathic pain, and it has unique molecular mechanisms [1, 4]. Whether spinal Cx43 is involved in BCP remains unclear.
Activated astrocytes may secrete various inflammatory mediators, including cytokines and chemokines, which may amplify the nociceptive signals in the CNS. Previous studies have demonstrated that spinal chemokine CXC motif ligand 12 (CXCL12) was upregulated in astrocytes after tumor cell implantation, which contributed to the initiation and maintenance of BCP [6, 12]. Cx43 can induce the production of CXCL12 in bone marrow stromal cells [13]. However, whether spinal Cx43 is able to regulate the secretion of CXCL12 to participate in the maintenance of BCP needs to be investigated.
In the present study, we examined Cx43 expression and distribution in the spinal dorsal horns after injection of Walker 256 carcinoma cells into the tibia. The roles of Cx43 and CXCL12 in pain hypersensitivity after Walker 256 cells inoculation were also being examined.
Materials and Methods
Animals
Female Wistar rats weighing between 150 and 180 g were purchased from the animal center of Shanghai Laboratory and housed individually at a temperature of 22 ± 2 °C with free access to both food and water. All animal procedures in this study were in accordance with the recommendations of the International Association for the Study of Pain [14], and approved by the Animal Care and Use Committee of the Jiangsu University.
Preparation of Tumor Cells
Walker 256 mammary gland carcinoma cells were kindly supplied by the Soochow University (China). The female rats were injected intraperitoneally with tumor cells (0.5 ml, 1 × 107 cells/ml). After 6–7 days, tumor cells were collected from the ascites, rinsed in Hanks’ solution and centrifugation for 5 min at 3000 g. Then, the pellet was resuspended in Hanks’ solution and the concentration was adjusted to 1 × 105 cells per 10 μL. The suspension of tumor cells was maintained on ice until injection.
Bone Cancer Pain Model
Our previously described model of rat BCP was used [15, 16]. In brief, under anesthesia with pentobarbital sodium (50 mg/kg, i.p), the animal was placed with the abdominal side up. A small skin incision was made in a disinfected area on the surface of the left tibia and the tibial plateau carefully exposed. A 23-gauge needle was used to drill a hole in the tibia, then, a volume of 10 μL containing 1 × 105 tumor cells was injected into the intramedullary space of the tibia. Subsequently, the injection hole was sealed with bone wax. Sham rats were injected with same amount of heat-killed tumor cells. Finally, the rats were placed on a heat pad until fully awake.
Intrathecal Cannulation
Intrathecal catheter implantation was carried out as our previously described [17, 18]. In brief, 3 days before any procedures, under anesthesia with pentobarbital sodium (50 mg/kg, i.p), a PE-10 polyethylene tube was inserted through the space between the L5 and L6 vertebrae. The cannula position was determined by intrathecal injection of 1 % lidocaine (10 μL). The animals were excluded from the study if insertion of the catheter caused an evidence of neurological damage.
Drug Application
43Gap26 (a selective Cx43 blocker) was purchased from AnaSpec (Fremont, CA, USA) and dissolved in phosphate buffered saline (PBS). Anti-CXCL12 neutralizing antibody, recombinant rat CXCL12, and normal goat IgG (for control) were obtained from Abcam (Cambridge, MA, USA). All drugs were delivered through the intrathecal catheter in a total volume of 10 μL. The dosage of drugs is mainly relied on the results from pilot experiments.
Radiology
The rats were radiographed at day18 after inoculation to verify cancer development in the tibia. The rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p), the hind limbs were exposed to X-ray for 1/20 s at 40 kVp. The images were taken from sham rats and BCP rats.
Behavioral Analysis
The general health situation of the animals was evaluated daily. The body weights were measured at day 0, 6, 12, 18 in both sham rats and BCP rats. A series of von Frey filaments (Stoelting, Wood Dale, IL, USA) were used to assess the mechanical allodynia, as described previously [19]. In brief, animals were put in separate test cages (25 × 20 × 15 cm) with a metal mesh floor, and allowed to adapt to the experimental environment for 30 min. The experimenter applied the tip of hair perpendicular to the plantar surface of the left hind paw for 5 s with enough force to cause a slight bend. A positive response was defined as brisk withdrawal or paw flinching, and the Dixon’s up-and-down method was used to determine the 50 % paw withdrawal threshold (PWT) to mechanical stimuli [20]. Behavioral testing was performed before and then at day 6, 12, and 18 post-carcinoma inoculation in a blinded manner.
Immunohistochemistry
Under deep anesthesia with pentobarbital sodium, the rats were transcardially perfused with PBS followed by 4 % paraformaldehyde. The L4 and L5 spinal segments were quickly removed, and post-fixed overnight with the same fixative at 4 °C, then, cut with the vibratome to a thickness of 30 μm. The sections were first blocked for 1 h using 2 % goat serum at room temperature, then incubated overnight at 4 °C with the following primary antibodies: phospho-Cx43 (Ser368) polyclonal antibody (rabbit, 1:100; Sigma-Aldrich, Shanghai, China), OX-42 monoclonal antibody (mouse, 1:400; Abcam, Cambridge, MA, USA), GFAP monoclonal antibody (mouse, 1:800; Millipore, Billerica, MA, USA), NeuN monoclonal antibody (mouse, 1:1000, Abcam), CXCL12 polyclonal antibody (rabbit, 1:400; Abcam, Cambridge, MA, USA). After three times washing with PBS, the sections were incubated for 1 h at room temperature with the DyLight™ 488 or 594 conjugated secondary antibodies (1:800, Jackson ImmunoResearch Europe Ltd, UK). For double staining, the sections were incubated with a mixture of primary antibodies followed by a mixture of conjugated secondary antibodies. Finally, the sections were detected by a laser scanning confocal microscopy. Image J software was used to analyze the fluorescence intensity of images. The intensities of immunoreactivity were measured in four to six randomly selected sections from each animal in laminae I and II of the spinal dorsal horn. The average intensity from each animal was calculated and these values from at least four animals in each group were averaged and are presented as group data [15].
Western Blot
Under deep anesthesia with pentobarbital sodium, the rats were sacrificed. The spinal dorsal horn segments (L4 and L5) were rapidly dissected and frozen in liquid nitrogen and stored at −70 °C. The spinal segments were homogenized in RIPA buffer plus the inhibitors of phosphatase and protease. Then, the homogenates were centrifuged for 15 min at 13,000g at 4 °C to get the supernatants for Western blot analysis. The concentrations of protein were measured by using the BCA method according to the kit’s instructions. Equivalent amounts of protein (50 μg) were separated on SDS-PAGE gel (10 %) and transferred to the PVDF membrane. Then, the membranes were incubated with p-Cx43 polyclonal antibody (rabbit, 1:1000; Sigma) overnight at 4 °C. After washed with PBST three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody. The blots were detected using a chemiluminescence detection system and exposed to film. β-actin (mouse, 1:5000; Sigma) was used as a loading control. Western blotting bands from four independent experiments were quantified with Image J. Each band density of phosphorylated Cx43 was compared to that of the β-actin and normalized to the intensity of the sham sample.
ELISA
Protein samples were prepared in the same way as for Western blot analysis. The expression level of CXCL12 was examined using a rat CXCL12 ELISA kit (Abbexa Ltd, Cambridge, UK) following the manufacturer’s protocol.
Statistical Analysis
Data were presented as the mean ± SEM and analyzed using SPSS16.0 software. The molecular data were analyzed by using one-way ANOVA followed by Bonferroni test, and the behavioral data were analyzed by using repeated-measures (RM) ANOVA followed by Bonferroni test. P < 0.05 was regarded statistically significant.
Results
Intramedullary Inoculation of Walker 256 Tumor Cells Induces Bone Cancer Pain
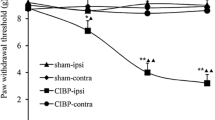
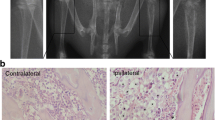
After Walker 256 tumor cells were injected into the tibia, the rats showed general good health and gradual increase in body weight during an 18-day observation period (Fig. 1c). Eighteen days after inoculation, major cortical destruction and severe loss of medullary bone were distinctly detected in the proximal epiphysis of the left tibia. However, there was no radiological change in the contralateral tibia or bilateral tibia of sham rats (Fig. 1a, b). The ipsilateral PWT to von Frey filaments was decreased from 8.0 ± 0.9 g before inoculation to 5.1 ± 0.8 g at day 6, 3.1 ± 0.7 g at day 12, and 2.3 ± 0.6 g at day 18. Neither the contralateral paw of BCP rats nor bilateral paws of sham rats showed a significant change in pain sensitivity (Fig. 1d). Pain behavioral results showed that injection of Walker 256 cells into the tibia led to the development of mechanical allodynia.
Intramedullary inoculation of Walker 256 tumor cells induces bone cancer pain. a, b Radiography showed severe loss of medullary bone and major cortical destruction of the left tibia (arrow) at day 18 after inoculation. No radiological changes were found in the contralateral tibia or bilateral tibia of sham rats. c The body weight was gradually increased in both sham rats and BCP rats during an 18-day observation period. d The ipsilateral PWT progressively decreased from day 6 to day 18 in BCP rats. Neither the contralateral paw of BCP rats nor bilateral paws of sham rats showed a significant change in pain sensitivity. Data were expressed as mean ± SEM. n = 8 rats in each group [*P < 0.05, **P < 0.01 vs. sham-ipsi., ▲ P < 0.05, ▲▲ P < 0.01 vs. baseline using repeated-measure (RM) ANOVA followed by Bonferroni test]. BCP bone cancer pain. Scale bar 1 mm (a, b)
Upregulation of p-Cx43 and CXCL12 Expression Levels in Spinal Dorsal Horn in BCP Rats
We first examined the expression and distribution of p-Cx43 in the spinal dorsal horn after Walker 256 tumor cells inoculation. The result of immunostaining showed that a significant increase of p-Cx43 protein expression in the ipsilateral spinal dorsal horn at day 6, 12, 18 after inoculation (Fig. 2a–d). Compare with sham rats, the intensity of p-Cx43 immunoreactivity in BCP rats was gradually increased from day 6 to day 18 (P < 0.01, Fig. 2h). In addition, Western blot analysis also revealed that tibia inoculation of Walker 256 cells induced a progressively increased expression of p-Cx43 protein from day 6 to day 18 after tumor cells inoculation (P < 0.01, Fig. 2i). To detect the cellular distribution of p-Cx43 in spinal dorsal horn after inoculation, we carried out double immunostaining of p-Cx43 with different cell markers: GFAP (for astrocytes), OX-42 (for microglia), and NeuN (for neurons). Confocal images indicated that p-Cx43 was extensively colocalized with GFAP (Fig. 2e), but not with OX-42 (Fig. 2f) or NeuN (Fig. 2g), which suggested that the expression of p-Cx43 by astrocytes, but not by microglia or neurons in BCP rats.
Upregulation of p-Cx43 and CXCL12 expression in spinal dorsal horn in BCP rats. a–d Immunostaining indicated that a significant increase of p-Cx43 expression in the ipsilateral spinal dorsal horn at day 6, 12, 18 after inoculation. Scale bar 100 μm. e–g Double immunostaining showed that p-Cx43 (red) was colocalized with GFAP (e, arrows indicate double-labeled, yellow), but not with OX-42 (green, f) or NeuN (green, g). Tissues were collected at day 18 after inoculation. Scale bar 50 μm. h Compare with sham rats, the intensity of p-Cx43 immunoreactivity in BCP rats was gradually increased from day 6 to day 18 after tumor cells inoculation. i Each image shows representative western blotting bands from four independent experiments. Western blot analysis showed that a progressively increased expression of p-Cx43 protein from day 6 to day 18 after inoculation. j The ELISA analysis showed that the concentration of CXCL12 significantly increased from day 6 to day 18 in BCP rats. All data were expressed as mean ± SEM. n = 4 rats in each group (**P < 0.01 vs. sham rats using one-way ANOVA followed by Bonferroni test) (Color figure online)
We then used ELISA analysis to measure CXCL12 produced in spinal dorsal horn in BCP rats. As shown in Fig. 2j, the concentration of CXCL12 significantly increased from day 6 to day 18 in BCP rats. The CXCL12 expression peaked at day 12, and increased expression was sustained up to day 18 in BCP rats. These results suggested that spinal CXCL12 was upregulated in inoculated rats.
Intrathecal Administration of 43Gap26 Attenuated Pain Behavior and Reduced p-Cx43 and CXCL12 Expression in BCP Rats
To investigate the exact role of Cx43 in bone cancer pain, 43Gap26, a selective Cx43 blocker, was administered intrathecally at day 18 after tumor cells inoculation. As shown in Fig. 3, intrathecal injection of 43Gap26 reversed mechanical allodynia for about 1.5 h, in a dose-dependent manner (P < 0.05 or 0.01). Intrathecal dose (10 μg) of 43Gap26 almost completely reversed mechanical allodynia at the 1.5 h time point after intrathecal injection (P < 0.01, Fig. 3).
Intrathecal administration of 43Gap26 attenuated pain behavior in BCP rats. Intrathecal injection of 43Gap26 reversed mechanical allodynia for about 1.5 h, in a dose-dependent manner. Intrathecal dose (10 μg) of 43Gap26 almost completely reversed mechanical allodynia at the 1.5 h time point after intrathecal injection. In CXCL12 0.1 μg + 43Gap26 10 μg group, CXCL12 was intrathecally administered 1.5 h before 43Gap26 administration. Pre-intrathecal injection of CXCL12 almost completely abolished the attenuated mechanical allodynia by 43Gap26. Data were expressed as mean ± SEM. n = 8 rats in each group [*P < 0.05, **P < 0.01 vs. PBS group using repeated-measure (RM) ANOVA followed by Bonferroni test]
To explore the roles of Cx43-CXCL12 interaction in modulation of BCP, the recombinant rat CXCL12 (0.1 μg) was intrathecally administered 1.5 h before 43Gap26 administration. As shown in Fig. 3, intrathecal CXCL12 (0.1 μg) exhibited no effects on the PWT compared with PBS group, however, in CXCL12 0.1 μg + 43Gap26 10 μg group, pre-intrathecal injection of CXCL12 almost completely abolished the attenuated mechanical allodynia by 43Gap26 administration.
To examine whether intrathecal 43Gap26 would affect the spinal p-Cx43 and CXCL12 expression, the spinal dorsal horn segments were collected at 1.5 h after intrathecal administration. Western blot analysis indicated that intrathecal 43Gap26 (10 μg) could markedly attenuate spinal p-Cx43 expression. The p-Cx43 expression in the PBS group was significant higher than that of sham rats (P < 0.01, Fig. 4a). ELISA analysis and immunostaining indicated that intrathecal 43Gap26 (10 μg) could markedly decrease CXCL12 expression in BCP rats (P < 0.01). The CXCL12 expression level in the PBS group was significant higher than that of Gap26-treated rats or sham rats (P < 0.01, Fig. 4b–f).
Intrathecal administration of 43Gap26 reduced p-Cx43 and CXCL12 expression in BCP rats. a Western blot analysis indicated that intrathecal 43Gap26 (10 μg) could markedly attenuate spinal p-Cx43 expression. The p-Cx43 expression in the PBS group was significant higher than that of sham rats (P < 0.01). Each image shows representative western blotting bands from four independent experiments. b ELISA and c–f immunostaining indicated that intrathecal 43Gap26 (10 μg) could markedly decrease CXCL12 expression in BCP rats (P < 0.01). The CXCL12 expression level in the PBS group was significant higher than that of Gap26-treated rats or sham rats (P < 0.01). Scale bar 100 μm. Tissues were harvested at 1.5 h after intrathecal injection. All data were expressed as mean ± SEM. n = 4 rats in each group (**P < 0.01 vs. sham rats, ▲▲ P < 0.01 vs. PBS group using one-way ANOVA followed by Bonferroni test)
Taken together, these results indicated that inhibition of spinal p-Cx43 upregulation resulted in attenuation of pain behavior as well as decrease of CXCL12 production from spinal dorsal horn in BCP rats.
Intrathecal Administration of Anti-CXCL12 Neutralizing Antibody Attenuated Pain Behavior and Decreased CXCL12 Expression in BCP Rats
To further verify Cx43 is an upstream molecule of CXCL12 for maintenance of bone cancer pain, anti-CXCL12 neutralizing antibody was used to confirm this hypothesis. Eighteen days after inoculation, intrathecal anti-CXCL12 neutralizing antibody (4 and 8 μg) could markedly reverse mechanical allodynia in a dose-dependent manner (P < 0.05 or 0.01, Fig. 5a). The spinal dorsal horn segments were collected at 6 h after intrathecal administration, and ELISA analysis showed that intrathecal anti-CXCL12 neutralizing antibody (8 μg) could significantly reduce spinal CXCL12 expression in BCP rats (P < 0.01, Fig. 5b). However, Western blot analysis showed that there was no influence on spinal p-Cx43 expression by intrathecal injection of anti-CXCL12 neutralizing antibody (Fig. 5c). Taken together, these findings further indicate that Cx43 mediates CXCL12 production to maintain bone cancer pain in rats.
Intrathecal administration of anti-CXCL12 neutralizing antibody attenuated pain behavior and decreased CXCL12 expression, but not influence on p-Cx43 expression in BCP rats. a At day 18 after inoculation, intrathecal anti-CXCL12 neutralizing antibody (4 and 8 μg) could markedly reverse mechanical allodynia in a dose-dependent manner (P < 0.05 or 0.01). Data were expressed as mean ± SEM. n = 8 rats in each group [*P < 0.05, **P < 0.01 vs. NS group using repeated-measure (RM) ANOVA followed by Bonferroni test]. NS normal saline. b ELISA analysis showed that intrathecal anti-CXCL12 neutralizing antibody (8 μg) could significantly reduce spinal CXCL12 expression in BCP rats (P < 0.01). c Western blot analysis showed that there was no influence on spinal p-Cx43 expression by intrathecal injection of anti-CXCL12 neutralizing antibody (8 μg). Tissues were collected at 6 h after intrathecal injection. Each image shows representative western blotting bands from four independent experiments. Data were expressed as mean ± SEM. n = 4 rats in each group (*P < 0.05, **P < 0.01 vs. sham rats, ▲▲ P < 0.01 vs. NS group using one-way ANOVA followed by Bonferroni test)
Discussion
Bone cancer pain is a complex pain state including background pain, spontaneous pain, and incident pain (movement-evoked) [1, 21]. To investigate the potential mechanisms underlying BCP, animal models have been developed using direct inoculation of tumor cells (Walker 256 mammary gland carcinoma cells, osterolytic 2472 murine osteosarcoma tumor cells, and prostate tumor cells) into the intramedullary space of long bones (tibia, femur, and calcaneus) [22, 23]. These models can well mimic the clinical conditions, and enable systematic examination of the molecular and cellular mechanisms of BCP. After inoculation of Walker 256 cells into the tibia, pain behavioral and radiological results indicated that the site-specific BCP model was well established. An interesting ‘mirror-image pain’ phenomenon was described in some BCP studies [24]. However, we found there was no marked change in the contralateral pain sensitivity in BCP rats. Studies have revealed that inoculation of different types tumor cells could lead to different types of pain behaviors [1, 25]. Female rats are more sensitive to Walker 256 mammary gland cells, therefore, only female rats were chosen in the present study.
Cx43, an integral membrane protein also known as gap junction alpha-1 protein, is broadly expressed in different tissues including the central nervous system (CNS), and especially expressed in astrocytes [5, 26]. In the current study, we found that spinal Cx43 was mainly expressed in astrocytes in the BCP model. The localization of Cx43 in astrocytes was also confirmed by other investigators in the different neuropathic pain models [10, 11]. Growing evidence indicates that gap junctions in astrocytes are involved in pain modulation. Spinal cord injury-induced heat hyperalgesia and mechanical allodynia were abolished in Cx43/Cx30 double-deletion mice, but not in only Cx30 deletion mice, suggesting a crucial role of Cx43 in the chronic neuropathic pain [27]. Consistently, inhibition of Cx43 expression by using antisense oligodeoxynucleotide led to decrease neuroinflammation and ameliorate pain hypersensitivity in two spinal cord injury models [28]. Intrathecal Cx43 siRNA alleviated mechanical allodynia in a rat model of L5 spinal nerve ligation [8]. Similarly, we found that intrathecal 43Gap26 (a selective Cx43 blocker) could attenuate established BCP at day 18 after inoculation, suggesting Cx43 may participate in the maintenance of BCP. Our data indicate that targeting spinal Cx43 may lead to a potential effective therapy for the treatment of the maintenance phase of BCP in clinical practice.
CXCL12, also known as stromal cell-derived factor 1 (SDF-1), is expressed in many kinds of cells including nervous cells, and plays a vital role in nociception regulation. Studies have shown that intrathecal or intraplantar injection of CXCL12 could induce mechanical hyperalgesia in naive rats [29, 30]. Inhibition of CXCR4, a major receptor of CXCL12, could attenuate pain-related behaviors [31–33]. In the current study, we found that intrathecal anti-CXCL12 neutralizing antibody could attenuate mechanical allodynia in BCP rats. Similar results were reported by Shen et al. [6], who found that CXCL12/CXCR4 contributed to the development and maintenance of BCP.
Cx43 assembles to constitute hemichannels and gap junction channels, which plays a vital physiological role in the behavior of astrocytes as cellular networks [34]. According to the earlier report, Cx43 and Cx45 gap junctions could regulate CXCL12 secretion in bone marrow stromal cells [13]. Increasing evidence suggests that astrocytes can produce proinflammatory cytokines, such as CXCL12 and CXCL1, which play a vital role in the maintenance of BCP [6, 7, 12]. The results from our present study showed that a causal relationship between Cx43 expression and secretion of CXCL12 in the maintenance of BCP. Astrocytic gap junctions may mediate the intercellular transmission of Ca2+ waves to activate neighboring cells [35]. So, we speculate that upregulating Cx43 may activate astrocytes, via intracellular Ca2+-cAMP-PKA signaling pathway, to induce CXCL12 secretion [13]. However, upstream factors for Cx43 activation are still not well documented. Activation of TLR4 via proinflammatory molecules was able to increase the expression of Cx43 in astrocytes [36]. Chen et al. [10] found that TNF-α-induced upregulation of Cx43 led to the chemokine CXCL1 release, which contributed to maintain late-phase neuropathic pain in mice. CXCR4 (a major receptor of CXCL12) expression has been detected in spinal neurons [6, 32]. So, Cx43–CXCL12–CXCR4 signaling might mediate astroglial–neuronal interaction in the maintenance of central sensitization in the BCP. Future studies are needed to verify this hypothesis.
In conclusion, our results suggest Cx43 mediates CXCL12 production from spinal dorsal horn in astrocytes to maintain bone cancer pain in rats. The findings may improve our understanding of the underlying mechanisms of BCP and provide a novel target for the treatment of BCP.
References
Falk S, Dickenson AH (2014) Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 32(16):1647–1654
Krzeszinski JY, Wan Y (2015) New therapeutic targets for cancer bone metastasis. Trends Pharmacol Sci 36(6):360–373
Mantyh P (2013) Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 154(Suppl 1):S54–S62
Mantyh PW (2014) Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 8(2):83–90
Bennett MV, Garré JM, Orellana JA et al (2012) Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res 1487:3–15
Shen W, Hu XM, Liu YN et al (2014) CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflamm 11:75
Xu J, Zhu MD, Zhang X et al (2014) NFκB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflamm 11:38
Xu Q, Cheong YK, He SQ et al (2014) Suppression of spinal connexin 43 expression attenuates mechanical hypersensitivity in rats after an L5 spinal nerve injury. Neurosci Lett 566:194–199
Wang H, Cao Y, Chiang CY et al (2014) The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain 155(2):429–435
Chen G, Park CK, Xie RG et al (2014) Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137(Pt 8):2193–2209
Yoon SY, Robinson CR, Zhang H et al (2013) Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain 14(2):205–214
Hu XM, Liu YN, Zhang HL et al (2015) CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 132(4):452–463
Schajnovitz A, Itkin T, D’Uva G et al (2011) CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat Immunol 12(5):391–398
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Hang LH, Yang JP, Yin W et al (2012) Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur J Neurosci 36(1):2107–2117
Hang LH, Yang JP, Shao DH et al (2013) Involvement of spinal PKA/CREB signaling pathway in the development of bone cancer pain. Pharmacol Rep 65(3):710–716
Hang LH, Shao DH, Chen Z et al (2013) Involvement of spinal CC chemokine ligand 5 in the development of bone cancer pain in rats. Basic Clin Pharmacol Toxicol 113(5):325–328
Hang LH, Li SN, Shao DH et al (2014) Evidence for involvement of spinal RANTES in the antinociceptive effects of triptolide, a diterpene triepoxide, in a rat model of bone cancer pain. Basic Clin Pharmacol Toxicol 115(6):477–480
Hang LH, Shao DH, Chen Z et al (2013) Spinal RhoA/Rho kinase signaling pathway may participate in the development of bone cancer pain. Basic Clin Pharmacol Toxicol 113(2):87–91
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462
Middlemiss T, Laird BJ, Fallon MT (2011) Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 23(6):387–392
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557:52–59
Hibberd C, Cossigny DA, Quan GM (2013) Animal cancer models of skeletal metastasis. Cancer Growth Metastasis 6:23–34
Lee BH, Seong J, Kim UJ et al (2005) Behavioral characteristics of a mouse model of cancer pain. Yonsei Med J 46(2):252–259
Sabino MA, Luger NM, Mach DB et al (2003) Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer 104(5):550–558
Nagy JI, Rash JE (2000) Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev 32(1):29–44
Chen MJ, Kress B, Han X et al (2012) Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60(11):1660–1670
Cronin M, Anderson PN, Cook JE et al (2008) Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci 39(2):152–160
Oh SB, Tran PB, Gillard SE et al (2001) Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21(14):5027–5035
Reaux-Le Goazigo A, Rivat C, Kitabgi P et al (2012) Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci 36(5):2619–2631
Menichella DM, Abdelhak B, Ren D et al (2014) CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Mol Pain 10:42
Dubový P, Klusáková I, Svízenská I et al (2010) Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 133(3):323–337
Luo X, Tai WL, Sun L et al (2014) Central administration of C-X-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PLoS ONE 9(8):e104860
Giaume C, Koulakoff A, Roux L et al (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11(2):87–99
Pannasch U, Rouach N (2013) Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci 36(7):405–417
Aguirre A, Maturana CJ, Harcha PA et al (2013) Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation. Mediators Inflamm 2013:893521
Acknowledgments
This work was supported by the Social Development Fund of Zhenjiang, Jiangsu Province (SH2015049, SH2011036), and the Natural Science Foundation of Jiangsu Province for Youths (BK2012280).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Li-Hua Hang and Shu-Na Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hang, LH., Li, SN., Luo, H. et al. Connexin 43 Mediates CXCL12 Production from Spinal Dorsal Horn to Maintain Bone Cancer Pain in Rats. Neurochem Res 41, 1200–1208 (2016). https://doi.org/10.1007/s11064-015-1815-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1815-7