Abstract
Purpose
E-cadherin is a calcium-dependent glycoprotein whose main role is cell–cell adhesion. Its transcriptional repressor TWIST1 is a basic helix–loop–helix (bHLH) protein that participates in gastrulation and formation of mesodermal tissues during embryogenesis. In adult tissues, the high expression of TWIST1 induces the epithelial–mesenchymal transition (EMT)—a process in which cells become motile and able to metastasize. In this paper, we investigated the involvement of E-cadherin and TWIST1 in the carcinogenesis of brain metastases originating from two different primary sites—breast and lung.
Methods
The localization and expression of E-cadherin and its transcriptional repressor TWIST1 were investigated using a DAB-labeled streptavidin–horseradish peroxidase immunohistochemical reaction and specific monoclonal antibodies against TWIST1 and E-cadherin. Image J software was used for semi-quantitative analysis while H-score served for statistical evaluations.
Results
Immunohistochemistry showed that the expression of E-cadherin was downregulated in 85.7% of brain metastases, while at the same time, 82.2% of them showed upregulated TWIST1. Statistical analysis confirmed a significant negative correlation between expressions of TWIST1 and E-cadherin (p = 0.001). When the brain metastases expression levels were compared to primary breast tumors in corresponding patients, E-cadherin showed higher expression in primary pairs compared to corresponding metastases. Consistent to its role, TWIST1 was downregulated in all primary tumor samples in comparison to corresponding metastases pairs (p = 0.034).
Conclusion
This research provides valuable data regarding molecular events involving two EMT key components that could give directions for new possibilities for brain metastases diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Autopsy-based epidemiological studies indicate the presence of secondary tumors (metastases) of the central nervous system in about 25% of patients who died of malignancies [1]. The most common primary sites that metastasize to central nervous system (CNS) are malignant lung and breast tumors (45% and 15%, respectively), followed by melanomas, kidney and colorectal cancers, non-Hodgkin’s lymphomas and multiple myelomas [2]. The majority (80%) of brain metastases is localized in the cerebral hemispheres at the border zone of gray and white matter, about 15% are found in the cerebellum and 5% in the brain stem, while the areas of the spinal cord and meninges are less commonly affected. However, the lack of epidemiological studies does not provide us with accurate data on the incidence of central nervous system metastases. Nevertheless, it is estimated that the incidence of brain metastases is about 3 times higher than the incidence of primary brain tumors [1]. In this study, we decided to investigate the involvement of two proteins, markers of epithelial–mesenchymal transition (EMT), in brain metastases originating from breast and lung cancers.
Epithelial cadherin or E-cadherin is one of the central cell adhesion glycoproteins that belong to the group of calcium-dependent adhesion molecules. Expression of epithelial cadherin begins at an early embryonic two-cell stage to serve for blastomere adhesion and subsequent cell polarization [3]. In mature epithelial tissues, E-cadherin is indirectly involved in cellular signaling, proliferation, apoptosis, and differentiation, but directly maintains cell adhesion, so it is not surprising that some call it “the guardian of the epithelial phenotype” [4]. Loss of E-cadherin is one of the main indicators of the EMT in which cells develop mesenchymal features by losing polarity and cell adhesion [5]. Expression of mesenchymal markers, such as N-cadherin, vimentin and fibronectin, gives the cells a greater ability to move, thereby providing them with invasive and metastatic potential [6, 7]. Based on the above, E-cadherin was characterized as a tumor suppressor that prevents the invasiveness and metastatic potential of neoplastic cells. However, some papers report that E-cadherin can be re-expressed in the metastatic deposits where it allows re-stabilization and growth of tumors at the secondary localization [8,9,10]. The today’s concept of EMT transient states and cellular plasticity replaced the classic view of EMT in tumor as the transformation of epithelial cells into mesenchymal. We have also learned that carcinoma cells can undergo a partial EMT where cells acquire mesenchymal but keep epithelial markers as well. Those intermediate EMT states are influenced by the microenvironment enabling cells to quickly adapt to novel “soils” [9, 10]. The alterations of E-cadherin expression indicate that other important factors, such as the extracellular matrix remodeling together with signaling mechanisms during tumor cell adaptation, are also important for the metastatic process [9, 11].
TWIST1 belongs to helix–loop–helix (HLH) transcription factors whose important roles in gastrulation, mesoderm differentiation, and dorsal–ventral axis formation have long been recognized in the fruit fly model. TWIST1 expression in adult human tissues is in general very low. The highest expression was observed in placenta, while in other tissues, it is negligible, present only in traces in healthy mesodermal tissues [11]. TWIST1 is often activated and upregulated during carcinogenesis [12,13,14] and its expression is regulated by many signaling pathways responsible for cell growth and proliferation including AKT, STAT3, MAPK, Ras, and Wnt pathways. TWIST1 is the transcriptional repressor of E-cadherin whose activation decreases cadherin expression levels, while at the same time increases the expression of neural cadherins. Because N-cadherin and E-cadherin are major markers of EMT, TWIST1 is involved in the regulation of EMT, both embryonic and oncogenic [15, 16]. In the process of EMT, the epithelial phenotype is gradually replaced by a more mobile and invasive mesenchymal one, prone to hematogenous dissemination and formation of metastases. TWIST1 acts on the expression of E-cadherin both directly and indirectly. Directly it reduces E-cadherin expression by binding to the E-cadherin promoter, and indirectly by inducing SNAI2 expression, a protein which also binds to the E-cadherin promoter region and inhibits its transcription [4, 17, 18]. Numerous studies have shown that enhanced expression of TWIST1 contributes to the production of highly resistant malignancies with small therapeutic potential [4].
Materials and methods
Sample collection and processing
Formalin-Fixed Paraffin-Embedded (FFPE) brain metastases samples and primary breast and lung tumors were collected from the Neurosurgery Clinics and the Departments of Pathology Hospital Centers “Zagreb”, and “Sisters of Mercy”, Zagreb. Patients’ tumors were identified by magnetic resonance imaging (MRI) and removed using a micro-neurosurgical technique. Tumors were histopathologically classified as small-cell lung carcinomas, non-small-cell lung carcinoma (NSCLC), and invasive ductal breast carcinoma. We analyzed 28 samples of metastatic brain tumors, 15 of which originated from primary lung carcinoma (12 non-small-cell and 3 small-cell lung carcinomas), whereas 13 originated from primary breast carcinoma (all invasive ductal carcinomas) and 10 primary tumor pairs. The samples were collected through surgical resection of brain metastases from 20 female and 8 male patients. The subjects' age ranged from 39 to 81, with an average age of 59.68. The median age at the time of diagnosis was 62.88 in men and 58.40 in women. Ethical approval was received from the Ethical Committees School of Medicine University of Zagreb (case number: 380-59-10106-14-55/147; class: 641-01/14-02/01) and University Hospital Centers “Sisters of Mercy” (Number EP-7426/14-9) and “Zagreb” (Number 02/21/JG, Class: 8.1.-14/54-2) and all patients provided informed consent. Six samples of primary lung cancer and 4 primary breast cancers were also collected and analyzed to compare gene expression between primary tumors and metastasis.
Immunohistochemistry
The protein expression levels and localizations were determined by an immunohistochemical method using TWIST1 and E-cadherin-specific antibodies, in separate reactions. Paraffin Sects. 5 μm in thickness were fixed onto coated FLEX microscope slides (DakoCytomation, Glostrup, Denmark), then deparaffinized in xylene/ethanol baths and rehydrated in citrate buffer which unmasked epitopes. This was followed by incubation with hydrogen peroxide solution (30% H2O2, methyl alcohol, and distilled water at a ratio of 3:3:1) in a humid chamber for 10 min at room temperature to avoid the false-positive signal caused by oxidation of 3,3′-diaminobenzidine tetrahydrochloride (DAB). Non-specific binding of the primary antibody was blocked by adding serum (Protein Block Serum-Free, Dako, Glostrup, Denmark) and incubating for 30 min at 4 °C. The serum was carefully removed from the slide and each primary antibody was applied. For E-cadherin detection, we used monoclonal mouse anti-human E-cadherin clone: NCH-38 code M3612 (Dako, Glostrup, Denmark), diluted 1:100; and for TWIST1 mouse monoclonal antibody 10E4E6 (Santa Cruz Biotechnology, Inc., USA), diluted 1:400.
The next step was the incubation at 4 °C overnight, followed by Dako REAL™EnVision™/HRP, Rabbit/Mouse (ENV) kit reagent for 45 min and visualization of the reaction by Dako REAL™ DAB + Chromogen (EnVisionTM, Dako REALTM) for 2–5 min. Also, the hematoxylin was applied for 3 min for counterstain. To exclude any non-specific staining negative control, a paraffin slide treated in the same manner as the slides tested, but without the addition of a primary antibody, was used in each of the staining series. Liver, placenta and colon samples were used as positive controls for E-cadherin and TWIST1, respectively, according to the manufacturer's instructions. The reaction was marked as positive if brown staining was observed in the nucleus or membrane and/or cytoplasm or negative if only blue hematoxylin staining persisted in the tumor hot spot.
The results of the immunohistochemical reactions of TWIST1 and E-cadherin proteins were determined by a semi-quantitative analysis technique, where samples were analyzed using a bright-field microscope (Olympus Provis Ax70). For each sample, 200 cells were counted in tumor hot spot area and their protein expression intensities were determined using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). Assessment of immunopositivity in membranes, cytoplasm and/or cell nuclei was based on histological score or H-score. The cell staining intensity was divided into four categories: (0) no immunopositivity—blue staining, (1) weak—light yellow staining, (2) moderate—brown staining and (3) strong—dark brown staining. The H-score is calculated according to formula (1) where % cell1 indicates the percentage of cells stained with intensity 1; % cell2 with intensity 2, and % cell3 with intensity 3.
The H-score, therefore, ranged 0–300. Subsequently, the H-score values were converted into the following categories: (0) = no expression (H-score = 0–30), (1) = weak expression (H-score = 31–180), (2) = moderate expression (H-score = 181–255) and (3) = strong expression (H-score = 256–300). The specimens were inspected by three independent observers, who were “blind” to experimental conditions.
Statistical analysis
All statistical evaluations including histological subtype of the primary lesion, sex, age, protein expression intensities, subcellular localization and number of cells showing the specific intensity of E-cadherin and TWIST1 were performed with the SPSS statistical package 23.0 (SPSS Inc., Chicago, IL, USA) with the statistical significance set at p < 0.05. Normal distribution of the data was tested using a Kolmogorov–Smirnov test for normality where significance p < 0.05 indicates that the distribution significantly differs from normal. Differences in the distribution of two independent samples were determined with the Mann–Whitney U test, while differences between two sets of observations on a single sample were obtained with the Wilcoxon test.
Analysis of public database cBioPortal
Our results were compared to data provided by the publicly available database cBioPortal [24]. To validate our findings, we explored the public datasets downloaded from the cBioPortal for Cancer Genomics web resource which compiles data from the most recent studies from The Cancer Genome Atlas (TCGA) (https://www.cancer.gov/tcga).
Results
Demographic and clinical parameters of metastases
The results of the Kruskal–Wallis test show that there was a statistically significant difference in the age of diagnosis between different pathohistological types of brain metastases (p < 0.001). Using the Mann–Whitney U test, we have established that metastases from NSCLC appeared at a later age than those from invasive ductal carcinoma (p < 0.001). All collected breast cancer samples were from the female population. Although our cohort included 20 females and 8 males, the variable sex was not significantly associated to any specific pathohistological origin of brain metastases.
E-cadherin expression
Our results showed that E-cadherin expression was weak (46.4%) or lost (39.3%) in the majority of brain metastases (85.7%). Moderate expression was observed in 10.7%, while only 3.6% showed strong expression of E-cadherin in the membrane and cytoplasm. However, protein expressions differed between brain metastases according to distinct primary tumor sites. In metastases originating from lung, 80.0% of samples showed complete loss or weak E-cadherin expression, while 13.3% showed moderate and only 6.7% strong expression of this protein (Fig. 1a). Slightly different distributions were observed in metastases originating from breast tumors, where 92.3% of samples showed complete loss of the protein or its low expression levels. Moderate expression displayed 7.7% of metastases while none of the samples showed strong expression of E-cadherin protein (Fig. 1a).
Percentage of metastases expressing (a) E-cadherin and (b) TWIST1. ML—metastases from lung; MB—metastases from the breast; 0—no expression 1—weak expression 2—moderate expression and 3—strong expression
TWIST1 expression
The overall expression levels of TWIST1 protein were higher than the levels of E-cadherin. TWIST1 expression was strong (42.9%) or moderate (39.3%) in the majority of brain metastases (82.2%), weak in only 7.1% of samples, while lack of its expression was observed in 10.7% of total metastases. The analysis of this protein also showed that the expressions of TWIST1 differed between brain metastases originating from different primary sites. The highest number of samples (53.3%) with the strong TWIST1 expression was confined to metastases from lung tumors. Moderate expression was observed in 20.0%, while weak expression and loss of the protein was seen in 26.7% of metastases from the lung. Meanwhile, 61.5% metastases from breast tumors showed moderate expression with an additional 30.8% with strong expression levels (Fig. 1b). Only 7.7% of metastases displayed weak expression, while complete loss of TWIST1 expression has not been observed in any of the analyzed samples. In spite of the somewhat heterogeneous primary origins, the molecular findings in metastases are surprisingly homogenous.
The correlations of E-cadherin and TWIST1 expression levels
Next, we counted the cells displaying different staining intensities for each patient and calculated the H-scores (Fig. 2). Such semi-quantitative analysis showed that metastases originating from breast cancer contained a significantly higher number of weakly stained cells for E-cadherin than the metastases originating from lung (p = 0.025). Differences in counted cells among other categories of staining intensities were not significant. For instance, in category 2—moderate expression, 38.5% of metastases from breast showed higher expression of TWIST1 compared to metastases from lung, but the difference between moderately stained cells was not significant (p = 0.102).
To determine the possible correlation between TWIST1 and E-cadherin expression, the H-score values were analyzed for each sample by Mann–Whitney U and Wilcoxon tests. The relationship between the H-score of E-cadherin and TWIST1 is shown in Fig. 2. The Wilcoxon test performed on the H-score values showed a statistically significant difference in the expression of TWIST1 and E-cadherin in brain metastases (Z = − 3.302; p = 0.001). Furthermore, when the H-scores were categorized into two categories: strong (H-score = 181–300) and weak (H-score = 0–180) expression, the Mann–Whitney U test showed that the samples with low E-cadherin expression had the highest H-score of TWIST1 protein (p = 0.002). The reverse analysis also confirmed this with an even more pronounced negative association between H-scores of TWIST1 and E-cadherin expressions (p = 0.001). The immunohistochemical staining of both proteins is shown in Fig. 3.
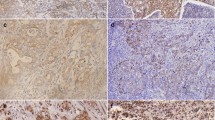
Immunohistochemical staining of brain metastases on E-cadherin and TWIST1. a metastasis sample showing poor staining intensity of E-cadherin and strong nuclear staining of TWIST1; b metastasis sample showing weak staining intensity of E-cadherin and strong of TWIST1; c metastasis sample showing strong staining intensity of E-cadherin and weak of TWIST1. d positive control sample (liver hepatocytes) with the strong E-cadherin staining of membranes and negative control for TWIST1 staining. Scale bar 20 μm
Comparison of TWIST1 and E-cadherin expression levels between primary tumors and corresponding metastases
The expression levels of E-cadherin and TWIST1 were also determined in 10 primary tumor pairs, 4 of which invasive ductal carcinomas and 6 NSCLC. Unfortunately, we were not able to collect all paired primary samples. Nevertheless, the collected group served for expression comparison between the primary tumor and its associated metastases. The significance was not established due to the small number of primary tumor samples. However, the following distribution was observed: E-cadherin expression was lost or weak in 7/10 primary tumors, moderate in 2/10 and strong in only 1/10 primary tumors. Similarly, TWIST1 expression was weak in 6/10 primary tumors, moderate in 3/10 and strong in 1 primary tumor (Fig. 4). When we divided our groups according to pathohistological origin, weak and negative E-cadherin expression was observed in 5/6 samples of primary lung cancer, moderate in 1/6, while none of the samples exhibited strong immunostain. Primary breast tumors showed weak E-cadherin expression in 2/4 samples, moderate in 1/4 and strong in 1/4 (Fig. 5a). TWIST1 protein expression in primary lung cancer was distributed as follows: weak or lack of expression in 4/6 samples, moderate in 1/6 and strong also in 1/6. Weak or lack of expression of TWIST1 in primary breast tumors was observed in 2/4 cases, while another 2/4 cases displayed moderate expression levels (Fig. 5b).
Expression of TWIST1 and E-cadherin in primary tumors; 0—no expression 1—weak expression 2—moderate expression and 3—strong expression
In spite of the fact that 5/6 of primary lung tumors showed lack or weak E-cadherin expression, the more detailed analysis based on counted cells and the comparison of H-scores revealed that the protein was more abundant in primary breast tumors than in metastatic pairs (Fig. 5a). The comparison of H-scores for primary breast and lung tumors showed that all of them had lower expression of TWIST1 in comparison with their metastatic pairs (Fig. 5b). In most of the metastases, E-cadherin expression was reduced in comparison to healthy tissue and primary tumors and only 4 metastatic samples showed higher expression than the primary NSCLC (Fig. 5a). The Mann–Whitney U test showed that H-scores of TWIST1 expression in metastases samples significantly exceeded those in primary tumors (p = 0.034). However, the same test showed no significant difference in H-scores of E-cadherin between metastases and primary tumors (p = 0.666). When comparing total lung category consisting of primary tumors and metastases to the total breast category consisting of primary tumor and metastases, the Mann–Whitney U test showed no statistical significance in the H-scores of E-cadherin (p = 0.253) and TWIST1 (p = 0.977) expression.
Alterations of TWIST1 and CDH1 genes reported on cBioPortal database
Publicly reported gene alterations, RNA sequencing (RNA- Seq) expression data as well as protein expression levels available for small-cell lung carcinoma, NSCLC and invasive ductal breast carcinoma were downloaded from the cBioPortal platform (https://www.cbioportal.org/) on August 27, 2020 and compared to our results. Combined collective public datasets from 23 studies on primary lung and breast cancer showed compatibility to our findings. The predominant TWIST1 change reported for NSCLC was gain in 42.78% and amplification in 1.98% of 4691 investigated cases, while somatic mutations were found in 0.13% and deep deletions in 0.26%. Data on TWIST1 for small-cell lung cancer investigated on 252 cases report on 1.19% of somatic mutations. Gene CDH1 was investigated on 6049 NSCLC cases and it predominately showed shallow deletions in 19.64% and mutations in 0.64%. Small cell lung carcinoma report on 0.79% of somatic mutations (2 out of 252 reported cases). The expression levels were available only for lung adenocarcinoma and cBioPortal reports on high mRNA levels of TWIST1 in 35.26% (850/2411) and low protein levels (negative Z score) of E-cadherin in 46.34% of cases (336/752). cBioPortal data on invasive ductal breast carcinoma encompassed 5699 cases. Here, TWIST1 predominantly showed gain in 23% (907/3944) and amplification in 1.83% (72/3944) of cases and was mutated in 0.13%. mRNA was tested on 2081 cases and 23.5% showed high levels of TWIST1 mRNA. As for CDH1 in breast carcinoma, there were predominantly shallow deletions found in 1934 out of 5699 cases (33.94%), while mutations were reported in 1.19% and low levels of mRNA and E-cadherin protein in 70.16% and 31.39%, respectively. The reported mutations are predominantly missense somatic mutations or truncating mutations characterized as potential drivers. The publicly available data for each primary site are shown in Fig. 6.
Publicly reported gene alterations, RNA sequencing (RNA- Seq) expression data as well as protein expression levels available for small-cell lung carcinoma, NSCLC and invasive breast carcinoma downloaded and adapted from the cBioPortal platform (https://www.cbioportal.org/). Percents represent number of alterations on total cancers profiled per gene
Discussion
The developmental program of EMT is regulated by numerous signaling pathways and microenvironmental signals and is now considered to be dysregulated in tumor invasion, intravasation of blood vessels, and metastasis formation [19]. Hypoxia, oxidative stress, and inflammatory processes can all activate transcription factors SNAIL, SLUG, ZEB1, ZEB2, and TWIST1 responsible for the E-cadherin down-regulation, consequent EMT onset and mesenchymal phenotype obtaining [20]. The adhesive activity of E-cadherin on the cell surface can be regulated by various molecules including growth factors. It plays an important role in the process of invasion and metastasis using similar mechanisms to those involved in embryonic morphogenesis and the formation of epithelial tissues [11]. Our findings show the involvement of two proteins, key markers of EMT, in the collection of brain metastases. Decreased expression of E-cadherin was found in 85.7% of our total metastases sample, while at the same time, a strong upregulation of its transcriptional repressor TWIST1 was observed in 82.2% of cases. Furthermore, metastases originating from both breast and lung tumors showed a statistically higher expression of TWIST1 in comparison with their primary tumor pairs (p = 0.034). Numerous studies have evidenced that a key event in EMT is the loss of E-cadherin which allows tumor cells to become motile, invasive, and spread to distant organs [19]. A significant difference in H-score values of the expression of TWIST1 and E-cadherin in brain metastases (Z = − 3.302; p = 0.001) was obtained by Wilcoxon test. This significant reverse expression in the same patients indicates that brain metastatic cells have acquired mesenchymal character.
Data from cBioPortal database are compatible to our findings.
In the majority of metastases investigated here, E-cadherin expression was reduced when compared to primary tumors. However, 4 metastasis samples originating from NSCLC showed higher expression than the primary tumor pairs. Several reports on the increased expression of E-cadherin as a feature of the re-stabilization of metastatic cells at a secondary localization could explain the observed higher levels. Other studies have shown that different mutations of the E-cadherin gene (CDH1) can cause changes in its tertiary structure, resulting in a decrease or increase in cell adhesion capacity depending on the context [21,22,23]. In spite of the high expression, the adhesion could still be reduced. Our finding on positive E-cadherin expression levels in metastases from NSCLC is in accordance with Zhang et al. [24], who found positive expression of E-cadherin in lung adenocarcinoma they investigated, more precisely in 79% of investigated patients. However, in their paper, positive expression of E-cadherin was found to be lower in tissues from poorly differentiated tumors.
Zhao et al. [25] showed that high expression of E-cadherin in tumor cells from pleural effusion is correlated with EGFR mutation and better prognosis in patients with lung adenocarcinoma. The alteration of E-cadherin expression has also been shown to play a role in suppressing cell proliferation via loss of HIF-1 and the tumors' inability to adapt to aerobic glycolysis [26]. Epigenetic studies have shown that CDH1 promoter methylation can also cause the reduction of the protein. It has been shown that this process is mediated by the transcriptional repressor SNAIL, which associates specific methyltransferases to the CDH1 promoter site. Abnormal DNA methylation in the promoter region of the CDH1 gene has been associated with loss of E-cadherin in a large number of invasive breast cancer samples [21]. The reversible process of demethylation enables the re-expression of protein, mesenchymal–epithelial transition (MET) and the return of the epithelial phenotype [9, 27].
Several studies are in accordance with our results. Matsubara et al. [28] determined the relationship between E-cadherin and ZEB1 using immunohistochemical analysis in 157 NSCLC. They found that the expression of E-cadherin was low in the 10% of adenocarcinomas and 11% of squamous cell carcinomas, while in poorly differentiated carcinomas, low levels of E-cadherin were more frequent. Thus, 50% of large cell carcinomas and 90% of pleomorphic carcinomas showed low expression of E-cadherin. Interestingly, they also found ZEB1 expression to be inversely correlated with that of E-cadherin. They concluded that the expression of ZEB1 was not necessarily associated with the low expression of E-cadherin in lung adenocarcinomas and squamous cell carcinomas. It seems that specific pathohistological tumor subtype alternates E-cadherin transcription suppressor. Furthermore, it has been demonstrated that in all of the EMT-associated transcription factors, ZEB1 uniquely promotes NSCLC progression. Manshouri et al. [29] defined ZEB1 interactors that are critical to metastatic NSCLC using two screens, BioID, and an Epigenome shRNA dropout screen, and identified the GTPase-activating protein TBC1D2B as a ZEB1 target. TBC1D2b has been shown to suppress E-cadherin internalization, thus hindering invasion and metastasis.
Because of E-cadherin and beta-catenin involvement in EMT, the role of the Wnt pathway has been suggested. Wnt is an evolutionarily conserved pathway intertwined with EMT via E-cadherin, which simultaneously acts as a β-catenin stabilizer and as an adhesion molecule. Elevated levels of unphosphorylated β-catenin cause its translocation to the karyoplasm, where it binds to TCF1/LEF1 transcription factors and promotes the transcription of genes for cell growth. Our previous work on the increased expression of Wnt pathway signaling components, DVL1, DVL3, and beta-catenin, in brain metastases from primary lung cancer support this suggestion [30].
TWIST1 has also been known to play important roles in the EMT mechanism primarily by reducing E-cadherin cellular levels [31]. The upregulation of this transcription factor has been shown to stimulate metastatic potential in invasive breast cancers [32, 33]. In our study, 73.3% of brain metastases from lung and 92.3% from breast cancer showed the upregulation of TWIST1. Besides E-cadherin, TWIST1 acts upon many other genes that control apoptosis and extracellular matrix remodeling. Vesuna et al. [32] showed that in breast cancer, TWIST1 enhanced transcription and translation of apoptosis genes, protease protein family, and extracellular matrix proteins. Further studies discovered that TWIST1 plays a role in reprogramming energy metabolism through mitochondrial mass change, glucose utilization, and lactate production in breast cancer cells [34]. The activation is mediated by β1-integrin/FAK/PI3K/AKT/mTOR pathways and repression of the p53 signaling [35, 36]. Such metabolic changes contribute to malignant transformation by promoting the development of cachexia in tumors expressing TWIST1. As for its known involvement in lung cancer [37], clinical studies have shown that TWIST1 expression has a significant correlation with survival and prognosis in patients with NSCLC associated with tumor stage, differentiation level, and metastasis in the surrounding lymph nodes [38,39,40].
Our results showed that TWIST1 was significantly (p = 0.034) lower in all primary cancer samples compared to the metastatic pairs, suggesting that TWIST1 expression is a later event associated to progression and metastasis. Overall E-cadherin expression levels were decreased in both primary and secondary tumors as compared to normal controls. Furthermore, a comparison of the primary specimens and their associated metastases showed a decreased E-cadherin expression level in 66.7% of primary NSCLC. This suggests that the loss of E-cadherin expression is an early event in primary cancer cells.
Several studies have shown that the genetic characteristics of metastatic tumors are similar to those of primary ones, which supports the hypothesis that metastatic gene potential may already exist in primary tumors and is not associated with the later stages of the disease, as has long been assumed [31, 41].
E-cadherin expression levels in primary breast cancers were higher than the levels found in brain metastatic pairs, while in the heterogeneous group of primary lung cancer, its expression levels were predominantly lower when compared to their metastatic pairs. Alsaleem et al. [22] studied E-cadherin in invasive ductal carcinoma using various platforms including immunohistochemistry, microarray analysis, next-generation sequencing, and copy number analysis. Their study showed that 27% of high-grade invasive ductal carcinoma showed reduced/loss of E-cadherin membranous expression. CDH1 copy number loss was seen in 21% of invasive ductal carcinoma, which also showed low CDH1 mRNA expression (p = 0.003). Furthermore, 79% percent of cases with reduced CDH1 mRNA showed elevated levels of TWIST2, ZEB2, NFKB1, LLGL2, CTNNB1 (p < 0.01) which are all repressors of E-cadherin transcription.
Conclusion
Brain metastases are a major medical problem due to the increasing incidence and stagnating therapeutic options. Several protective mechanisms have developed in the brain in the course of human evolution, such as the blood–brain barrier and supporting glial cells, which, in addition to many other roles, regulate transport from blood to brain tissue. Therefore, tumor cells have adapted to survive in a highly competitive brain environment.
Our study demonstrates that the expression level of the epithelial marker E-cadherin and its transcriptional repressor TWIST1 plays a central role in EMT, the process of hematogenous dissemination and the formation of secondary brain metastases. Future research in this direction will improve our understanding of the molecular basis of the metastatic process into the brain and uncover new directions in the treatment of secondary brain tumors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKT:
-
Protein kinase B
- APC:
-
Adenomatous polyposis coli
- CTNNB1:
-
Beta-Catenin
- EGFR:
-
Epidermal growth factor receptor
- HIF1:
-
Hypoxia-inducible factor 1
- LEF1:
-
Lymphoid-enhancer factor 1
- LLGL2:
-
Lethal Giant Larvae Homolog 2, Scribble Cell Polarity Complex Component
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- NFKB1:
-
Nuclear Factor Kappa B Subunit 1
- RB1:
-
Retinoblastoma 1
- SNAI2:
-
Snail Family Transcriptional Repressor 2
- STAT3:
-
Signal transducer and activator of transcription 3
- TBC1D2B:
-
TBC1 Domain Family Member 2B
- TCF1:
-
T-cell transcription factor 1
- TWIST1:
-
Twist Family BHLH Transcription Factor 1
- ZEB1:
-
Zinc finger E-box binding homebox 1
- ZEB2:
-
Zinc finger E-box binding homebox 2
- DVL:
-
Dishevelled
References
Wesseling P, von Deimling A, Aldape KD, Preusser M, Rosenblum MK, Mittelbronn M, et al. Metastatic tumours of the CNS. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system (IARC WHO classification of tumours). 4th ed. Lyon: The International Agency for Research on Cancer (IACR); 2016.
Lowery FJ, Yu D. Brain metastasis: Unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer. 2017;1867:49–57. https://doi.org/10.1016/j.bbcan.2016.12.001.
Pećina-Šlaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. https://doi.org/10.1186/1475-2867-3-17.
Bastid J, Ciancia C, Puisieux A, Ansieau S. Role of TWIST proteins in cancer progression. Atlas Genet Cytogenet Oncol Haematol. 2010;14:898–907. https://doi.org/10.4267/2042/44856.
Cervantes-Arias A, Pang LY, Argyle DJ. Epithelial-mesenchymal transition as a fundamental mechanism underlying the cancer phenotype. Vet Comp Oncol. 2013;11:169–84. https://doi.org/10.1111/j.1476-5829.2011.00313.x.
Mrozik KM, Blaschuk OW, Cheong CM, Zannettino ACW, Vandyke K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer. 2018;18:939. https://doi.org/10.1186/s12885-018-4845-0.
Battaglia RA, Delic S, Herrmann H, Snider NT. (2018) Vimentin on the move: new developments in cell migration. F1000Res. 2018;7:F1000 Faculty Rev-1796. Published 2018 Nov 15. https://doi.org/10.12688/f1000research.15967.1
Bukholm IK, Nesland JM, Bùrresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gama-catenin, in metastatic tissue from breast cancer patients. J Pathol. 2000;190:15–9. https://doi.org/10.1002/(SICI)1096-9896(200001)190:1<15:AID-PATH489>3.0.CO;2-L.
Williams ED, Gao D, Redfern A, Thompson EW. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019;19:716–32. https://doi.org/10.1038/s41568-019-0213-x.
Gloushankova NA, Rubtsova SN, Zhitnyak IY. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers. 2017;5(3):e1356900. https://doi.org/10.1080/21688370.2017.1356900.
Petrova YI, Schecterson L, Gumbiner BM. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell. 2016;27:3233–44. https://doi.org/10.1091/mbc.e16-01-0058.
Nieszporek A, Skrzypek K, Adamek G, Majka M. Molecular mechanisms of epithelial to mesenchymal transition in tumor metastasis. Acta Biochim Pol. 2019;66(4):509–20. https://doi.org/10.18388/abp.2019_2899.
Zhang P, Hu P, Shen H, Yu J, Liu Q, Du J. Prognostic role of twist or snail in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2014;44:1072–94. https://doi.org/10.1111/eci.12343.
Zhu QQ, Ma C, Wang Q, Song Y, Lv T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biol. 2016;37:185–97. https://doi.org/10.1007/s13277-015-4450-7.
Zhang H, Gong J, Kong D, Liu HY. Anti-proliferation effects of twist gene silencing in gastric cancer SGC7901 cells. World J Gastroenterol. 2015;21:2926–36. https://doi.org/10.3748/wjg.v21.i10.292613.
Navas T, Kinders RJ, Lawrence SM, Ferry-Galow KV, Borgel S, Hollingshead MG, et al. Clinical evolution of epithelial-mesenchymal transition in human carcinomas. Cancer Res. 2020;80:304–18. https://doi.org/10.1158/0008-5472.CAN-18-3539.
Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene. 2018;37:4769–80. https://doi.org/10.1007/s10741-014-9462-7.
Hui L, Zhang S, Dong X, Tian D, Cui Z, Qiu X. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS ONE. 2013;8:e62171. https://doi.org/10.1371/journal.pone.0062171.
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805–23. https://doi.org/10.1002/1878-0261.12095.
Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11:718–38. https://doi.org/10.1002/1878-0261.12091.
Caldeira JR, Prando EC, Quevedo FC, Neto FA, Rainho CA, Rogatto SR. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6:48. https://doi.org/10.1186/1471-2407-6-48.
Alsaleem M, Toss MS, Joseph C, Aleskandarany M, Kurozumi S, Alshankyty I, et al. The molecular mechanisms underlying reduced E-cadherin expression in invasive ductal carcinoma of the breast: high throughput analysis of large cohorts. Mod Pathol. 2019;32:967–76. https://doi.org/10.1038/s41379-019-0209-9.
Shen M, Kang Y. Role reversal: a pro-metastatic function of E-cadherin. Dev Cell. 2019;51:417–9. https://doi.org/10.1016/j.devcel.2019.10.028.
Zhang Y, Wang LF, Gao JH, et al. Clinical significance of epithelial-mesenchymal transition-related molecules in lung adenocarcinoma. Curr Oncol. 2019;26:e121–e127127. https://doi.org/10.3747/co.26.4471.
Zhao C, Li X, Su C, Li J, Cheng N, Ren S, et al. High expression of E-cadherin in pleural effusion cells predicts better prognosis in lung adenocarcinoma patients. Int J Clin Exp Pathol. 2015;8:3104–9.
Hugo HJ, Gunasinghe NPAD, Hollier BG, Tanaka T, Blick T, Toh A, et al. Epithelial requirement for in vitro proliferation and xenograft growth and metastasis of MDA-MB-468 human breast cancer cells: oncogenic rather than tumor-suppressive role of E-cadherin. Breast Cancer Res. 2017;19:86. https://doi.org/10.1186/s13058-017-0880-z.
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, et al. G9a interacts with snail and is critical for snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122:1469–86. https://doi.org/10.1172/JCI57349DS1.
Matsubara D, Kishaba Y, Yoshimoto T, Sakuma Y, Sakatani T, Tamura T, et al. Immunohistochemical analysis of the expression of E-cadherin and ZEB1 in non-small cell lung cancer. Pathol Int. 2014;64:560–8. https://doi.org/10.1111/pin.12214.
Manshouri R, Coyaud E, Kundu ST, Peng DH, Stratton SA, Alton K, et al. ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat Commun. 2019;10:5125. https://doi.org/10.1038/s41467-019-12832-z.
Kafka A, Tomas D, Beroš V, Pećina HI, Zeljko M, Pećina-Šlaus N. Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int J Mol Sci. 2014;15:10635–51. https://doi.org/10.3390/ijms150610635.
Liao TT, Yang MH. Hybrid epithelial/mesenchymal state in cancer metastasis: clinical significance and regulatory mechanisms. Cells. 2020;9:E623. https://doi.org/10.3390/cells9030623.
Vesuna F, Bergman Y, Raman V. Genomic pathways modulated by twist in breast cancer. BMC Cancer. 2017;17:52. https://doi.org/10.1186/s12885-016-3033-3.
Devanand P, Sundaramoorthy S, Ryu MS, Jayabalan AK, Ohn T, Lim IK. Translational downregulation of Twist1 expression by antiproliferative gene, B-cell translocation gene 2, in the triple negative breast cancer cells. Cell Death Dis. 2019;10:410. https://doi.org/10.1038/s41419-019-1640-z.
Yang X, Hu J, Shi C, Dai J. Activation of TGF-β1 pathway by SCUBE3 regulates TWIST1 expression and promotes breast cancer progression. Cancer Biother Radiopharm. 2019. https://doi.org/10.1089/cbr.2019.2990(published online ahead of print).
Yang L, Hou Y, Yuan J, Tang S, Zhang H, Zhu Q, et al. Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways. Oncotarget. 2015;6:25755–699. https://doi.org/10.18632/oncotarget.4697.
Tang H, Massi D, Hemmings BA, Mandalà M, Hu Z, Wicki A, et al. AKT-ions with a TWIST between EMT and MET. Oncotarget. 2016;7:62767–77. https://doi.org/10.18632/oncotarget.11232.
Xia W, Mao Q, Chen B, Wang L, Ma W, Liang Y, et al. The TWIST1-centered competing endogenous RNA network promotes proliferation, invasion, and migration of lung adenocarcinoma. Oncogenesis. 2019;8:62. https://doi.org/10.1038/s41389-019-0167-6.
Duan F, Hao D, Xu W, Zhong X, Luo T. Correlations of twist expression with pathological and computed tomography (CT) characteristics and prognosis of Non-Small Cell Lung Cancer (NSCLC). Med Sci Monit. 2019;25:977–83. https://doi.org/10.12659/msm.912674.
Luo T, Duan F, Hao D, Xu W, Zhong X. Correlations of twist expression with pathological and computed tomography (CT) characteristics and prognosis of Non-Small Cell Lung Cancer (NSCLC). Med Sci Monit. 2019;25:977–83. https://doi.org/10.12659/msm.912674.
Wang Y, Shi L, Li J, Wang H, Yang H. Involvement of twist in NNK exposure-promoted lung cancer cell migration and invasion. Toxicol In Vitro. 2020;63:104740. https://doi.org/10.1016/j.tiv.2019.104740.
Plygawko AT, Kan S, Campbell K. Epithelial–mesenchymal plasticity: emerging parallels between tissue morphogenesis and cancer metastasis. Phil Trans R Soc. 2020;B375:20200087. https://doi.org/10.1098/rstb.2020.0087.
Funding
This work was supported by grants 6625 from Croatian Science Foundation and co-financed by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience (project “Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
PB performed experimental work, performed analyses, contributed to study design and wrote the manuscript. AB contributed to the data interpretation, manuscript editing, revised the manuscript for important intellectual content. AK contributed to data acquisition and analysis and revised the manuscript for important intellectual content. NPŠ produced the idea, designed the study, contributed to analysis and interpretation of the results, wrote the manuscript and approved the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose regarding this manuscript.
Ethical approval
This study was conducted in compliance with the declaration of Helsinki regarding ethical principles for medical research involving human subjects. This research was approved by Ethics Committees of the School of Medicine University of Zagreb (Case number: 380-59-10106-14-55/147; Class: 641-01/14-02/01) and University Hospital Centers “Sisters of Mercy” (Number EP-7426/14-9) and “Zagreb” (Number 02/21/JG, Class: 8.1.-14/54-2) and all patients provided informed consent.
Informed consent
Informed consent has been obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brlek, P., Bukovac, A., Kafka, A. et al. TWIST1 upregulation affects E-cadherin expression in brain metastases. Clin Transl Oncol 23, 1085–1095 (2021). https://doi.org/10.1007/s12094-020-02496-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02496-3