Abstract
Purpose
This study investigated the effect of an isocitrate dehydrogenase 1 (IDH1) mutation (mutIDH1) on the invasion and angiogenesis of human glioma cells.
Methods
Doxycycline was used to induce the expression of mutIDH1 in glioma cells. Transwell and wound healing assays were conducted to assess glioma cell migration and invasion. Western blotting and cell immunofluorescence were used to measure the expression levels of various proteins. The influence of bone morphogenetic protein 2 (BMP2) on invasion, angiogenesis-related factors, BMP2-related receptor expression, and changes in Smad signaling pathway-related proteins were evaluated after treatment with BMP2. Differential gene expression and reference transcription analysis were performed.
Results
Successful infection with recombinant lentivirus expressing mutIDH1 was demonstrated. The IDH1 mutation promoted glioma cell migration and invasion while positively regulating the expression of vascularization-related factors and BMP2-related receptors. BMP2 exhibited a positive regulatory effect on the migration, invasion, and angiogenesis of mutIDH1-glioma cells, possibly mediated by BMP2-induced alterations in Smad signaling pathway-related factors.After BMP2 treatment, the differential genes of MutIDH1-glioma cells are closely related to the regulation of cell migration and cell adhesion, especially the regulation of Smad-related proteins. KEGG analysis confirmed that it was related to BMP signaling pathway and TGF-β signaling pathway and cell adhesion. Enrichment analysis of gene ontology and genome encyclopedia further confirmed the correlation of these pathways.
Conclusion
Mutation of isocitrate dehydrogenase 1 promotes the migration, invasion, and angiogenesis of glioma cells, through its effects on the BMP2-driven Smad signaling pathway. In addition, BMP2 altered the transcriptional patterns of mutIDH1 glioma cells, enriching different gene loci in pathways associated with invasion, migration, and angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastomas are the most common and malignant primary brain tumors, accounting for approximately 30% of all primary and 80% of all malignant brain tumors [1]. Their continuous proliferation, migration, invasion, angiogenesis, and chemoresistance contribute to poor prognosis in patients with glioblastoma [2]. Mutations in isocitrate dehydrogenase (IDH) are frequently observed in glioblastoma and represent early genetic events in tumor progression. IDH enzymes convert isocitrate to α-ketoglutarate. In humans, three subtypes of IDH enzymes are encoded by IDH1, IDH2, and IDH3; all of which play crucial roles in metabolic reactions. IDH1 is present in the cytoplasm and peroxisomes, whereas IDH2 and IDH3 are present in the mitochondrial matrix [3]. In glioblastomas, mutations in IDH enzymes primarily occur in the IDH1 and IDH2, with the most common mutations located in codons R132 and R172, respectively [4]. IDH1 mutations in glioblastomas are closely associated with tumor proliferation, migration, invasiveness, and angiogenesis [5]. However, the interpretation of these phenomena remains controversial because clinical studies have shown that glioblastomas with IDH mutations have a better prognosis [5]. Therefore, understanding the effects and underlying mechanisms of IDH mutations on glioma cell migration, invasion, and angiogenesis is crucial.
Bone morphogenetic proteins (BMPs) are capable of inducing bone and cartilage formation at ectopic sites. Structurally, and in terms of amino acid sequences, BMPs are similar to transforming growth factor-β (TGF-β), and with the exception of BMP1, they all belong to the multifunctional growth factor family of TGF-β [6]. BMP2 is a cytokine that promotes osteoblast differentiation and bone matrix synthesis by activating intracellular signaling pathways. IDH mutations activate the BMP signaling pathway in gliomas [7], an evolutionarily conserved pathway with diverse functions in different tissue types, including cell proliferation,differentiation control, epithelial-mesenchymal transition (EMT), bone metabolism. [8].
During tumor metastasis, cancer cells interact with the tumor microenvironment through various growth factors. BMP has a dual role in the development and progression of cancer [9] and can either inhibit or promote tumorigenesis, promoting metastasis in most cases [10]. However, whether BMP affects the biological behavior of IDH1 mutant gliomas remains unclear. Therefore, this study investigated the characteristics and mechanisms involved in IDH1 mutant glioma cell migration, invasion, and angiogenesis and the regulatory role of BMP2 in these processes.
Materials and methods
Reagents and instruments
Experimental reagents: exposure solution purchased from Shanghai Shangbao Biology Co., Ltd.; crystal purple dye solution; 4% paraformaldehyde; phosphate buffer solution PBS (American HyClone); mouse anti-IDH1 (Japanese Sigma); mouse anti-GAPDH (# 51332S) Antibody HO-1 was purchased from American CST company, antibody E-cadherin was purchased from Santa cruz company, antibody N-cadherin, Vimentin, GFAP, Snal1, Snal2, Twist1, Zeb2, BMPR1A, BMPR1B, BMPR2, SOX2, Nestin, HIF1a, Nrf2, VEGF, P-CX43 were purchased from Affinty company, and anti-rabbit and goat anti-mouse 603 antibodies labeled with horseradish peroxide (should) were purchased from Proteintech company. Bone morphogenetic protein 2 was purchased from Beijing Yiqiao Shenzhou Technology Co., Ltd.
Experimental instruments: optical microscope (OLYMPUS, Japan); cell incubator (Shanghai Likang Company); inverted microscope (Olympus company, Japan); cell culture bottle (Corning, USA); cell cryopreservation tube (Corning, USA); freezing centrifuge: ST16R,Thermo; gel imaging system: Tanon 2500, Tianneng Company; desktop centrifuge eppendorf Centrifuge 5418R; various models of tip, centrifugal tube Axygen.
Cell lines
Glioma U87,U251 cells expressing a doxycycline (dox)-inducible mutant IDH1 (including IDH1 R132H) were generously provided by Professor Jing Ye at the Air Force Medical University.
Cell culture and doxycycline induction
Cells were maintained as adherent monolayers in Minimum Essential Medium supplemented with 10% fetal bovine serum (FBS; HyClone; Cytiva) at 37 °C and 5% carbon dioxide (CO2).For doxycycline-inducible expression, the glioma cells were cultured in DMEM-ALPPHA containing 1% penicillin–streptomycin and 10% FBS before 1 × 106 cells were seeded into culture dishes. After 8 h, when the cells adhered, the medium in the dox + group was replaced with complete medium containing 10 μg/μL dox. The cells were then incubated for 48 h and observed under a microscopeThe basic doxycycline-inducible expression system involved lentiviral expression vector containing IDH1 R132H, pLVX-Tight-IDH1(R132H) with G418 selection marker; Tet-On lentiviral expression vector, pLVX-Tet-On with a puromycin selection marker.
A stock solution of 0.15 mg/mL human BMP2 was prepared using ultrapure water. Forty-eight hours after dox-induction, BMP2 was added to give an optimal final concentration of 80 ng/mL [11].
Transwell assay
After cell digestion and counting, the cells were seeded in Transwell chambers with culture medium containing 20% serum for 24 h. The remaining cells in the upper chamber were removed using a cotton swab, and the chambers were washed twice with phosphate-buffered saline (PBS). The cells were fixed with 4% paraformaldehyde, stained with crystal violet for 16 min, and washed with PBS. Cell migration was observed under a microscope, images were captured at various locations, and the number of migrating cells in each group was calculated.
Wound healing assay
A cross was drawn at the bottom of a six-well plate, and 5 × 105 cells were added to each well. After the cells adhered, a scratch was made at the bottom of each well using a 200 µL pipette tip. The wells were washed three times with PBS, and photographs were taken to record the initial (0 h) scratch width. Subsequently, photographs were taken at 12 h and 24 h in the same location to measure the scratch healing rate, which was calculated as
Western blot analysis
Forty micrograms of protein were loaded onto 10% polyacrylamide gels for electrophoresis at 80 V before transferring onto membranes and blocking with milk. Primary antibodies (mutIDH1 1:1000, GAPDH 1:2000, and others) were incubated at 4 °C overnight, followed by incubation with secondary antibody at room temperature for 1.5 h. After washing, the membranes were exposed and imaged using a gel imaging system, and grayscale values were measured.
Cellular immunofluorescence
Cell climbing tablets were placed in a 24-well plate and 1 mL cell suspension at a density of 1 × 105/mL was added for culture. The tablets were then removed and the cells washed with PBS three times before fixing for 15 min and washing with PBS three times. The cells were then incubated with the diluted primary antibody (E-cadherin 1:50; N-cadherin, vimentin, and GFAP 1:100) at room temperature for 30 min, washed with PBS three times, and incubated with the secondary antibody (1:200) for 1 h protected from light. The results were then visualized using fluorescence microscopy.
mRNA sequencing analysis process
RNA Sequencing and Differentially Expressed Genes Analysis.The libraries were sequenced on an llumina Novaseq 6000 platform and 150 bp paired-end reads were generated.Differential expression analysis was performed using the DESeq2. Q value < 0.05 and foldchange > 2 or foldchange < 0.5 was set as the threshold for significantly differential expression gene (DEGs). Hierarchical cluster analysis of DEGs was performed using R (v 3.2.0) to demonstrate the expression pattern of genes in different groups and samples. The radar map of top 30 genes was drew to show the expression of up-regulated or down-regulated DEGs using R packet ggradar.Based on the hypergeometric distribution, GO,Footnote 1 KEGGFootnote 2 pathway, Reactome and WikiPathways enrichment analysis of DEGs were performed to screen the significant enriched term using R (v 3.2.0), respectively.
Statistical analysis
Statistical analyses were performed using SPSS 23.0 software (IBM Corp., Armonk, NY, USA). Data were presented as means ± standard deviations (x ± s) for continuous variables. Differences between the two groups were analyzed using t-tests, whereas one-way analysis of variance was used for multiple group comparisons, followed by least significant difference post hoc tests for pairwise comparisons. All independent experiments were repeated three times, and p < 0.05 was considered statistically significant.
Results
IDH1 mutations alter the expression of invasion, migration, and EMT-related genes
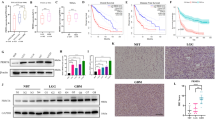
After the establishment of doxycycline (dox) induced expression system, IDH mutant (mutIDH1) protein was successfully induced expression (Fig. 1A, B). Transwell assay showed a significant increase in cell invasion in the IDH mutant group (Fig. 1C). Scratch healing experiments showed a significant increase in migration at 12 h and 24 h in the IDH mutant group compared to the non-mutant group (Fig. 1D). Western blot experiments showed that the expression of EMT-related transcription factors Snal1, Snal2, Twist1 and Zeb2 increased in the IDH mutant group compared with the non-mutant group (Fig. 1E, F). The expressions of EMT-related factors N-cadherin, vimentin and GFAP were significantly increased in the IDH mutant group (Fig. 1G, H). Immunofluorescence results showed that green fluorescence was the target protein, targeted at cytoplasm, and blue fluorescence DAPI stained the nucleus. Compared with the non-mutant group, the fluorescence intensity of E-cadherin in the cytoplasm of IDH mutant group was weaker, while the fluorescence intensity of N-cadherin, vimentin and GFAP was stronger (Fig. 1I). The difference among all groups was statistically significant (*p < 0.05, **p < 0.01).
IDH1 mutation affects glioma cell invasion, migration and expression of EMT-related genes. A, B Western blot analysis of IDH mutant protein expression and bar statistical analysis diagram. C Transwell experiment and bar chart. D Wound healing tests and bar charts. (E–H)Western blot analysis and statistical analysis of EMT-related factors. (I) Immunofluorescence showed the expression of E-cadherin, N-cadherin, vimentin and GFAP in the cytoplasm (200 ×)
IDH mutations affect the tumor microenvironment, including angiogenesis, intercellular connectivity, and expression of stem cell-related genes and BMP-related genes
Western blot experiments showed that the expressions of angiogenic factors HIF, NRF2, VEGF, HO-1, VE-cadherin and EphA2 in the IDH mutant group were higher than those in the non-mutant group (Fig. 2A, D), suggesting that IDH1 mutation up-regulated the angiogenic capacity of glioma cells. Compared with the non-mutant group, the expression of gap junction related factors, including ZO-1, claudin-5, CX43, p-CX43, aquaporin, collagen II, laminin and occludin, was significantly decreased in the IDH mutant group (Fig. 2E, F). The expressions of BMP2-related receptors BMPR1A, BMPR1B and BMPR2 were increased, while the expressions of stem cell-related genes SOX2 and nestin were decreased, while the expressions of BMP2 and BMP4 were significantly increased (*p < 0.05, **p < 0.01, Fig. 2G–N).
IDH mutations affect the tumor microenvironment, including angiogenesis, intercellular connectivity, and expression of stem cell-related genes and BMP-related genes. A–N Western blot analysis was performed to detect and statistically analyze the expression of angiogenesis related factors, intercellular junction factors, stem cell related genes and BMP related genes
BMP2 promotes the expression of EMT and angiogenesis related genes and activates the Smad signaling pathway
Exogenous BMP2 promoted the differentiation of glioma cells, and Western blot analysis showed increased expression of BMP2-related receptors (including BMPR1A, BMPR1B, and BMPR2, Fig. 3A, B) in the IDH mutant group compared with the non-mutant group. After BMP2 intervention, the expression of E-cadherin and N-cadherin, vimentin and GFAP were significantly increased. The expressions of angiogenic factors HIF, NRF2, VEGF and HO-1 were significantly increased (Fig. 3C–F). In the non-mutant group, the expression of SMAD2 and SMAD3 decreased after BMP2 intervention, while the expression of SMAD4 and P-SMAD2/3 increased. In the mutant group, the expression of SMAD signaling pathway gene was significantly increased after BMP2 intervention (*p < 0.05, **p < 0.01, Fig. 3G, H).
BMP2 alters transcription in mutIDH1 glioma cells, enriching genes associated with cell invasion and biological function of BMP-related signaling pathways
Paired transcriptome sequencing was performed on Mut + BMP2 and Mut samples. We identified 1782 differentially expressed genes (DEGs), of which 902 were down-regulated and 879 up-regulated (Fig. 4A). We then analyzed the top 30 most important DEGs and identified SMAD6, SMAD11, and HIF1A that were directly relevant for this study (Fig. 4B). The GO enrichment analysis identified four related terms, namely "BMP signaling pathway," "positive regulation of chondrogenesis," "positive regulation of cell migration," and "cell adhesion factor," which all belong to the top 30 enrichment terms for up-regulated and down-regulated genes. These terms are closely related to the regulation of cell migration, cell adhesion, and function of BMP-related proteins (Fig. 4C, D). KEGG enrichment analysis of up-regulated genes showed that some genes were related to transforming growth factor-β and signaling pathways regulating stem cell potential, while KEGG enrichment analysis of down-regulated genes showed that some genes were related to cell adhesion factors (Fig. 4E, F). The scratch healing experiment showed an increase in the amount of migration at 24 h and 48 h in the BMP2 intervention group compared with no BMP2 intervention (Figures G, H). This suggests that BMP2 affects the migration, invasion, and angiogenesis of IDHmut glioma cells, possibly by altering their transcriptional patterns,affecting associated cellular functions.
BMP2 alters transcription in IDH-mutated glioma cells. A Volcano map, with gray indicating non-significant differentially expressed genes (DEGs) and red and blue indicating significantly differentially expressed genes. B DEGs radar maps showing minimum P-values for 30 up-regulated or down-regulated genes. C GO enrichment analysis revealed the top 30 up-regulated genes. D GO enrichment analysis of the top 30 down-regulated genes. Arrange the 10 items in descending order according to the corresponding − log 10p value. E and F KEGG enrichment analysis was performed for the top 20 factors, and the corresponding log 10p values were sorted from largest to smallest. Wound healing tests and bar charts (G, H).
Discussion
Brain tumors are characterized by high incidence and mortality owing to their localized and locally invasive growth, with gliomas being the most common primary brain tumors [12]. Invasion and angiogenesis of gliomas are driven by multiple growth factors [13]. The discovery of IDH mutation as a "driver mutation" in gliomas has provided a theoretical foundation to develop clinical methods targeting this mutation [14]. IDH mutation leads to the accumulation of the "tumor metabolite" D-2-hydroxyglutarate, and these abnormally elevated levels competitively inhibit α-ketoglutarate/Fe(II)-dependent dioxygenases; representing a unique pattern in cancer biology [15]. Studies have found a close association between IDH mutations and the invasive and angiogenic characteristics of gliomas [16].
This study investigated the effects of IDH mutation on the migration and invasion of glioma cells. Our research suggests that IDH1 mutation may promote glioma migration and invasion, possibly by driving EMT. EMT is characterized by E-cadherin downregulation, which is associated with a more invasive phenotype and increased metastasis. Additionally, the expression of markers such as vimentin and N-cadherin is upregulated. N-cadherin is believed to promote cell motility and migration [17], ultimately contributing to the formation of a malignant tumor phenotype. Transcription factors involved in EMT activation, such as Snail, Slug, TWIST1, and ZEB1, regulate EMT and can drive epithelial cells to exhibit a mesenchymal phenotype [18, 19]. The invasive capacity of human brain gliomas is significantly promoted during EMT, driven by Twist1 [20]. In our study, mutIDH1 glioma cells showed a significant increase in the expression of EMT-related factors and transcription factors, and a significant decrease in cell junction protein expression. This indicates that IDH mutation causes glioma cells to be more invasive and migratory and that the tumor microenvironment becomes more conducive to tumor invasion and metastasis. Our analysis suggests that IDH1 mutation promotes glioma cell migration by activating the AKT-mTOR signaling pathway [21], which increases matrix metalloproteinases, decreases E-cadherin expression, and plays a crucial role in tumor migration and invasion [22]. In the tumor microenvironment, the expression levels of cell junction proteins play a significant role in determining tumor cell behavior because migratory and invasive cells disrupt cell–cell connections [23]. Major proteins of tight cell junctions, ZO-1, claudin, and occludin [24]; epithelial cell extracellular matrix, collagen and laminin [25]; and aquaporin-4, which is closely related to ion homeostasis in astrocytes [26], were all downregulated in cells expressing mutIDH1. Studies have shown a close relationship between changes in glioma cell junction proteins and EMT [27]. Cx43 is the main junction protein in astrocytes and glioma cells and regulates neuronal differentiation through its C-terminal [28]. High expression of Cx43 promotes the migration and invasion of brain tumor cells [29, 30]. Our results found that CX43 was significantly downregulated in mutIDH1 glioma cells, together with a decrease in other factors related to cell–cell gap junctions.
Glioma angiogenesis plays a crucial role in tumor development [31]. Hypoxia-inducible factor-1 (HIF-1) is an important regulator of angiogenesis [32]. VEGF is a fundamental regulator of angiogenesis and vascular permeability, and its high expression in brain gliomas is associated with their invasiveness [33]. High expression of heme oxygenase-1 (HO-1), a stress-inducible enzyme, suggests an association with cancer as it is closely associated with cell proliferation, migration, immune regulation, and angiogenesis [34]. Nuclear factor-erythroid 2-related factor 2 (Nrf2) plays a central role in inhibiting or inducing inflammation and angiogenesis [35] and regulates the expression of its downstream factor HO-1 [36]. During tumor development, Nrf2 promotes EMT and invasion and migration cascade reactions by downregulating E-cadherin [37]. This study found that VEGF, Nrf2, HO-1, and HIF were significantly upregulated in mutIDH1 gliomas. We postulate that IDH1 mutations in gliomas induce the HIF-1 pathway, leading to increased expression of the angiogenic factor VEGF and thus driving carcinogenesis [38]. Metabolic enzymes affected by IDH1 mutations promote tumor growth by stimulating the HIF-1 pathway and tumor angiogenesis [39]. Our results revealed that IDH1 mutation not only upregulated HIF and VEGF expression but also increased NRF2 and HO-1 expression. Therefore, we believe that the IDH1 mutation-driven angiogenesis process is due to the activation of HIF-1, which in turn triggers the VEGF/Nrf2/HO-1 pathway.
The differentiation/stemness status differs between wild-type and mutant IDH gliomas and may contribute to their distinct behaviors. The stem cell markers nestin and CD133 were significantly upregulated in IDH wild-type compared to mutIDH1 gliomas. Overexpression of IDH mutant protein downregulates the levels of nestin and CD133 expression via the Wnt/β-catenin pathway and promotes the differentiation of glioma stem cells [40]. Our study demonstrates that induction of IDH mutant expression leads to a decrease in the stem cell markers nestin and SOX2, an increase in the differentiation marker GFAP, and upregulation of BMP2, BMP4, and BMP receptor expression. Aberrant BMP expression can lead to the development of various diseases, and the BMP-Smad signaling pathway is a key negative regulator of cancer stem cells [41]. BMP receptors, including BMPRIa, BMPRIb subtypes, and BMPRII, synergistically interact with BMP binding. BMPRI proteins serve as substrates for Smad proteins, which play a core role in transmitting BMP signals from receptors to target genes in the cell nucleus. After release from the receptor, phosphorylated Smad proteins associate with the Co-Smad4 protein and, together with other transcription factors, participate in gene transcription [42]. Research has indicated that BMP2 and BMP4 are overexpressed in gliomas, and that their expression is highly correlated [43]. Activation of the BMP2 signaling pathway is a critical indicator of initiation of tumor differentiation. BMP4 induces the differentiation of human primary glioma stem cells into astrocytes [44]. BMP2/4 can induce neural stem cells to differentiate into neurons and astrocytes through the expression of BMPRIb [45]. Our results confirmed that BMP2 treatment promoted the expression of EMT- and angiogenesis-related genes and activated the Smad signaling pathway in mutIDH1 glioma cells. This finding is consistent with previous studies showing that BMP2 promotes the differentiation of gynecological tumors and promotes EMT by inducing Snail, leading to increased cell migration [46]. BMP also promotes the expression of EMT transcription factors Snail and Slug, thereby enhancing cell migration and invasion [47]. BMP may function in vivo as both a proangiogenic and antiangiogenic factor [9] and can promote the motility and invasion of various cancer cell types [48]. Similar to most other tumors, gliomas exhibit a dual response to BMP, with proliferation and self-renewal promoted by BMP2, resulting in a faster and more invasive tumor phenotype [49].
Analysis by DEGs showed that BMP2 is closely related to the regulation of cell migration and cell adhesion, especially the regulation of SMAD-related proteins. KEGG analysis confirmed that it was related to BMP signaling pathway and TGF-β signaling pathway and cell adhesion. These findings imply that the effects of BMP2 on angiogenesis, migration, and invasion of mutIDH1 cells are mediated by alterations in their transcriptional patterns, thereby affecting relevant cell functions.
Data availability
The data used to support the findings of this study are included within the article.
No datasets were generated or analysed during the current study.
Notes
The Gene Ontology Resource: 20 years and still GOing strong[J]. Nucleic Acids Res. Jan 2019;47(D1): D330-D338.
Kanehisa M, Araki M, Goto S, et al. KEGG for linking genomes to life and the environment[J]. Nucleic acids research, 2008, 36(suppl 1): D480-D484.
References
Weller M et al (2015) Glioma. Nat Rev Dis Primers 1(1):1–18
Cen L et al (2021) Competing endogenous RNA networks in glioma. Front Genet 12:675498
Kayabolen A, Yilmaz E, Bagci-Onder T (2021) IDH mutations in glioma: double-edged sword in clinical applications? Biomedicines 9(7):799
Hartmann C et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118(4):469–474
Vallée A, Lecarpentier Y, Vallée J (2021) Opposed interplay between IDH1 mutations and the WNT/β-catenin pathway: added information for glioma classification. Biomedicines 9(6):619
Chen D, Zhao M, Mundy GR (2009) Bone morphogenetic proteins. Growth Factors 22(4):233–241
Katsuno Y et al (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through smad pathway. Oncogene 27(49):6322–6333
Davis H et al (2016) Mechanisms of action of bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev 27:81–92
Ehata S et al (2013) Bi-directional roles of bone morphogenetic proteins in cancer: another molecular Jekyll and Hyde? Pathol Int 63(6):287–296
Fukuda T et al (2020) BMP signaling is a therapeutic target in ovarian cancer. Cell Death Discov 6(1):139
cao, X., (2021) The role of recombinant human bone morphogenetic protein-2 in promoting glioblastoma stem cell differentiation. 104.
Ostrom QT et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. https://doi.org/10.1093/neuonc/nou223
Groblewska M, Litman-Zawadzka A, Mroczko B (2020) The role of selected chemokines and their receptors in the development of gliomas. Int J Mol Sci 21(10):3704
Gatto L et al (2021) IDH inhibitors and beyond: the cornerstone of targeted glioma treatment. Mol Diagn Ther 25(4):457–473
Chou F et al (2021) D-2-Hydroxyglutarate in glioma biology. Cells 10(9):2345
Kang S et al (2020) Interaction between IDH1 WT and calmodulin and its implications for glioblastoma cell growth and migration. Biochem Biophys Res Commun 524(1):224–230
Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4(2):118–132
Stemmler MP et al (2019) Non-redundant functions of EMT transcription factors. Nat Cell Biol 21(1):102–112
Brlek P et al (2021) TWIST1 upregulation affects E-cadherin expression in brain metastases. Clin Transl Oncol 23(6):1085–1095
Kuhnol CD et al (2017) Snail homolog 1 is involved in epithelial-mesenchymal transition-like processes in human glioblastoma cells. Oncol Lett 13(5):3882–3888
Zhu H et al (2017) IDH1 R132H mutation enhances cell migration by activating AKT-mTOR signaling pathway, but sensitizes cells to 5-FU treatment as NADPH and GSH are reduced. PLoS ONE 12(1):e0169038
Kim D et al (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 15(11):1953–1962
de Rooij J et al (2005) Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171(1):153–164
Qiu L et al (2016) Perfluorooctane sulfonate (PFOS) disrupts blood-testis barrier by down-regulating junction proteins via p38 MAPK/ATF2/MMP9 signaling pathway. Toxicology. https://doi.org/10.1016/j.tox.2016.11.003
Borghi N et al (2010) Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci 107(30):13324–13329
Rao SB et al (2021) Orchestrating aquaporin-4 and connexin-43 expression in brain: differential roles of α1- and β1-syntrophin. Biochimica et Biophysica Acta (BBA)—Biomembranes 8:183616
Wei L et al (2021) Inhibition of cathepsin s restores TGF-β-induced epithelial-to-mesenchymal transition and tight junction turnover in glioblastoma cells. J Cancer 12(6):1592–1603
Santiago MF et al (2010) The carboxyl-terminal domain of connexin43 is a negative modulator of neuronal differentiation. J Biol Chem 285(16):11836–11845
Oliveira R et al (2005) Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol 6(1):7
Zhang W et al (2003) Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J Neurosurg 99(6):1039
Shimizu T et al (2016) Adhesion molecules and the extracellular matrix as drug targets for glioma. Brain Tumor Pathol 33(2):97–106
Diaz-Gonzalez JA et al (2005) Targeting hypoxia and angiogenesis through HIF-1alpha inhibition. Cancer Biol Ther 4(10):1055–1062
Veganzones S et al (2017) Genetic alterations of IDH1 and vegf in brain tumors. Brain and Behavior 7(9):e00718
Lu J et al (2021) Heme oxygenase 1: a novel oncogene in multiple gynecological cancers. Int J Biol Sci 17(9):2252–2261
Krajka-Kuźniak V, Baer-Dubowska W (2021) Modulation of Nrf2 and NF-κB signaling pathways by naturally occurring compounds in relation to cancer prevention and therapy. are combinations better than single compounds. Int J Mole Sci 22(15):8223
Li W, Kong A (2009) Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48(2):91–104
Shin JH et al (2019) Nrf2-heme oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β signaling. J Diabetes Res 2019:1–8
Yalaza C et al (2017) R132H mutation in IDH1 gene is associated with increased tumor HIF1-alpha and serum VEGF levels in primary glioblastoma multiforme. Ann Clin Lab Sci 47(3):362–364
Zhao S et al (2009) glioma-derived mutations inIDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science 324(5924):261–265
Yao Q et al (2018) IDH1 mutation diminishes aggressive phenotype in glioma stem cells. Int J Oncol 52(1):270–278
Sneddon JB et al (2006) Bone morphogenetic protein antagonist gremlin 1 Is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci—PNAS 103(40):14842–14847
Nishimura R et al (1998) Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem 273(4):1872–1879
Augustus M et al (2021) Identification of CRYAB+ KCNN3+ SOX9+ astrocyte-like and EGFR+ PDGFRA+ OLIG1+ oligodendrocyte-like tumoral cells in diffuse IDH1-mutant gliomas and implication of NOTCH1 signalling in their genesis. Cancers 13(9):2107
Piccirillo SGM et al (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444(7120):761–765
Pera MF et al (2004) Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci 117(Pt 7):1269–1280
Fukuda T et al (2021) BMP2-induction of FN14 promotes protumorigenic signaling in gynecologic cancer cells. Cell Signal 87:110146
Fukuda T et al (2021) Tumor promoting effect of BMP signaling in endometrial cancer. Int J Mol Sci 22(15):7882
Gao H et al (2012) The BMP inhibitor coco reactivates breast cancer cells at lung metastatic site. Cell 151(6):1386–1388
Guo M et al (2014) miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis 35(8):1698–1706
Acknowledgements
NP is president of Medimatics, a company that provides consulting services on medical information systems located in Maastricht, the Netherlands. The remaining authors declare no conflicts of interest. English language editing was performed by Editage (www.editage.com).
Funding
The author(s) disclosed receipt of the following financial support for Research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (82260509), the Key Research and Development Program of Ningxia (Grant No. 2023BEG02009) and Natural Science Foundation of Ningxia Province (Grant No. 2022AAC03161).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hui Xu and Yu Cao; Methodology: Hui Xu and Yu Cao; Validation: Jianqiao Ruan and Fei Wang; Resources: Ningmei Zhang and Xiangmei Cao; Data Curation: Lina Yang, Tian Yu, Fang Du; Writing—Original Draft Preparation: Hui Xu and Xiangmei Cao; Paper submission: Yuhong He and Hui Xu; Writing—Review & Editing: Fang Du and Xiangmei Cao; Funding Acquisition:Xiangmei Cao and Ningmei Zhang.
Corresponding authors
Ethics declarations
Conflict of interest
The Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, H., Cao, Y., Ruan, J. et al. The effects of BMP2 and the mechanisms involved in the invasion and angiogenesis of IDH1 mutant glioma cells. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04789-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04789-x