Abstract
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults. The extraordinary invasion of human GBM into adjacent normal brain tissues contributes to treatment failure. However, the mechanisms that control this process remain poorly understood. Increasing evidence has demonstrated that microRNAs are strongly implicated in the migration and invasion of GBM. In this study, we found that microRNA-98 (miR-98) was markedly downregulated in human glioma tissues and cell lines. Functional experiments indicated that restored expression of miR-98 attenuated glioma cell invasion and migration, whereas depletion of miR-98 promoted glioma cell invasion and migration. Subsequent investigation showed that pre-B-cell leukemia homeobox 3 (PBX3), an important transcription factor that controls tumor invasion, was a direct and functional target of miR-98 in GBM cells. Consistently, an orthotopic mouse model also demonstrated the suppressive effects of miR-98 overexpression on tumor invasion and PBX3 expression. Silencing of PBX3 using small interfering RNA inhibited the migratory and invasive capacities of glioma cells, whereas reintroduction of PBX3 into glioma cells reversed the anti-invasive function of miR-98. Furthermore, depletion of PBX3 phenocopied the effects of miR-98 overexpression in vivo. Finally, quantitative real-time polymerase chain reaction results showed that miR-98 was negatively correlated with PBX3 expression in 24 glioma tissues. Thus, we propose that PBX3 modulation by miR-98 has an important role in regulating GBM invasion and may serve as therapeutic target for GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) represents one of the most frequently occurring and aggressive forms of primary brain tumors in adults (Dolecek et al. 2012). Despite recent advances in multimodal therapies, including surgical resection followed by radiation and chemotherapy, the prognosis for patients afflicted with this disease remains extremely poor, with median survival being on average a little over a year (Stupp et al. 2005; Van Meir et al. 2010). The highly invasive nature of GBM, which contributes to the poor prognosis, favors its infiltration into the surrounding normal brain parenchyma, making complete surgical resection impossible (Lefranc et al. 2005; Onishi et al. 2011). Therefore, it is essential to determine the novel molecular mechanisms underlying and supporting glioma cell invasion.
MicroRNAs (miRNAs) are a class of evolutionarily conserved, small non-coding RNAs of 19–23 nucleotides in length, which negatively regulate gene expression at the post-transcriptional level via imperfect base pairing with the 3′-untranslated regions (3′-UTRs) of corresponding messenger RNAs (mRNAs) (Bartel 2004). Mounting evidence indicates that miRNAs play pivotal regulatory roles in cancer initiation and progression, and aberrant miRNA expression profiles are a common hallmark of human cancers including GBM (Calin and Croce 2006; Lu et al. 2005). Moreover, evidence suggests that miRNAs regulate glioma cell migration and invasion (Wei et al. 2016a; Chen et al. 2015b). MiRNA-98 (miR-98), a member of the let-7 family, is located on chromosome Xp11.22. It predominantly has tumor-suppressing properties and is downregulated in a number of malignancies, including salivary adenoid cystic carcinoma (Liu et al. 2016), lung cancer (Yang et al. 2015; Ni et al. 2015), oral squamous cell carcinoma (Du et al. 2015), melanoma (Li et al. 2014a), breast cancer (Siragam et al. 2012), and esophageal squamous cell carcinoma (Huang et al. 2012). Recently, it has been reported that miR-98 inhibits tumor growth by enhancing the anti-proliferative effects of vitamin D (Ting et al. 2013). Moreover, downregulation of miR-98 has been associated with the invasive nature of glioma, and several target genes have been identified, such as IκB Kinase (IKKε) (Fan et al. 2015), high mobility group A2 (HMGA2) (Chen et al. 2013), and enhancer of zeste 2 (EZH2) (Smits et al. 2010). Because one miRNA can regulate hundreds of mRNAs, we hypothesized that other target genes may exist and contribute to glioma invasion. In this study, we focused on miR-98 and its target gene, pre-B-cell leukemia homeobox 3 (PBX3), in GBM.
PBX3 is an important member of the highly conserved PBX group of proteins, which belong to the three amino acid loop extension homeobox (HOX) gene family. PBX3 is expressed ubiquitously in various mammalian cells and plays an important role in regulation of gene transcription by interacting with a subset of HOX proteins and facilitating their DNA-binding affinities and specificities, thereby enabling downstream target gene expression (Shah and Sukumar 2010; Chang et al. 1996; Li et al. 2013). Aberrant expression of PBX3 has been observed in various types of human cancers including prostate cancer (Ramberg et al. 2016), multiple myeloma (Lu et al. 2016), gastric cancer (Li et al. 2014b), hepatocellular carcinoma (Han et al. 2016), and colorectal cancer (Han et al. 2014). Furthermore, overexpression of PBX3 is associated with cancer cell migration and invasion (Han et al. 2014, 2016, 2012). However, the expression prolife and potential role of PBX3 in GBM have not been investigated thus far.
In the current study, we demonstrated that miR-98 was appreciably downregulated in human glioma cells and tumor tissue compared with their normal counterparts. We identified PBX3 as a direct downstream target of miR-98, and knockdown of PBX3, or overexpression of miR-98 significantly inhibited glioma invasion and migration. Collectively, our results suggest that the miR-98/PBX3 axis may be a clinically relevant therapeutic target for GBM.
Materials and Methods
Chinese Glioma Genome Atlas (CGGA) Database and Human Tissue Samples
A total of 158 glioma cases with microarray data for miRNA expression were downloaded from the CGGA data portal (http://www.cgga.org.cn.portal.phpg). The 158 glioma samples included 48 astrocytomas (A, World Health Organization (WHO) grade II), 13 oligodendrogliomas (O, WHO grade II), eight anaplastic astrocytomas (AA, WHO grade III), 10 anaplastic oligodendrogliomas (AO, WHO grade III), 15 anaplastic oligoastrocytomas (AOA, WHO grade III), and 64 GBMs (WHO grade IV). Twenty-four glioma tissue specimens were obtained postoperatively from the Department of Neurosurgery, First Affiliated Hospitals of Nanjing Medical University, from 2014 to 2015. Of these 24 samples, eight were low-grade glioma samples (grades I and II) and sixteen were high-grade glioma samples (grades III and IV). The histological features of all specimens were confirmed by pathologists according to the WHO criteria. Prior to tumor resection, none of the patients had received chemotherapy or radiotherapy. In addition, eight normal brain tissues (NBTs) were collected as negative controls from patients who underwent decompressive craniotomy for severe traumatic brain injury (TBI). All tissue samples collected from glioma or TBI patients were immediately frozen in liquid nitrogen. This study was reviewed and approved by Institutional Review Board of Nanjing Medical University. Written informed consent was obtained from all patients who were included in this study.
Cell Culture
Human GBM cell lines U87 and U251 were purchased from the Chinese Academia Sinica Cell Repository (Shanghai, China). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, CA, USA). Normal human astrocytes (NHAs) were obtained from Lonza (Walkersville, MD, USA) and cultured following the manufacturer’s instructions. All cells were incubated at 37 °C in a fully humidified atmosphere containing 5% CO2 in filtered air.
Oligonucleotides, Lentiviral Vectors, Plasmid Construction, and Cell Transfection
An miR-98 inhibitor and miRNA inhibitor negative control (NC) were obtained from RiboBio (Guangzhou, China) and used to evaluate the effects of miR-98 loss-of-function. Lentiviruses carrying hsa-miR-98 or hsa-miR-NC or siRNA-PBX3 or siRNA-NC were purchased from Genepharma (Shanghai, China). Stable U87 and U251 cell lines were established by lentiviral infection and puromycin selection according to the manufacturer’s protocol. These stable cell lines were used to examine the effects of miR-98 overexpression. siRNA-PBX3 oligonucleotides and control siRNA-NC were obtained from Santa Cruz Biotechnology. The PBX3-overexpression plasmid was generated by inserting the PBX3-coding region into a pcDNA3.1 vector. The plasmid was sequenced verified by Genepharma. All oligonucleotides and plasmids were transfected into cells using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions.
RNA Extraction and Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
Total RNA in human tissue samples and cultured cells was extracted using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. cDNA was synthesized from 1 μg total RNA with an miScript II Reverse Transcription Kit (Qiagen) for miRNA and TaqMan Reverse Transcription Reagents (Applied Biosystems) for mRNA. qRT-PCR for miRNA and mRNA expression analyses was performed using an ABI-7500 Fast RT-PCR system (Applied Biosystems). A miScript SYBR Green PCR Kit (Qiagen) was used for miRNA, and SYBR Green PCR Mixture (Invitrogen) was used for mRNA. U6 and β-actin mRNAs were also amplified as internal controls for miR-98 and PBX3, respectively. Primers for qRT-PCR obtained from Genepharma were as follows: miR-98 forward 5′-ATCCAGTGCGTGTCGTG-3′ and reverse 5′-TGCTTGAGGTAGTAAGTTG-3′; U6 forward 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse 5′-GGAACGCTTCACGAATTTG-3′; PBX3 forward 5′-CAAGTCGGAGCCAATGTG-3′ and reverse 5′-ATGTAGCTCAGGGAAAAGTG-3′; β-actin forward 5′-TCATGAAGTGTGACGTGGACATC-3′ and reverse 5′-CAGGAGGAGCAATGATCTTGATCT-3′. All experiments were performed in triplicate and repeated three times. Relative gene expression was calculated by the 2\(- \Delta \Delta {\text{Ct}}\) method.
Western Blot Analysis
Cultured cells were washed twice with phosphate buffered saline (PBS) and lysed in ice-cold radio-immunoprecipitation assay buffer (KenGEN, China). The samples were centrifuged at 14000g for 15 min at 4 °C, and the supernatant of each sample was collected. A bicinchoninic acid assay kit (Pierce, Rockford, IL, USA) was used to measure the protein concentration. Equal amounts of proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride-membranes (Millipore, MA, USA). The membranes were blocked in 5% dry skim milk at room temperature for 2 h and then incubated with primary antibodies overnight at 4 °C. The antibodies used in this study were rabbit anti-PBX3 (1:1000, ab109173, Abcam, Cambridge, UK), rabbit anti-PBX1 (1:500, ab154285, Abcam), rabbit anti-PBX2 (1:1000, ab55498, Abcam), and mouse anti-β-actin (1:2000, ab8226, Abcam). The membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The bound antibodies were detected using an enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ, USA). The optical density of the resultant bands was analyzed using the Image J software (National Institutes of Health, Bethesda, MD, USA) and normalized to the β-actin signal.
Invasion Assay
Cellular invasiveness was examined in a 24-well plate using 8-μm-pore-size polycarbonate insets (BD Biosciences, Bedford, MA, USA) according to the manufacturer’s instructions. Cells (4 × 104) were seeded on top of polymerized matrigel in serum-free DMEM, whereas DMEM supplemented with 15% FBS was placed in lower chambers to stimulate invasion. After incubation for 24 h, non-invading cells on the top of the membrane were removed by scraping. Invasive cells on the bottom of the insert were fixed with 4% paraformaldehyde for 20 min. The cells were then stained with 0.1% crystal violet for 2 h. Invasion was quantified by counting the number of cells in three independent 10 × magnification fields. Experiments were performed in triplicate.
Wound Healing Assay
At 100% confluence, an artificial wound was made by scratching with a standard 200 μL pipette tip. Cells were washed twice with PBS and maintained in serum-free DMEM. Images were captured under an inverted microscope (Leica) at 0 and 48 h after scratching. Scratch closure was evaluated as relative to the total wound area.
Dual Luciferase Reporter Assay
Wild-type (WT) and mutated putative miR-98-binding sites in the PBX3 3′-UTR region were amplified and cloned into the XbaI site of a pGL3 control vector (Invitrogen). For the reporter assay, cells were cultured in 96-well plates and co-transfected with WT or mutated 3′-UTR luciferase reporters and miR-98 mimics (RiboBio). After 48 h of incubation, luciferase activity was measured with a Dual Luciferase Reporter Assay Kit (Promega, Madison, USA) according to the manufacturer’s protocol. Renilla luciferase activity was used as an internal control.
Immunohistochemistry (IHC)
The mouse brain was fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 3.5-μm-thick sections. The sections were stained with Mayer’s hematoxylin and subsequently with eosin (Biogenex Laboratories) or an antibody against PBX3 (1:75, ab56239, Abcam). Brown staining in cells was considered as positive signaling.
Orthotopic Glioma Model
Bagg albino (BALB)/c nude mice at 6 weeks of age were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences. LV-miR-98- or LV-miR-NC- or LV-siRNA-PBX3- LV-siRNA-NC-transfected U87 cells (2.5 × 105) were injected intracranially into individual mice (n = 10). At 3 weeks after injection, all mice were sacrificed by rapid decapitation. The brain was removed quickly, followed by flash freezing or embedding in paraffin. For survival analysis, signs of disease progression in each group (n = 10) were monitored until the last mouse had died. All procedures were approved by the Animal Care and Use Committee of Nanjing Medical University and in line with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Statistical Analysis
The two-tailed Student’s t-test was used for pairwise comparison and one-way analysis of variance for multivariate analysis. Survival analysis was performed by the Kaplan–Meier method in Graphpad Prism 5 software. Differences with p < 0.05 were considered to be significant.
Results
Reduced miR-98 Expression in Glioma Specimens and Cell Lines
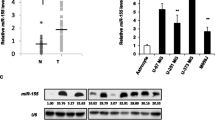
To explore the potential function of miR-98 in glioma, we initially analyzed the expression patterns of miR-98 in the CGGA database. As shown in Fig. 1a, miR-98 levels in high-grade glioma (grades III and IV) were dramatically decreased compared with those in low-grade glioma (grade II). To verify these findings, we investigated miR-98 expression in 24 samples of various grades of glioma, including eight low-grade glioma cases and 16 high-grade glioma cases and eight NBTs by qRT-PCR. A significant decrease in miR-98 expression was observed in low- and high-grade glioma compared with NBTs. In addition, high-grade glioma had lower miR-98 expression than low-grade glioma (Fig. 1b), confirming that miR-98 is associated with the glioma grade. Finally, we examined the expression levels of endogenous miR-98 in U87 and U251 cell lines as well as NHAs. Compared with NHAs, miR-98 was notably downregulated in U87 and U251 cell lines (Fig. 1c). These findings supported the hypothesis that miR-98 may act as a tumor suppresser in glioma.
miR-98 expression is decreased in human glioma tissues and cell lines. a CGGA database indicating reduced miR-98 expression in high-grade glioma tissues compared with that in low-grade glioma tissues. b Thirty-two samples of various grades of glioma and NBTs showed low miR-98 expression in high-grade glioma. ***p < 0.001. c miR-98 was notably downregulated in U87 and U251 cell lines compared with NHAs. **p < 0.01
miR-98 Attenuates Glioma Cell Invasion and Migration
The aberrant expression of miR-98 led us to explore its roles in regulating glioma cell invasion and migration. In vitro gain-of-function analysis by overexpressing miR-98 with a lentiviral vector and loss-of-function by depleting endogenous miR-98 with antisense oligonucleotides were performed in U87 and U251 cells. qRT-PCR confirmed that miR-98 was significantly increased in cells transfected with lentiviral-miR-98 (LV-miR-98) compared with LV-miR-NC. In addition, miR-98 was remarkably reduced in cells transfected with the miR-98 inhibitor compared with the scramble control (Anti-miR-NC) (Fig. 2a). Transwell invasion assays were performed on the miR-98-transduced cells. The results indicated that ectopic expression of miR-98 notably reduced the ability to migrate across the membrane of transwell chambers coated with matrigel, whereas those transfected with Anti-miR-98 had a significantly increased invasive ability (Fig. 2b, c). Moreover, wound healing assays were employed to evaluate the function of miR-98 in glioma cell migration. Consistent with the results of transwell assays, LV-miR-98 markedly attenuated the migration of U87 and U251 cells. In contrast, gap widths were much narrower in Anti-miR-98-treated cell cultures than Anti-miR-NC-treated cell cultures (Fig. 2d, e). Collectively, our results demonstrate that miR-98 may attenuate glioma cell invasion and migration in vitro.
miR-98 inhibits glioma cells invasion and migration in vitro. a qRT-PCR analysis of miR-98 expression in U87 and U251 cells transfected with LV-miR-98, LV-miR-NC, anti-miR-98, or anti-miR-NC. ***p < 0.001; **p < 0.01. b Representative images of transwell assays using U87 and U251 cells stably expressing miR-98, or miR-NC or transiently transfected with anti-miR-98 or anti-miR-NC. c Quantification of transwell assays. ***p < 0.001; **p < 0.01. d Representative images of wound healing assay using U87 and U251 cells stably expressing miR-98 or miR-NC or transiently transfected with anti-miR-98 or anti-miR-NC. e Quantification of wound healing assays. ***p < 0.001
PBX3 is a Direct Target of miR-98 in Glioma
After observing the inhibitive effect of miR-98 on glioma cell invasion and migration, we next investigated its potential specific targets by performing in silico analysis using several prediction algorithms including miRanda, PicTar, and miRWalk. According to the prediction analysis, we identified several dozen to hundreds of putative miR-98 target genes. Among these genes, experimentally validated targets, such as IKKε (Fan et al. 2015), HMGA2 (Hebert et al. 2007), interleukin-6 (IL-6) (Li et al. 2014a), neuroblastoma RAS viral oncogene homolog (N-RAS) (Liu et al. 2016), and EZH2 (Alajez et al. 2010), could be implicated in the invasive phenotype of glioma. However, our aim was to focus on novel miR-98 targets, which are potentially involved in glioma invasiveness, and not experimentally validated miR-98 targets. To this end, we focused on PBX1, PBX2, and PBX3 that are involved in carcinogenesis (Magnani et al. 2015; Errico et al. 2013; Han et al. 2014). To test whether PBX1, PBX2 and PBX3 are direct targets of miR-98, we evaluated the levels of the encoded proteins in both miR-98-overexpressing and -depleted glioma cells. We found that ectopic expression of miR-98 caused a decrease in PBX3, but not PBX1 or PBX2 expression. In contrast, suppression of miR-98 resulted in higher levels of PBX3 protein, while the protein levels of PBX1 and PBX2 were unchanged (Fig. 3a). To confirm that PBX3 is a direct target of miR-98, we performed a luciferase report assay. Figure 3b shows the base pairing between miR-98 and WT and mutant 3′-UTR fragments of PBX3. Relative luciferase activity was significantly inhibited by miR-98 transfection in U87 and U251 cells when the PBX3 plasmid containing the WT 3′-UTR was present. However, mutations in the tentative miR-98 binding sites of the PBX3 3′-UTR abrogated this suppressive effect of miR-98 (Fig. 3c). Taken together, these results suggest that miR-98 downregulates PBX3 expression by directly targeting its 3′-UTR in glioma.
PBX3 is a direct target of miR-98 in glioma cells. a Western blot analysis of PBX1, PBX2 and PBX3 protein levels in miR-98-overexpressing or -depleting glioma cells. b Predicted binding sites of wild-type (WT) and mutant sequences of miR-98 in the 3′-UTR of PBX3 mRNA. c Luciferase assay results showed that luciferase activity in cells co-transfected with miR-98 mimics and pGL3-PBX3-3′UTR-WT plasmids decreased than that in cells co-transfected with miR-98 mimics and pGL3-PBX3-3′-UTR-mut plasmids, and luciferase activity in cells co-transfected with miR-98 mimics and pGL3-PBX3-3′-UTR-mut plasmids showed no obvious differences compared with that in cells co-transfected with miR-98 mimics and PGL3-vectors. ***p < 0.001. d Thirty-two samples of various grades of glioma and NBTs revealed high PBX3 expression in high-grade glioma. ***p < 0.001. e Pearson’s correlation analysis indicated that miR-98 expression was negatively associated with PBX3 expression in glioma tissues
We further examined PBX3 mRNA levels by qRT-PCR in 24 glioma specimens and eight NBTs. Our results showed that PBX3 mRNA levels were higher in glioma specimens than NBTs and increased with ascending pathological grade (Fig. 3d). Pearson’s correlation analysis revealed a significant and negative correlation between miR-98 and PBX3 (Fig. 3e). Therefore, these results indicate elevation of PBX3 expresssion in glioma tissues, and its enhancement is correlates with reduced miR-98 expression.
Inhibition of PBX3 is Essential for miR-98-Induced Attenuation of Invasion and Migration
Previous studies have demonstrated that PBX3 plays a critical role in the invasion and migration of various types of human cancers. To investigate the effect of PBX3 on glioma cell invasion and migration, we specifically suppressed expression of PBX3 in U87 and U251 cells using siRNA targeting PBX3 mRNA. The silencing effects were confirmed by western blotting (Fig. 4a). As expected, knockdown of PBX3 expression dramatically decreased the invasion and migration of glioma cells (Fig. 4b–e). Of note, the reduced invasive effect of PBX3 knockdown was similar to that of ectopic miR-98 expression, indicating that inhibition of PBX3 might be a key mechanism by which miR-98 attenuates glioma invasion and migration. To test this hypothesis, we transfected PBX3 plasmids (without the 3′-UTR) into U87 and U251 cells stably expressing miR-98 or miR-NC. Both transwell and wound healing assays demonstrated that restoration of PBX3 expression antagonized the inhibitory effects of miR-98 (Fig. 4f–j). Moreover, the increased invasion and migration capabilities were reversed by inhibition of PBX3 in miR-98-downregulated cells (Fig. 5a–e). Taken together, these results suggest that PBX3 is a functional target of miR-98 in glioma cells.
Knockdown of PBX3 affects the anti-invasive effects of miR-98 in glioma cells. a Western blot analysis showed that PBX3 expression was significantly decreased in cells transfected with siRNA-PBX3 (Si-PBX3). β-actin served as the loading control. b Representative images of transwell assays using U87 and U251 cells transiently transfected with Si-PBX3 and Si-NC. c Quantification of transwell assays. **p < 0.01. d Representative images of wound healing assays using U87 and U251 cells transiently transfected with Si-PBX3 and Si-NC. e Quantification of wound healing assay. ***p < 0.001. f Western blot analysis of PBX3 expression in cells transfected with pcDNA3.1-PBX3 or pcDNA3.1-vector plasmids in the presence or absence of ectopic expression of miR-98 or miR-NC. g Representative images of transwell assays of the above cells. h Quantification of transwell assays of the above cells was shown. ***p < 0.001; **p < 0.01. i Representative images of wound healing assay of the above cells. j Quantification of wound healing assays of the above cells. ***p < 0.001
PBX3 is involved in the enhanced miR-98 depletion-induced invasive ability. a Western blot analysis of PBX3 expression in cells transfected with Si-PBX3 or Si-Ctrl in the presence or absence of anti-miR-98 or anti-miR-NC. b Representative images of transwell assays of the above cells. c Quantification of transwell assays of the above cells. **p < 0.01; *p < 0.05. d Representative images of wound healing assays of the above cells. e Quantification of wound healing assay of the above cells. **p < 0.01; ***p < 0.001
Overexpression of miR-98 or Depletion of PBX3 Inhibits Glioma Invasion in an Orthotopic Glioma Model
To further verify the role of miR-98 and PBX3 in glioma invasion, LV-miR-98- or LV-miR-NC- or LV-siRNA-PBX3- or LV-siRNA-NC-transfected U87 cells were intracranially injected into nude mice. As shown in Fig. 6a, miR-98 overexpression inhibited both tumor growth and invasion. IHC of PBX3 showed decreased expression in LV-miR-98 tumors compared with LV-miR-NC tumors (Fig. 6b). Moreover, survival of mice injected LV-miR-98-U87 cells was significantly longer than that of mice injected with LV-miR-NC-U87 cells (Fig. 6c). Furthermore, depletion of PBX3 significantly inhibited glioma invasion and growth in vivo similar to miR-98 overexpression (Fig. 6d–f). The detailed experimental procedures and design were shown in Fig. 6g.
miR-98 inhibits glioma invasion and growth in vivo. a Representative images of Hematoxylin and eosin staining of tissues from mice with orthotopic tumors derived from LV-miR-NC-U87 or LV-miR-98-U87 cells. b Representative IHC images of PBX3 in tissues from mice with orthotopic tumors derived from LV-miR-NC-U87 or LV-miR-98-U87 cells. c Survival of mice with tumors derived from LV-miR-NC-U87 or LV-miR-98-U87 cells was demonstrated by Kaplan–Meier survival curves. d Representative images of Hematoxylin and eosin staining of tissues from mice with orthotopic tumors derived from LV-siRNA-NC-U87 or LV-siRNA-NC-U87 cells. e Representative IHC images of PBX3 in tissues from mice with orthotopic tumors derived from LV-siRNA-NC-U87 or LV-siRNA-NC-U87 cells. f Survival of mice with tumors derived from LV-siRNA-Ctrl-U87 or LV-siRNA-PBX3-U87 cells was demonstrated by Kaplan–Meier survival curves. g Schematic illustrating the in vivo experimental procedures and design
Discussion
The invasive ability of GBM is a key contributing factor leading to poor prognoses, but the involved mechanisms remain unclear. Thus, identification of critical factors that are misexpressed in glioma samples and elucidation of mechanisms that lead to the aberrant expression of genes promoting GBM invasion are essential to develop successful management of GBM (Paw et al. 2015). Mounting evidence suggests that miRNAs play pivotal roles in invasion and migration of human cancers, including GBM (Auffinger et al. 2013). In our study, we investigated the biological roles of miR-98 and its target gene PBX3 in glioma invasion and migration.
Emerging evidence has demonstrated that miR-98 plays a key role in tumor invasion and migration by regulating multiple target genes, such as insulin-like growth factor 1 receptor (IGF1R) (Du et al. 2015), IKKε (Fan et al. 2015), HMGA2 (Hebert et al. 2007), EZH2 (Alajez et al. 2010; Huang et al. 2012),-IL-6 (Li et al. 2014a), and N-RAS (Liu et al. 2016). Moreover, ectopic expression of miR-98 inhibits epithelial-mesenchymal transition that is essential for cancer metastasis (Liu et al. 2016; Zhou et al. 2016). The finding that miRNAs regulate various target genes simultaneously might explain the complex mechanisms underlying GBM. In this study, we analyzed expression of miR-98 in the CGGA database, clinical tissue samples, two glioma cell lines (U87 and U251), and NHAs. Our results showed that miR-98 was frequently downregulated in both glioma tissues and cell lines and low miR-98 levels were linked to higher incidence of high-grade gliomas compared with the high-expression group (Fig. 1). Subsequent functional assays revealed that restoration of miR-98 induced a significant reduction in cell invasion and migration abilities (Fig. 2), suggesting that miR-98 functions as a tumor suppressor and is involved in glioma invasion. A previous study has revealed that overexpression of miR-98 inhibits glioma cell migration and invasion (Fan et al. 2015), which is consistent with our results. However, they did not explore the effect of inhibiting endogenous miR-98 on glioma cell invasion and migration. Furthermore, lower miR-98 expression enhanced glioma cell invasion and migration (Fig. 2), further supporting the hypothesis that miR-98 is a tumor suppressor in glioma. Although we focused on determining the role of miR-98 in glioma cell invasion and migration, little is known about the mechanism underlying downregulation of miR-98 in glioma. Previous studies have shown that transactivation of lin-28 homolog B (LIN2B) is essential to inhibit miR-98 biogenesis (Li et al. 2014a; Wei et al. 2016b; Lin et al. 2015). Moreover, overexpression of LIN28B has been implicated in metastasis of various types of human cancers (Chen et al. 2015a; Li et al. 2014a; Lin et al. 2015). Hence, we hypothesize that decreased miR-98 in glioma may be due to LIN28B activation. Thus, more study should be performed to address this issue in the future.
Extensive studies have demonstrated that PBX3 is a key transcriptional regulator that performs a variety of biological functions. Overexpression of PBX3 is associated with many kinds of malignancies. PBX3 protein is also known to play an important role in the regulation of cancer migration and invasion partially through activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway (Han et al. 2014). Furthermore, patients with high PBX3 expression are more likely to have poor overall survival (Han et al. 2014, 2015). The expression of PBX3 is also negatively regulated by multiple miRNAs, such as let-7c (Han et al. 2012), let-7d (Ramberg et al. 2011), miR-320a (Lu et al. 2016), and miR-33a-3p (Han et al. 2016). These findings suggest that miRNA-mediated post-transcriptional silencing mechanisms play a crucial role in regulating the expression of PBX3. In the current study, we showed for the first that miR-98 exerts its effects by specifically targeting the 3′-UTR of PBX3 mRNA in glioma cells, which was demonstrated by luciferase reporter assays (Fig. 3b, c). Additionally, our findings demonstrated that downregulation of miR-98 increased PBX3 expression, whereas upregulation of miR-98 reduced PBX3 expression (Fig. 3a). Furthermore, we found that PBX3 expression was frequently upregulated in glioma specimens (Fig. 3d), and the expression levels of PBX3 mRNA were inversely correlated with miR-98 in glioma tissues (Fig. 3e). Therefore, we conclude that overexpression of PBX3 in glioma may be the result of decreased miR-98 expression.
To test whether PBX3 is a functional target of miR-98, we knocked down endogenous PBX3 by small interfering RNA (siRNA) (Fig. 4a). Our observations showed that the reduction in PBX3 expression decreased invasion and migration of glioma cells as effectively as miR-98 restoration (Fig. 4b–e). More importantly, reintroduction of PBX3 into LV-miR-98-transfected cells abrogated the effect of inhibition of cell invasion and migration by miR-98 (Fig. 4f–j). In addition, miR-98 depletion-induced cell invasion and migration were reversed by inhibition of PBX3 (Fig. 5). Finally, our in vivo experiments confirmed the in vitro results (Fig. 6). Although a previous in vitro study has demonstrated that miR-98 exerts no effect on glioma cell proliferation (Chen et al. 2013), our in vivo results suggested that overexpression of miR-98 inhibited tumor growth. The different results between in vitro and in vivo experiments suggest that the microenvironment of tumor growth is important for the functions of proteins (Swartz et al. 2012). Collectively, our findings provide the first evidence that PBX3 is a key mediator of the miR-98-induced anti-invasive function in glioma. Thus, we propose that elevated PBX3 induced by decreased miR-98, may facilitate glioma invasion and migration and consequently drive the progression of glioma.
The underlying mechanism of PBX3 in glioma invasion and migration remains unclear. It has been reported that PBX3 activates the MAPK/ERK signaling pathway, a major regulator of cancer cell invasion and migration (Han et al. 2014). Whether PBX3 promotes glioma invasion by activating the MAPK/ERK pathway requires further investigation. Additionally, PBX3 directly interacts with HOX genes, strengthening their DNA-binding affinities and activating downstream target genes. HOX family genes are upregulated in multiple cancers and associated with cancer metastasis (Hong et al. 2015; Duan et al. 2015; Wang et al. 2015). The interaction of PBX3 with HOX genes and their role in glioma invasion and migration should be explored in the future.
In conclusion, our current data clearly demonstrate that downregulation of miR-98 is a common event in glioma, and reduced miR-98 expression is associated with a high incidence of high-grade gliomas and inversely correlates with PBX3 expression in glioma tissue samples. Our findings suggest that loss of miR-98 results in gaining expression of the oncogene PBX3, which in turn favors glioma migration and invasion. This novel miR-98/PBX3 axis may further our understanding of the molecular mechanisms involved in glioma invasion, and targeting miR-98/PBX3 may serve as a promising therapeutic strategy for glioma treatment.
References
Alajez NM, Shi W, Hui AB, Bruce J, Lenarduzzi M, Ito E, Yue S, O’Sullivan B, Liu FF (2010) Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis 1:e85. doi:10.1038/cddis.2010.64
Auffinger B, Thaci B, Ahmed A, Ulasov I, Lesniak MS (2013) MicroRNA targeting as a therapeutic strategy against glioma. Curr Mol Med 13(4):535–542
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866. doi:10.1038/nrc1997
Chang CP, Brocchieri L, Shen WF, Largman C, Cleary ML (1996) Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol 16(4):1734–1745
Chen Z, Cheng Q, Ma Z, Xi H, Peng R, Jiang B (2013) Overexpression of RKIP inhibits cell invasion in glioma cell lines through upregulation of miR-98. BioMed research Int 2013:695179. doi:10.1155/2013/695179
Chen C, Cao F, Bai L, Liu Y, Xie J, Wang W, Si Q, Yang J, Chang A, Liu D, Liu D, Chuang TH, Xiang R, Luo Y (2015a) IKKbeta enforces a LIN28B/TCF7L2 positive feedback loop that promotes cancer cell stemness and metastasis. Cancer Res 75(8):1725–1735. doi:10.1158/0008-5472.can-14-2111
Chen W, Zhang B, Guo W, Gao L, Shi L, Li H, Lu S, Liu Y, Li X (2015b) miR-429 inhibits glioma invasion through BMK1 suppression. J Neurooncol 125(1):43–54. doi:10.1007/s11060-015-1887-x
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology 14(Suppl 5):v1–49. doi:10.1093/neuonc/nos218
Du Y, Li Y, Lv H, Zhou S, Sun Z, Wang M (2015) miR-98 suppresses tumor cell growth and metastasis by targeting IGF1R in oral squamous cell carcinoma. Int J Clin Exp Pathol 8(10):12252–12259
Duan R, Han L, Wang Q, Wei J, Chen L, Zhang J, Kang C, Wang L (2015) HOXA13 is a potential GBM diagnostic marker and promotes glioma invasion by activating the Wnt and TGF-beta pathways. Oncotarget 6(29):27778–27793. doi:10.18632/oncotarget.4813
Errico MC, Felicetti F, Bottero L, Mattia G, Boe A, Felli N, Petrini M, Bellenghi M, Pandha HS, Calvaruso M, Tripodo C, Colombo MP, Morgan R, Care A (2013) The abrogation of the HOXB7/PBX2 complex induces apoptosis in melanoma through the miR-221&222-c-FOS pathway. Int J Cancer 133(4):879–892. doi:10.1002/ijc.28097
Fan YH, Ye MH, Wu L, Lv SG, Wu MJ, Xiao B, Liao CC, Ji QK, Chai Y, Zhu XG (2015) Overexpression of miR-98 inhibits cell invasion in glioma cell lines via downregulation of IKKepsilon. Eur Rev Med Pharmacol Sci 19(19):3593–3604
Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li M, Ji DB, Lu YY, Zhang ZQ (2012) Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J Pathol 226(3):544–555. doi:10.1002/path.3014
Han HB, Gu J, Ji DB, Li ZW, Zhang Y, Zhao W, Wang LM, Zhang ZQ (2014) PBX3 promotes migration and invasion of colorectal cancer cells via activation of MAPK/ERK signaling pathway. World J Gastroenterol 20(48):18260–18270. doi:10.3748/wjg.v20.i48.18260
Han H, Du Y, Zhao W, Li S, Chen D, Zhang J, Liu J, Suo Z, Bian X, Xing B, Zhang Z (2015) PBX3 is targeted by multiple miRNAs and is essential for liver tumour-initiating cells. Nat Commun 6:8271. doi:10.1038/ncomms9271
Han SY, Han HB, Tian XY, Sun H, Xue D, Zhao C, Jiang ST, He XR, Zheng WX, Wang J, Pang LN, Li XH, Li PP (2016) MicroRNA-33a-3p suppresses cell migration and invasion by directly targeting PBX3 in human hepatocellular carcinoma. Oncotarget. doi:10.18632/oncotarget.9886
Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ (2007) High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer 6:5. doi:10.1186/1476-4598-6-5
Hong CS, Jeong O, Piao Z, Guo C, Jung MR, Choi C, Park YK (2015) HOXB5 induces invasion and migration through direct transcriptional up-regulation of beta-catenin in human gastric carcinoma. Biochem J 472(3):393–403. doi:10.1042/bj20150213
Huang SD, Yuan Y, Zhuang CW, Li BL, Gong DJ, Wang SG, Zeng ZY, Cheng HZ (2012) MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer 11:51. doi:10.1186/1476-4598-11-51
Lefranc F, Brotchi J, Kiss R (2005) Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol 23(10):2411–2422. doi:10.1200/jco.2005.03.089
Li Z, Zhang Z, Li Y, Arnovitz S, Chen P, Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H, He C, Wang CZ, Elkahloun AG, Valk PJ, Dohner K, Neilly MB, Bullinger L, Delwel R, Lowenberg B, Liu PP, Morgan R, Rowley JD, Yuan CS, Chen J (2013) PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood 121(8):1422–1431. doi:10.1182/blood-2012-07-442004
Li F, Li XJ, Qiao L, Shi F, Liu W, Li Y, Dang YP, Gu WJ, Wang XG, Liu W (2014a) miR-98 suppresses melanoma metastasis through a negative feedback loop with its target gene IL-6. Exp Mol Med 46:e116. doi:10.1038/emm.2014.63
Li Y, Sun Z, Zhu Z, Zhang J, Sun X, Xu H (2014b) PBX3 is overexpressed in gastric cancer and regulates cell proliferation. Tumour Biol 35(5):4363–4368. doi:10.1007/s13277-013-1573-6
Lin X, Chen L, Yao Y, Zhao R, Cui X, Chen J, Hou K, Zhang M, Su F, Chen J, Song E (2015) CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget 6(24):20485–20499. doi:10.18632/oncotarget.4107
Liu X, Zhang W, Guo H, Yue J, Zhuo S (2016) miR-98 functions as a tumor suppressor in salivary adenoid cystic carcinomas. OncoTargets Ther 9:1777–1786. doi:10.2147/OTT.S98534
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838. doi:10.1038/nature03702
Lu Y, Wu D, Wang J, Li Y, Chai X, Kang Q (2016) miR-320a regulates cell proliferation and apoptosis in multiple myeloma by targeting pre-B-cell leukemia transcription factor 3. Biochem Biophys Res Commun 473(4):1315–1320. doi:10.1016/j.bbrc.2016.04.069
Magnani L, Patten DK, Nguyen VT, Hong SP, Steel JH, Patel N, Lombardo Y, Faronato M, Gomes AR, Woodley L, Page K, Guttery D, Primrose L, Fernandez Garcia D, Shaw J, Viola P, Green A, Nolan C, Ellis IO, Rakha EA, Shousha S, Lam EW, Gyorffy B, Lupien M, Coombes RC (2015) The pioneer factor PBX1 is a novel driver of metastatic progression in ERalpha-positive breast cancer. Oncotarget 6(26):21878–21891. doi:10.18632/oncotarget.4243
Ni R, Huang Y, Wang J (2015) miR-98 targets ITGB3 to inhibit proliferation, migration, and invasion of non-small-cell lung cancer. OncoTargets Ther 8:2689–2697. doi:10.2147/ott.s90998
Onishi M, Ichikawa T, Kurozumi K, Date I (2011) Angiogenesis and invasion in glioma. Brain Tumor Pathol 28(1):13–24. doi:10.1007/s10014-010-0007-z
Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW (2015) Mechanisms regulating glioma invasion. Cancer Lett 362(1):1–7. doi:10.1016/j.canlet.2015.03.015
Ramberg H, Alshbib A, Berge V, Svindland A, Tasken KA (2011) Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer 10:50. doi:10.1186/1476-4598-10-50
Ramberg H, Grytli HH, Nygard S, Wang W, Ogren O, Zhao S, Lovf M, Katz B, Skotheim RI, Bjartell A, Eri LM, Berge V, Svindland A, Tasken KA (2016) PBX3 is a putative biomarker of aggressive prostate cancer. Int J Cancer 139(8):1810–1820. doi:10.1002/ijc.30220
Shah N, Sukumar S (2010) The Hox genes and their roles in oncogenesis. Nat Rev Cancer 10(5):361–371. doi:10.1038/nrc2826
Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, Yang BB (2012) MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget 3(11):1370–1385. doi:10.18632/oncotarget.717
Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J, Krichevsky AM, Noske DP, Tannous BA, Wurdinger T (2010) miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget 1(8):710–720. doi:10.18632/oncotarget.101207
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J Med 352(10):987–996. doi:10.1056/NEJMoa043330
Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA (2012) Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 72(10):2473–2480. doi:10.1158/0008-5472.can-12-0122
Ting HJ, Messing J, Yasmin-Karim S, Lee YF (2013) Identification of microRNA-98 as a therapeutic target inhibiting prostate cancer growth and a biomarker induced by vitamin D. J Biol Chem 288(1):1–9. doi:10.1074/jbc.M112.395947
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60(3):166–193. doi:10.3322/caac.20069
Wang H, Liu G, Shen D, Ye H, Huang J, Jiao L, Sun Y (2015) HOXA1 enhances the cell proliferation, invasion and metastasis of prostate cancer cells. Oncol Rep 34(3):1203–1210. doi:10.3892/or.2015.4085
Wei F, Wang Q, Su Q, Huang H, Luan J, Xu X, Wang J (2016a) miR-373 inhibits glioma cell U251 migration and invasion by down-regulating CD44 and TGFBR2. Cell Mol Neurobiol. doi:10.1007/s10571-016-0338-3
Wei YB, Liu JJ, Villaescusa JC, Aberg E, Brene S, Wegener G, Mathe AA, Lavebratt C (2016b) Elevation of Il6 is associated with disturbed let-7 biogenesis in a genetic model of depression. Transl Psychiatry 6:e869. doi:10.1038/tp.2016.136
Yang G, Zhang X, Shi J (2015) MiR-98 inhibits cell proliferation and invasion of non-small cell carcinoma lung cancer by targeting PAK1. Int J Clin Exp Med 8(11):20135–20145
Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S, Cong N (2016) MicroRNA-98 acts as a tumor suppressor in hepatocellular carcinoma via targeting SALL4. Oncotarget. doi:10.18632/oncotarget.12190
Acknowledgements
This work was funded by the National Natural Science Foundation of China (81300998 and 81471269), National Natural Science Foundation of Jiangsu Province (BK20131022 and BK20160047), Jiangsu Province's Key Discipline of Medicine (XK201117), Jiangsu Province and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Specially Appointed Professor Foundation of Jiangsu Province (ky216r201307).
Author contributions
Xiupeng Xu and Zhongyuan Bao wrote the manuscript. Xiupeng Xu and Zhongyuan Bao performed and analyzed experiments and prepared the figures. Yinlong Liu and Jing Ji conceived experiments. Ning Liu and Jing Ji assisted with data analysis. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Xiupeng Xu and Zhongyuan Bao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, X., Bao, Z., Liu, Y. et al. MicroRNA-98 Attenuates Cell Migration and Invasion in Glioma by Directly Targeting Pre-B Cell Leukemia Homeobox 3. Cell Mol Neurobiol 37, 1359–1371 (2017). https://doi.org/10.1007/s10571-017-0466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0466-4