Abstract
Glioma is a brain tumor deriving from the neoplastic glial cells or neuroglia. Due to its resistance to anticancer drugs and different disease progress of individuals, patients with high-grade glioma are difficult to completely cure, leading to a poor prognosis and low overall survival. Therefore, there is an urgent need to look for prognostic and diagnostic indicators that can predict glioma grades. P53 is one of the widely studied biomarkers in human glioma. The purpose of this study was to comprehensively evaluate the significance of p53 expression in glioma grades and overall survival. We searched commonly used electronic databases to retrieve related articles of p53 expression in glioma. Overall, a total of 21 studies including 1322 glioma patients were finally screened out. We observed that the frequency of p53 immuno-positivity was higher in high-grade patients than that in low-grade category (63.8 vs. 41.6 %), and our statistic analysis indicated that p53 expression was associated with pathological grade of glioma (OR 2.93, 95 % CI 1.87–4.60, P < 0.00001). This significant correction was also found in 1-, 3- and 5-year overall survival. However, no positive relationship was found between age, sex, tumor size and p53 expression in patients with glioma. In conclusion, our results suggested that p53 immunohistochemical expression might have an effective usefulness in predicting the prognosis in patients with glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma, originates from glial cells, is the most common type of primary brain tumor in adults [1, 2]. It accounts for 81 % of all malignant cerebral tumors, leading to significant mortality and morbidity with a poor survival outcome [3]. The incidence of glioma varies by gender, race, age at diagnosis and histologic type [4]. According to the World Health Organization (WHO) classification in 2007, glioma is divided into grade I, II, III and IV, among which low-grade gliomas (I and II) are well-differentiated with a better prognosis, while high-grade gliomas (III and IV) are undifferentiated with a worse prognosis [5]. Although major progresses have made in treatment, it remains the public concern especially in oncology and neurosurgery, with the overall survival (OS) rate decrease year after year [6]. Thus, it is necessary to explore effective biomarkers which would be useful in predicting the gliomas status thereby increasing the OS.
Several molecular markers have been identified to be beneficial to the varying prognoses of gliomas [7–9]. P53 gene, located on human chromosome 17p13, is a tumor suppressor, and has been detected as possible predictive and prognostic factor in gliomas [10]. It is a nuclear phosphoprotein that functions as a transcription factor, and can inhibit DNA replication [11], regulate apoptosis [12], slow proliferation [13], and control cell motility and invasion [14]. P53 expression was shown to be upregulated in patients with colorectal cancer [15], endometrial cancer [16], breast cancer [17], and head and neck squamous cell carcinoma [18]. Furthermore, the network of p53 target genes thus functions as an important regulator of cancer prevention and aging [19]. Understanding the role of p53 in grade and prognosis of gliomas, which can provide clinical insights into the efficacious therapeutic strategy is urgent crucial.
Recent studies confirmed that p53 played an important role in regulating glioma. However, the results remain inconclusive. Pollack et al. [20] found a significant relationship between overexpression of p53 and progression-free survival at five years (P < 0.001). Mokhtari et al. [21] showed that p53 expression might be used in current classification of gliomas for clinical studies. While Newcomb et al. [22] demonstrated that altered expression of p53 gene did not influence the survival of patients with glioblastoma. Antonelli et al. [23] identified that TP53 mutations but not p53 expression might correlate with pediatric high-grade gliomas. Therefore, we conducted this meta-analysis to systematically review and evaluate the role of p53 expression in patients with glioma based on all published articles.
Materials and Methods
Search Strategy
We searched the commonly used electronic databases of Medline, Emabase, PubMed, CNKI and Wanfang to retrieve related articles published between January 2000 and 2015. The following MeSH: “glioma”, “p53 expression”, “prognosis”, and “survival” as well as their combinations were employed as the searching keywords. References of retrieved studies were searched manually. We only focused on studies that conducted in humans. When the same authors reported two or more articles on the same issue, only the most recent full-text was included.
Inclusion Criteria
Eligible studies included must meet the following criteria: (1) patients were confirmed with the diagnostic criteria of glioma by the department of pathology, and were classified based on current WHO guidelines [5]; (2) p53 expression was evaluated by using immunohistochemistry (IHC) methods or RT-PCR; (3) the main results focused on WHO grade and OS; and (4) the relevant data of each studies was available to extract.
Data Extraction
Two experts independently estimated the data from each relevant studies, any disagreement was resolved by discussing with a third expert to reach a consensus on each item. The following information was extracted from each included studies: the name of first author, published year, mean age, sample size, cutoff point for protein positivity, WHO grades of glioma patients and their positive rate.
Statistical Analysis
The RevMan5.2 program was employed to conduct the statistical analysis. The significance of p53 expression in glioma patients was estimated by risk ratios (RRs) or odds ratios (ORs) and its 95 % confidence intervals (CI). The Z-test was used to determine the statistical significance with a P value less than 0.05 considered significant. Between-study heterogeneity was assessed by the Q-test and the I2 test. The random-effect model was used when the effect were heterogeneous (P-value ≤0.01 for the Q-test and I2 ≥ 50 % for the I2 test), while the fixed-effect model was used when it was homologous.
Results
Characteristics of Included Studies
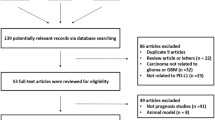
We firstly identified 159 articles. After deleting the duplicate ones and applying the inclusion criteria, only 21 articles were finally screened out, including 1322 glioma patients. The selection process was shown in Fig. 1. Of the 21 studies, one were written in English [24] and 20 in Chinese [25–44]; All of them were conducted in Chinese population. The sample size ranged from 38 to 152. P53 expression was evaluated by using IHC method in all articles. Positive expression of p53 protein was detected in 711 glioma patients (53.8 %, range from 33.3 to 68.9 %). The cutoff points for p53 expression selected in most studies was that 10 % or less positive cell percentage was scored as no staining (−); 10–25 % as weak intensity (+); 26–50 % as moderate intensity (++); 51 % or more as strong intensity (+++). The main characteristics of included studies were presented in Table 1.
Association Between p53 Expression and WHO Grade of Glioma
Patients with glioma were divided into two groups according to WHO grade: low-grade (I+II) group and high-grade (III+IV) group. There were 739 high-grade and 583 low-grade patients in all included studies, respectively. A significant heterogeneity was found between-studies, and the random-effect model was used. The frequency of p53 immunopositivity was shown to be higher in high-grade patients than that in low-grade group (63.8 vs. 41.6 %), and our statistical analysis indicated there was a significant difference between p53 expression and grades of glioma patients. Overall, our results demonstrated that p53 expression was associated with pathological grade of glioma (OR 2.93, 95 % CI 1.87–4.60, P < 0.00001) as shown in Fig. 2.
We also examined the correlation of p53 expression in glioma and normal tissues. A total of 12 studies contained 815 glioma patients and 130 controls. This results showed that p53 expression was connected with glioma as well (OR 23.16, 95 % CI 10.51–51.03, P < 0.00001) in the fixed-effect model as shown in Fig. 3.
Correlation Between p53 Expression with Sex, Age and Tumor Size in Patients with Glioma
Three studies including 86 male patients and 65 female patients concerned the sex issue. Our result did not find a significant association between p53 expression and sex variable (OR 1.19, 95 % CI 0.62–2.29, P = 0.60) in the fixed-effect model as shown in Fig. 4a.
Three studies focused on the age issue. For different age stages were presented, we divided ages into two comparable groups (≥50 and <50 year-old). As shown in Fig. 4b, our result detected no difference between p53 expression and age difference (OR 1.56, 95 % CI 0.81–3.03, P = 0.18).
Three articles concerned the tumor size, including 148 glioma patients. Our result showed no relationship between tumor size and p53 expression (OR 0.99, 95 % CI 0.19–5.04, P = 0.99) in the random-effect model as shown in Fig. 4c.
Correlation of p53 Expression with Overall Survival (OS)
Four articles were obtained, including 310 glioma patients. Two articles concerned the 1-year OS, two in the 3-year OS, two in the 5-year OS. Our result showed that p53 expression was significantly associated with 1-year OS (RR 3.32, 95 % CI 1.46–7.53, P = 0.004) in the fixed-effect model. This significant association was also found with 3-year OS (RR 2.10, 95 % CI 1.25–3.53, P = 0.005), and 5-year OS (RR 1.40, 95 % CI 1.13–1.74, P = 0.002). Figure 5 showed the relationship of p53 expression with OS in glioma patients.
One article concerned the survival times of patients with glioma. The data from the study conducted by Xiao et al. could not be extracted, but the result revealed a significant effect of p53 expression on the cumulative survival time (P < 0.01), indicating that p53 expression played a role on survival time.
Sensitivity Analysis and Publication Bias
Each individual study was deleted one time to observe whether the single study would affect the pooled OR. Our result showed that the OR was not affected by omitting the included studies. The funnel plots were employed to reveal the publication bias, as shown in Fig. 6, no obvious asymmetry was presented, further indicating no publication bias in this study.
Discussion
In this meta-analysis, we identified 21 articles that investigated the effect of p53 expression in glioma prognosis and pathology. Overall, our results showed that p53 expression was associated with pathological grades of glioma. This significant association was also found in 1-, 3-, and 5-year OS. However, no correction was found between p53 expression and sex, age and tumor size. These results suggested that positive p53 expression could effectively predict patient with high-grade glioma (III+IV) and OS. Our results were not consistent with previous meta-analysis which did not find the significance of p53 expression as a prognostic marker in patients with astrocytomas (one type of the high-grade gliomas) [45]. This was the first meta-analysis that systematically evaluated the role of p53 expression in patients with glioma grades.
P53, the tumor suppressor gene, plays a vital role in control of the cell cycle and apoptosis [46]. It also regulates the transcription of multiple genes which involved in a complex carcinogenesis signaling pathway. The p53 protein is a promise cancer therapeutic target, and drug target for novel therapies in glioma [47]. Recent studies have showed that p53 expression was common in glioma, which involved in the pathogenesis, patient prognosis, and therapeutic targeting [48]. Glioma stem cells depended on signaling pathways to regulate survival and tumor radio resistance in a p53-dependent manner [49]. P53 expression appeared to be helpful in the setting of diffuse low-grade glioma phenotype diagnosis [50], and were maximally noted in patients with poorer outcome in pediatric glioblastoma multiforme [51]. IHC staining with p53 might serve as an additional useful tool in determining the clinical course in combination with and as an adjunct to tumor grade [52], and studies have demonstrated a positive correlation between p53 expression with the grade of malignancy, according with the WHO classification [53].
P53 may play a role in glioma through interacting with other gene expression. Studies have identified that p53-induced miR-107 could suppresses proliferation of glioma cell [54]. Wild-type p53-induced phosphatase 1 was shown to be conferred poor prognosis of patients with glioma, which was related with pathological diagnosis and prognosis evaluation for malignant glioma [55]. Isocitrate dehydrogenase 1 mutation in diffuse glioma correlated significantly with p53 expression [56]. Activation of p53 might be a therapeutic option in patients with high-grade glioma which expressed both a high level of α5β1 integrin and functional p53 [57].
P53 may involve in glioma therapeutic strategy. P53 overexpression was found to be the only significant molecular prognostic factor for outcome in patients with glioblastoma [58]. P53 status may influence response to temozolomide in differentiated cells in a glioblastoma [59]. The beneficial effect of combination treatment with temozolomide and CQ in glioma via differential autophagy-associated mechanisms, depending on p53 status [60]. Combining SGT-53 with temozolomide appears to limit development of temozolomide resistance, prolonging its anti-tumor effect and could be a more effective therapy for glioblastoma [61].
Recently, mutant p53 was proven to be associated with a broad range of cancers risk, and might be useful in developing new therapeutic approaches [62]. P53 variants might influence p53 gene statue and protein expression, thus involving in glioma risk and relating with tumor pathology. This gene mutations were frequently found in all malignancy stages of glioma, and corrected with increased susceptibility to glioma [63]. The association between global statistical test of glioblastoma and p53 haplotypes was shown to be significant (P = 0.02) [64]. Glioma tumor grade correlated with parkin depletion in mutant p53-linked tumors due to loss of p53 transcriptional activity [65]. Previous meta-analysis proved that he polymorphism of p53 codon 72 Arg/Pro might play a protective role in the development of glioblastoma [66]. P53 mutations seen in pediatric glioblastoma multiforme were associated with a poor prognosis [67].
P53 expression was also associated with prognosis in patients with other diseases. Yao et al. [68] demonstrated that p53 overexpression was an independent predictor of poorer OS and prognosis in patients with early stage esophageal squamous cell carcinoma. Gunia et al. [69] identified that the five-year cancer-specific survival of patients with penile cancer was higher in p53-negative than that in p53-positive, indicating that p53 expression predicted poor prognosis and was negatively associated with cancer specific survival.
Several limitations were presented in our study. Firstly, all of the included studies were conducted in Chinese population, patients in other ethnicities were also should be considered. Secondly, p53 statue should be divided into p53 expression normal or p53 overexpression. Moreover, p53 mutation which would affect its expression should be included in the future researches. Thirdly, patients with glioma might be in different conditions (receiving different treatment or not). Fourthly, expression of other genes which may interact with p53 should be considered. Lastly, the cutoff point for p53 expression was different which might influence our results.
In conclusion, our results suggested that p53 expression was corrected with glioma grade and OS, not associated with age, sex and tumor size. These outcomes indicated that p53 might be a prognostic biomarker in patients with glioma. Further studies with large scale, more ethnicities, and other interacted genes should be included.
References
Hofer S, Rushing E, Preusser M, Marosi C (2014) Molecular biology of high-grade gliomas: What should the clinician know? Chin J Cancer 33:4–7
Chen Y, Gutmann D (2014) The molecular and cell biology of pediatric low-grade gliomas. Oncogene 33:2019–2026
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 16(7):896–913
Butowski NA, Berger M (2012) Malignant gliomas: Part I: epidemiology, risk factors, prognostic factors, and imaging findings. Contemp Neurosurg 34:1–5
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Chowdhary SA, Ryken T, Newton HB (2015) Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis. J Neurooncol 122(7):367–382
Noble M, Dietrich J (2004) The complex identity of brain tumors: emerging concerns regarding origin, diversity and plasticity. Trends Neurosci 27:148–154
Lv S, Dai C, Liu Y, Shi R, Tang Z, Han M, Bian R, Sun B, Wang R (2015) The impact of survivin on prognosis and clinicopathology of glioma patients: a systematic meta-analysis. Mol Neurobiol 51:1462–1467
Yang X, Lv S, Liu Y, Li D, Shi R, Tang Z, Fan J, Xu Z (2015) The clinical utility of matrix metalloproteinase nine in evaluating pathological grade and prognosis of glioma patients: a meta-analysis. Mol Neurobiol 52:38–44
Nieder C, Petersen S, Petersen C, Thames H (2000) The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat Rev 26:67–73
Misiewicz-Krzeminska I, Sarasquete ME, Quwaider D, Krzeminski P, Ticona FV, Paíno T, Delgado M, Aires A, Ocio E, García-Sanz R (2012) Restoration of miR-214 expression reduces growth of myeloma cells through a positive regulation of P53 and inhibition of DNA replication. Haematologica:haematol. 2012.070011
Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Ramirez FG, Rizzuto R, Di Virgilio F, Zito E (2015) p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci 112:1779–1784
e Silva RDF, dos Santos NFG, Pereira VRA, Amaral A (2014) Simultaneous analysis of P53 protein expression and cell proliferation in irradiated human lymphocytes by flow cytometry. Dose-Response 12:110–120
Hwang C-I, Matoso A, Corney DC, Flesken-Nikitin A, Körner S, Wang W, Boccaccio C, Thorgeirsson SS, Comoglio PM, Hermeking H (2011) Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci 108:14240–14245
He Z-Y, Shi C-B, Wen H, Li F-L, Wang B-L, Wang J (2011) Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig 29:208–213
Kularatne B, Capitanio A, Arora R, Jones P, Lopes A, Paterson J, Kristeleit R (2013) Abstract C32: PTEN and p53 expression in endometrial cancer correlated with clinicopathological phenotype. Mol Cancer Ther 12:C32–C32
Yamamoto M, Hosoda M, Nakano K, Jia S, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y, Yamashita H (2014) p53 accumulation is a strong predictor of recurrence in estrogen receptor-positive breast cancer patients treated with aromatase inhibitors. Cancer Sci 105:81–88
Sivars L, Näsman A, Tertipis N, Vlastos A, Ramqvist T, Dalianis T, Munck-Wikland E, Nordemar S (2014) Human papillomavirus and p53 expression in cancer of unknown primary in the head and neck region in relation to clinical outcome. Cancer Med 3:376–384
Reinhardt HC, Schumacher B (2012) The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet 28:128–136
Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R (2002) Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med 346:420–427
Mokhtari K, Paris S, Aguirre-Cruz L, Privat N, Criniere E, Marie Y, Hauw JJ, Kujas M, Rowitch D, Hoang-Xuan K, Delattre JY, Sanson M (2005) Olig2 expression, GFAP, p53 and 1p loss analysis contribute to glioma subclassification. Neuropathol Appl Neurobiol 31:62–69
Newcomb EW, Cohen H, Lee SR, Bhalla SK, Bloom J, Hayes RL, Miller DC (1998) Survival of patients with glioblastoma multiforme is not influenced by altered expression of P16, P53, EGFR, MDM2 or Bcl-2 genes. Brain Pathol 8:655–667
Antonelli M, Buttarelli FR, Arcella A, Nobusawa S, Donofrio V, Oghaki H, Giangaspero F (2010) Prognostic significance of histological grading, p53 status, YKL-40 expression, and IDH1 mutations in pediatric high-grade gliomas. J Neurooncol 99:209–215
Hu X, Miao W, Zou Y, Zhang W, Zhang Y, Liu H (2013) Expression of p53, epidermal growth factor receptor, Ki-67 and O6-methylguanine-DNA methyltransferase in human gliomas. Oncol Lett 6:130–134
Xiao Q, Huang S (2004) PDGFRa, MMP-2, MMP-9, p53 expression is associated with the infiltrative nature and prognosis of glioma. Sichuan University, Chengdu
Hong L, Li Q, Chen G, Lin S (2005) Expression and relationship of RGS16 and p53 in human glioma. Chin J Neurosurg Dis Res 4:248–251
Kong X, Cao H, Cui W, Liang G (2005) Relevant research of MDM2 and p53 expression in human gliomas. J Jining Med Coll 29:27–28
Lin Y, Huang S, Liao D (2005) Expression of DNA repair enzyme MGMT in human brain gliomas and its biological implication. J Mod Clin Med Bioeng 11:187–201
Wang J, Guan X, Zhan X, Luo B (2005) Expression of PCNA, Ki-67 and p53 in human glioma. Chin J Cancer Prev Treat 12:753–755
Ma L, Xu G, Song L, Zhang J (2006) The study of Survivin and p53 expression in glioma. J Basic Clin Oncol 19:4–6
Wang J, Z-c YUAN, Shi Y (2007) Expression of p53, Ki67, VEGF and MMP-9 in glioma and their clinical significances [J]. J Jiangsu Univ (Medicine Edition) 3:012
Wu Y, Dong L, Gu X, Yu B (2007) Expression of β-catenin, p53 and Ki67 in the human glioma tissue tested by tissue chip. J Mod Oncol 15:1396–1399
Wei Y (2007) Correlation analysis of p53 and MDM2 expression in human brain glioma. Shanxi Medical University, Taiyuan
Cheng A, Wan F, Jin Z, Wang J, Xu X (2008) Nitrite oxide and inducible nitric oxide synthase were regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J Ethnopharmacol 118:59–64
Long X, Xu H, Zeng Y, You C (2009) Expression of p14ARF, p53 and p21WAF1 protein in gliomas and their significance. J Mod Oncol 17:2090–2093
Zhang J (2010) Study of relationship between CatB and p53 expression and malignancy in human glioma. Kunming Medical College, Kunming
Ji L, Zhou F (2011) The correlation of the expression of MGMT and p53 in human brain gliiomas with tumor grade or prognosis. Chin J Clin Neurosci 19:588–593
M-y PAN, Z-m FANG, Y-b KANG, Z-p LIN (2011) Expression of p33 ~ (ING1b) and p53 gene in brain glioma and their relations. J Shanxi Med Univ 8:014
Gu W, Pei H (2012) The relationship between Ki-67, p53, PCNA and the prognosis of gliomas. Chin J Mod Drug Appl 6:16–18
Zhou K, Zhang M, Liu B (2012) Expression of p53 and Ki67 in the gliomas and its clinical significance. Pract J Cancer 27:572–573
Zeng R, Deng J, Xia L, Gong M (2013) Expression of p53, p15 and VEGF in human brain glioma and their relationship with malignace degree. J Xi’an Jiaotong Univ 34:365–370
He J, Feng H, Wang T, Zhang X (2014) Expression and clinical significance of SPARC, p53 and VEGF in the gliomas. J Shanxi Med Univ 45:1140–1143
Liu L, Li W, Xia H, Luan X (2014) Expression and clinical significance of MGMT, p53 and Ki-67 in XINJIANG gliomas. Xinjiang Med J 44:10–13
Luo Z, Zhou C, Li P (2014) The expression of p53, MGMT and EGFR in glioma and their clinical significance. Sichuan Med J 35:551–554
Levidou G, El-Habr E, Saetta AA, Bamias C, Katsougiannis K, Patsouris E, Korkolopoulou P (2010) P53 immunoexpression as a prognostic marker for human astrocytomas: a meta-analysis and review of the literature. J Neurooncol 100:363–371
Case AJ, Domann FE (2014) Absence of manganese superoxide dismutase delays p53-induced tumor formation. Redox Biol 2:220–223
Kim SH, Dass CR (2011) p53-targeted cancer pharmacotherapy: move towards small molecule compounds. J Pharm Pharmacol 63:603–610
England B, Huang T, Karsy M (2013) Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumor Biol 34:2063–2074
Gu C, Banasavadi-Siddegowda YK, Joshi K, Nakamura Y, Kurt H, Gupta S, Nakano I (2013) Tumor-Specific activation of the C-JUN/MELK pathway regulates glioma stem cell growth in a p53-dependent manner. Stem Cells 31:870–881
Gillet E, Alentorn A, Doukouré B, Mundwiller E, van Thuij H, Reijneveld JC, Medina JAM, Liou A, Marie Y, Mokhtari K (2014) TP53 and p53 statuses and their clinical impact in diffuse low grade gliomas. J Neurooncol 118:131–139
Ganigi P, Santosh V, Anandh B, Chandramouli B, Sastry KV (2004) Expression of p53, EGFR, pRb and bcl-2 proteins in pediatric glioblastoma multiforme: a study of 54 patients. Pediatr Neurosurg 41:292–299
Arshad H, Ahmad Z, Hasan SH (2010) Gliomas: correlation of histologic grade, Ki67 and p53 expression with patient survival. Asian Pac J Cancer Prev 11:1637–1640
Ranuncolo SM, Varela M, Morandi A, Lastiri J, Christiansen S, de Kier Joffé EB, Pallotta MG, Puricelli L (2004) Prognostic value of Mdm2, p53 and p16 in patients with astrocytomas. J Neurooncol 68:113–121
Chen L, Zhang R, Li P, Liu Y, Qin K, Z-q Fa, Y-j Liu, Y-q Ke, X-d Jiang (2013) P53-induced microRNA-107 inhibits proliferation of glioma cells and down-regulates the expression of CDK6 and Notch-2. Neurosci Lett 534:327–332
Liang C, Guo E, Lu S, Wang S, Kang C, Chang L, Liu L, Zhang G, Wu Z, Zhao Z (2012) Over-expression of Wild-type p53-induced phosphatase 1 confers poor prognosis of patients with gliomas. Brain Res 1444:65–75
Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M (2011) Expression of mutated isocitrate dehydrogenase-1 in gliomas is associated with p53 and EGFR expression. Folia Neuropathol 49:88–93
Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F (2012) Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res 72:3463–3470
Malkoun N, Chargari C, Forest F, Fotso M-J, Cartier L, Auberdiac P, Thorin J, Pacaut C, Peoc’h M, Nuti C (2012) Prolonged temozolomide for treatment of glioblastoma: preliminary clinical results and prognostic value of p53 overexpression. J Neurooncol 106:127–133
Blough MD, Beauchamp DC, Westgate MR, Kelly JJ, Cairncross JG (2011) Effect of aberrant p53 function on temozolomide sensitivity of glioma cell lines and brain tumor initiating cells from glioblastoma. J Neurooncol 102:1–7
Lee SW, Kim H-K, Lee N-H, Yi H-Y, Kim H-S, Hong SH, Hong Y-K, Joe YA (2015) The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett 360:195–204
Kim S-S, Rait A, Kim E, Pirollo KF, Chang EH (2015) A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine 11(2):301–311
Muller PA, Vousden KH (2014) Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 25:304–317
van Meyel DJ, Ramsay DA, Casson AG, Keeney M, Chambers AF, Cairncross JG (1994) p53 mutation, expression, and DNA ploidy in evolving gliomas: evidence for two pathways of progression. J Natl Cancer Inst 86:1011–1017
Malmer BS, Feychting M, Lönn S, Lindström S, Grönberg H, Ahlbom A, Schwartzbaum J, Auvinen A, Collatz-Christensen H, Johansen C (2007) Genetic variation in p53 and ATM haplotypes and risk of glioma and meningioma. J Neurooncol 82:229–237
Viotti J, Duplan E, Caillava C, Condat J, Goiran T, Giordano C, Marie Y, Idbaih A, Delattre J, Honnorat J (2014) Glioma tumor grade correlates with parkin depletion in mutant p53-linked tumors and results from loss of function of p53 transcriptional activity. Oncogene 33:1764–1775
He F, Xia Y, Liu H, Li J, Wang C (2013) P53 codon 72 Arg/Pro polymorphism and glioma risk: an updated meta-analysis. Tumor Biol 34:3121–3130
Cage TA, Mueller S, Haas-Kogan D, Gupta N (2012) High-grade gliomas in children. Neurosurg Clin N Am 23:515–523
Yao W, Qin X, Qi B, Lu J, Guo L, Liu F, Liu S, Zhao B (2014) Association of p53 expression with prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7:7158
Gunia S, Kakies C, Erbersdobler A, Hakenberg OW, Koch S, May M (2012) Expression of p53, p21 and cyclin D1 in penile cancer: p53 predicts poor prognosis. J Clin Pathol 65:232–236
Acknowledgments
This study was supported by Shanghai Pudong Science and Technology Commission, China. (Grant No.: PKJ2014-Y23).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Weizhong Xiao and Tingting Song are co-first authors of this article.
An erratum to this article is available at http://dx.doi.org/10.1007/s11064-016-2083-x.
Rights and permissions
About this article
Cite this article
Jin, Y., Xiao, W., Song, T. et al. Expression and Prognostic Significance of p53 in Glioma Patients: A Meta-analysis. Neurochem Res 41, 1723–1731 (2016). https://doi.org/10.1007/s11064-016-1888-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1888-y