Abstract
Purpose
Stereotactic body radiotherapy (SBRT) has proven to be a highly effective treatment for selected patients with spinal metastases. Randomized evidence shows improvements in complete pain response rates and local control with lower retreatment rates favoring SBRT, compared to conventional external beam radiotherapy (cEBRT). While there are several reported dose-fractionation schemes for spine SBRT, 24 Gy in 2 fractions has emerged with Level 1 evidence providing an excellent balance between minimizing treatment toxicity while respecting patient convenience and financial strain.
Methods
We provide an overview of the 24 Gy in 2 SBRT fraction regimen for spine metastases, which was developed at the University of Toronto and tested in an international Phase 2/3 randomized controlled trial.
Results
The literature summarizing global experience with 24 Gy in 2 SBRT fractions suggests 1-year local control rates ranging from 83-93.9%, and 1-year rates of vertebral compression fracture ranging from 5.4-22%. Reirradiation of spine metastases that failed prior cEBRT is also feasible with 24 Gy in 2 fractions, and 1-year local control rates range from 72-86%. Post-operative spine SBRT data are limited but do support the use of 24 Gy in 2 fractions with reported 1-year local control rates ranging from 70-84%. Typically, the rates of plexopathy, radiculopathy and myositis are under 5% in those series reporting mature follow up, with no cases of radiation myelopathy (RM) reported in the de novo setting when the spinal cord avoidance structure is limited to 17 Gy in 2 fractions. However, re-irradiation RM has been observed following 2 fraction SBRT. More recently, 2-fraction dose escalation with 28 Gy, with a higher dose constraint to the critical neural tissues, has been reported suggesting improved rates of local control. This regimen may be important in those patients with radioresistant histologies, high grade epidural disease, and/or paraspinal disease.
Conclusion
The dose-fractionation of 24 Gy in 2 fractions is well-supported by published literature and is an ideal starting point for centers looking to establish a spine SBRT program.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal metastases are a common manifestation of advanced cancer, manifesting in up to 30% of patients. Of all bone metastases, 70% are located in the spine and symptomatic spinal metastases may be the initial manifestation of malignancy in 12–20% of cases [1,2,3]. In addition, 10–20% of patients with spinal disease will develop malignant epidural spinal cord compression (MESCC) which may result in severe pain and debilitating neurologic deficits [4].
Painful spinal metastases have been traditionally treated with conventional external beam radiotherapy (cEBRT), with adjuvant palliative cEBRT in those requiring initial surgery [5]. However, cEBRT is associated with low rates of complete pain response (CPR) that range from 10 to 20%, and efficacy with respect to local control is limited [6,7,8]. In the modern era, patients with metastatic disease are surviving longer, and there are increasing demands on radiation oncology to provide a treatment that controls sites of metastatic disease while preserving quality-of-life and systemic therapy administration. This rationale is of greater importance in those histologies considered radioresistant, those with paraspinal and/or epidural disease (mass-type) and patients who have undergone surgery, given the poor results associated with cEBRT [9, 10].
Recently, the Canadian Cancer Trials Group (CCTG) symptom control-24 (SC.24) phase 2/3 randomized controlled trial (RCT) proved superiority with 24 Gy in 2 Stereotactic Body Radiotherapy (SBRT) fractions, as compared to 20 Gy in 5 cEBRT fractions, with respect to 3- and 6-month CPR rates post-radiation [11]. However, there is variability in spine SBRT dose-fractionation schemes with no RCTs interrogating the optimal approach with respect to pain control, local control and adverse events [9]. High-dose single-fraction approaches have been found to be associated with high rates of local control at the expense of an increased risk of vertebral compression fracture (VCF), while 3–5 fraction regimens commit patients to a more prolonged treatment course that may be less biologically effective, particularly for radioresistant histologies [12, 13]. As compared to 5 cEBRT fractions, 24 Gy in 2 SBRT fractions was not only efficacious but yielded less financial strain on patients and has been globally adopted [14,15,16,17,18]. This review will provide an overview of the technical details and planning parameters specific to spine SBRT, and summarize the current literature in those series reporting outcomes following 24 Gy in 2 SBRT fractions.
Indications
Selecting patients most appropriate for spine SBRT is critical as it is more resource intensive compared to cEBRT [19]. Patient and disease factors must be taken into consideration and involves a thorough evaluation of pain severity, neurological status, spine instability, presence and grade of epidural disease, tumor histology, systemic therapy options, overall metastatic burden, performance status and life expectancy. Zeng et al. performed an analysis of 605 patients treated with spine SBRT and found that having polymetastatic disease, a non-breast or prostate primary, ECOG performance status ≥ 2, poor neurological status, pain and paraspinal disease were significant predictors of shorter survival [20]. Based on the SC-24 RCT, only patients with a life expectancy of more than 3 months should be considered candidates for spine SBRT, as at 1-month the CPR rates were equivocal.
There have been well-established framework published in literature to inform clinical decisions and patient selection when considering spine SBRT [21, 22]. Specifically, the neurologic, oncologic, mechanical and systemic (NOMS) decision framework serves as an excellent guide to facilitate decision making in the care of patients with spinal metastases [23]. This is a multidisciplinary approach that incorporates radiation/medical oncology, surgery and interventional radiology, and integrates the use of spine SBRT within the decision-making tree for emerging practices.
Evaluating spinal stability is essential to administering spine SBRT safely and maintaining patients’ quality of life. The Spinal Instability Neoplastic Score (SINS) can help guide clinicians in selecting those patients who may be better suited for upfront surgical intervention [24]. Frank spinal instability based on a SINS > 12 should warrant assessment by a spine surgeon and can be considered in those with potential instability with a SINS of 7–12. In the SC-24 RCT, patients with frank instability were excluded. For patients with a baseline VCF, cement augmentation in conjunction with local tumor ablative procedures is becoming more prevalent and may be utilized in order to optimize pain control and maintain stability long-term [25, 26]. As evaluated by the Bilsky criteria, patients with high-grade epidural disease should be referred for surgical decompression, followed by postoperative spine radiotherapy as appropriate [27, 28]. Furthermore, with the advancement of minimally-invasive surgery, the combination of separation surgery and planned SBRT is emerging as an effective approach for the purposes of maximizing ablative dose to the target disease while minimizing toxicity and surgical complications [29, 30].
Technical specifications
Simulation
Spine SBRT is a precision-driven treatment technique that delivers high doses of radiation to metastatic lesions while minimizing dose to surrounding organs-at-risk (OAR), the most critical being the spinal cord and nerve roots, together referred to as the critical neural tissues (CNT). Near-rigid body immobilization with acceptable visualization of the CNT is essential for safe and effective treatment delivery. For lesions in the upper spine (cranial to T5), a thermoplastic mask immobilizing the head and shoulders is typically implemented. For lesions in the spine below this level, a device that maximizes repositioning accuracy and intrafraction stability is recommended such as the BodyFIX dual vacuum-assisted body cushion (Elekta AB, Stockholm, Sweden).
Computed tomography (CT) simulation is acquired with 1-mm slice thickness, and this is coupled with magnetic resonance imaging (MRI) simulation which consists of axial thin-slice volumetric T1 and T2 sequences with 1–2 mm slice thickness. MR images acquired around the time of treatment planning are essential to provide accurate visualization to assist with delineating the extent of the target lesion, as well as the CNT. The MRI should cover the affected segments and at least one vertebra above and below the target level. In the cervical spine, this can be expanded to two levels above and below given the short height of the individual cervical vertebral segments.
If a CT myelogram is required for accurate delineation of the CNT, it is recommended that the myelogram is performed just prior to simulation in order to acquire a simulation CT myelogram, as opposed to a diagnostic CT myelogram that is fused to the planning CT. Importantly, a treatment planning MRI is still required for disease visualization. Rarely, the level of artifact is too significant despite the MRI and CT myelogram and in such cases, treatment with cEBRT is recommended. Lastly, there have been concerns with respect to dosimetric compromises associated with the hardware, however, a recent phantom based study confirms that the effect is negligible [31].
Volume and OAR delineation
Gross tumor volume (GTV) is defined as the gross disease within the spinal segment, including any epidural or paravertebral extension. MRI should be used to assist with contouring of the gross tumor, with the T1 non-gadolinium sequence most helpful in determining the extent of intra-osseous disease. The T1 gadolinium enhanced sequence may be used in selected cases to better identify the extent of para-spinal disease.
Delineation of the clinical target volume (CTV) should be conducted using the international consensus guidelines based on anatomical classification. Current published guidelines inform reproducible contouring for metastases of the cervical, thoracic and lumbar spine, in addition to sacral lesions and in the post-operative setting [32,33,34]. Figure 1 illustrates an intact spine SBRT case with the CTV and planning target volume (PTV) delineated. For paraspinal disease, it is recommended to use a 5-mm anatomically respectful margin to any soft tissue extension in order to encompass possible microscopic disease. In addition, in cases with epidural disease, a 5-mm margin will be adapted in the cranio-caudal direction within the spinal canal given that the axis is at risk of spread [21]. Recently, an analysis of 283 patients with 360 spine metastases treated with SBRT was conducted for the purposes of validating the aforementioned contouring guidelines [35]. After adjusting for confounding variables, they found that deviation from guidelines was the strongest predictor of inferior local control. Marginal failure rate was 42% among those with deviations, the majority of which were in the adjacent vertebral compartment that should have been included if the treatment had been contouring guideline-compliant. These data support covering adjacent vertebral compartments as stipulated in consensus recommendations.
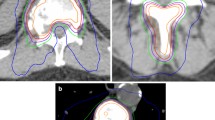
a Axial CT isodose distribution for an intact L1 spine SBRT plan (24 Gy in 2 fractions) showing CTV (blue color-wash), PTV (orange color-wash), thecal sac (purple color-wash), bowel bag (brown line) and kidneys (dark red and teal lines). b Sagittal CT isodose distribution showing CTV (blue color-wash), PTV (orange color-wash), thecal sac (purple color-wash) and spinal cord PRV (green color-wash). c Dose volume histogram of target volumes and organs-at-risk for intact SBRT plan
Use of a simultaneous integrated boost technique can be considered, particularly when using single-fraction dose prescriptions. Certain institutions deliver a lower integral dose to the CTV and boost the GTV with the intention of maintaining local control while minimizing rates of VCF [36]. Dosing can vary depending on technique, ranging from 8 to 18 Gy in 1 fraction to the low-dose volume and 18–24 Gy in 1 fraction to the high-dose volume [36,37,38].
For postoperative cases, the CTV is based on the treatment planning MRI. However, the preoperative MRI extent of disease should be accounted for, as it was shown by Chan et al., that the patterns of recurrence are determined by the preoperative extent of disease as opposed to the post-operative residual [39]. As the majority of patients are operated on due to MESCC, this typically leads to either a donut or horseshoe CTV shape (Fig. 2). Redmond et al. have reported on post-operative contouring guidelines and the International Stereotactic Radiosurgery Society have provided recommendations on the technical and clinical considerations specific to post-operative SBRT [33, 40]. Important considerations include the surgical hardware as it causes artifact that can compromise visualization of the CNT and disease extent. In our experience, an optimized T2 weighted axial image can overcome the limitations from the T1 sequence. Similarly, a CT myelogram is rarely required. From the surgical perspective, avoiding cross links at the levels requiring treatment, limiting screws in the index segments and implementation of carbon fiber implants can significantly reduce artifacts.
a Axial CT isodose distribution for a post-operative T10 spine SBRT plan (28 Gy in 2 fractions) showing CTV (blue color-wash), PTV (orange color-wash), spinal cord PRV (green-yellow color-wash), liver (green line), bowel (brown line), esophagus (dark red line) and stomach (pink line). b Sagittal CT isodose distribution showing CTV (blue color-wash), PTV (orange color-wash), spinal cord (yellow line) and spinal cord PRV (green-yellow color-wash). c Dose volume histogram of target volumes and organs-at-risk for post-operative SBRT plan
The PTV is typically a margin of 2 mm but this will vary depending on immobilization used, technology available and expertise with the approach. If there is overlap with OAR, the PTV is not modified and coverage will be dictated by dose limits to OAR. For treatment planning purposes, the OAR may be subtracted from the PTV to create an OPT_PTV which can be used for optimization. Li et al. reported on the utility of near-rigid body immobilization in their analysis of cone-beam computed tomography (CBCT) setup variability for 102 spinal metastatic lesions [41]. They recommended that a 3-mm margin for planning target volume (PTV) and OAR be used when using a thermoplastic mask for immobilization, and a 2-mm margin for semi-rigid vacuum body fixation, though this was without a 6 degrees-of-freedom couch. A technical evaluation of an institutional approach has been reported using the HexaPOD couch (Elekta AB, Stockholm, Sweden) [42]. A total of 42 patients were treated with spine SBRT and each underwent 4 total CBCTs. After the initial correct CBCT setup, 90% and 97% of shifts were observed within 1 mm and 1 degree, respectively, and based on a 1-mm and 1 degree correction threshold, the target was localized within 1.2 mm and 0.9 degree with 95% confidence. From these data, a 2 mm PTV was established as the institutional standard and has shown to be safe in SBRT with up to 3 contiguous vertebral segments [43]. When treating > 3 vertebral bodies, a 3 mm PTV margin should be used.
Ultimately, optimizing local control while maintaining dose limits to OAR will be achieved by a rapid dose fall-off between target disease and the CNT. Given the close proximity of the high-dose gradient, precise contouring of surrounding structures is critical to ensure safe treatment delivery and it is recommended that OAR delineation be based off of guidelines and protocols for contouring [44, 45]. The spinal cord and/or thecal sac should be contoured based on the T1 and/or T2 axial MRI image data sets fused to the planning CT [46]. Nerve roots will be contoured at the level of the brachial plexus and lumbosacral plexus. There is yet to be consensus on nerve root tolerance but generally dose is limited to < 105% of the prescription dose. If there is direct encroachment of the nerve roots by tumor, target coverage is not compromised to spare the nerves though hotspots on the nerve roots should be avoided.
A planning organ-at-risk volume (PRV) is expanded from the spinal cord contour and the dose limit is applied to this volume. Based on cord imaging analyses, a 1.5–2 mm margin is required as a cord PRV to account for motion during spine SBRT [47, 48]. For spinal segments below the level of the spinal cord, or at the transition of the cord to cauda equina, the thecal sac is contoured without a PRV.
Treatment planning and delivery
For de novo irradiation, a maximum point dose (Dmax) constraint to the spinal cord PRV and/or thecal sac should range between 17 and 19.3 Gy in 2 fractions [49, 50]. In cases of reirradiation, the Dmax to the spinal cord PRV and/or thecal sac is 12.2 Gy, which may be increased to 14.6 Gy in cases with epidural disease [50,51,52]. Dose delivered to the spinal cord PRV and/or thecal sac is optimized to the Dmax constraint (i.e., 17 Gy in 2 fractions), with the secondary objective of maximizing isotoxic dose to the PTV. The SC.24 protocol required a Dmax of 17 Gy with an allowed deviation of − 5% and 0% over 17 Gy [11]. Of note, the use of Dmax as a planning metric is system-dependent and is affected by which treatment planning system, dose calculation engine and dose grid size used. For our center and treatment planning system, Dmax has been consistently applied for all SBRT treatments.
Step-and-shoot intensity-modulated radiation therapy (IMRT) with > 6 fields or volumetric modulated arc therapy (VMAT) are used in the planning of spine SBRT [53]. See Figs. 1 and 2 for appropriate dose distributions and dose volume histograms for intact and post-operative cases. The SC.24 trial mandated that coverage of CTV should be at least 80% of the prescribed dose in order to maximize isotoxic coverage of the PTV [11]. However, in practice, this may not always be achievable, especially in cases with smaller spinal segments in close proximity to OAR such as in the cervical spine.
Kilovoltage CBCT is used for image guidance prior to treatment delivery which is overlaid with the planning CT to verify patient position through matching of bony anatomy and contours. A robotic couch top with 6 degrees-of-freedom is used to allow for correction of translational and rotational errors [42]. If treatment time is longer than 20 min as defined by the institutional protocol, or if there is suspected patient movement, intrafractional verification imaging may be done [42].
Data
De novo radiation
Recently, the CCTG published completed results of the SC.24 clinical trial [54]. This was a multicenter, randomized, controlled, phase 2/3 trial including patients with spinal metastases from a solid primary tumor with a pain score of ≥ 2 on the brief pain inventory and SINS of ≤ 12. Patients had no more than three consecutive spinal segments in the treatment field and no prior radiotherapy or surgery to target segments. Participants were randomized to cEBRT which consisted of 20 Gy delivered in 5 fractions or SBRT which was 24 Gy in 2 daily fractions. At 3 months, 35% of the SBRT arm had CPR, compared to 14% in the cEBRT cohort (p = 0.0002), and significance was maintained in multivariable-adjusted analysis (p = 0.0003). At 6 months, there were significantly more patients who achieved CPR with SBRT compared to the cEBRT arm (p = 0.0036). There was no difference in radiation site-specific progression-free survival between the SBRT and cEBRT arms at 3 months (p = 0.18) and 6 months (p = 0.34). There were two observed cases of progression to MESCC in the cEBRT arm and none in the SBRT arm.
Following completion of SC.24, Zeng et al. examined a subset of patients enrolled in the CCTG study and reported mature outcomes with continued routine clinical and radiographic surveillance past the designated trial follow-up period [55]. With 119 spinal segments (66 patients) in the SBRT cohort and 169 spinal segments (71 patients) in the cEBRT arm, 12- and 24-month local failure (LF) rates were 6.1% and 14.8% in the SBRT cohort, compared to 28.4% and 35.6% in the cEBRT cohort (p < 0.001). One-year reirradiation rates following SBRT and cEBRT were 2.2% and 15.8% (p = 0.002), respectively, and there was no significant difference in the rate of VCF between cohorts.
Data in the de novo setting are summarized in Table 1. One-year local control rates ranged from 83% (with renal cell carcinoma metastases) up to 93.9%. Rates of VCF were from 5.4 to 22% at 1 year. In the largest single-institution series of spine SBRT using 24 Gy in 2 fractions, the most common pattern of failure was progression within the epidural space or paraspinal tissues, and the presence of epidural disease predicted for LF [56].
Reirradiation
In regards to reirradiation, Detsky et al. examined 43 patients with 83 spinal segments treated with salvage SBRT [57]. Local failure at 6-, 12- and 24-months were 7%, 14% and 19%, respectively and overall survival (OS) at 1- and 2-years were 53% and 36%, respectively. Similarly, Thibault et al. reviewed 56 spinal metastases in 40 patients who were treated with salvage SBRT [58]. Overall, 23% of spinal metastases had local failure with a cumulative incidence of local failure of 19.4% at 1 year. In both studies, paraspinal disease predicted for local failure.
Hashmi et al. conducted a multi-institutional pooled analysis of 215 patients undergoing salvage spine SBRT following cEBRT, with 40% of patients receiving multi-fraction treatment [52]. With a median reirradiation interval of 13.5 months and median follow-up of 8.1 months, 6- and 12-month local control rates were 93% and 93%, respectively. VCF rate of 4.5% and there were no cases of RM.
The Japanese outcomes of spine SBRT reirradiation using 24 Gy in 2 fractions have also been published [15, 16, 18]. At 1 year, local control ranged from 72 to 74% and 1-year OS was 61–65%. Rates of CPR varied between 64 and 86% and VCF at 1 year was 8–14%. The incidence of RM was 3%.
Post-op SBRT
Data for post-operative spine SBRT is relatively limited. One of the first reports was published by Al-Omair et al. where authors analyzed 80 patients treated with post-operative SBRT to a median dose of 24 Gy in 2 fractions [59]. At 1 year, the local control rate was 84% and OS was 64%. Likewise, Alghamdi et al. reported on 47 patients who underwent postoperative SBRT to 83 spinal metastases and 1-year local control was 83% with an OS of 55% [17]. Both studies found that a lower grade of post-operative epidural disease predicted for local control. Finally, Ito et al. reviewed a Japanese cohort of 28 spinal lesions treated with postoperative SBRT in the reirradiation setting using 24 Gy in 2 fractions [60]. At 1 year, local control was 70% and OS was 63%.
Toxicities
Pain flare
A transient increase in pain shortly after spine SBRT is referred to as a pain flare. In published literature, rates of pain flare range from 14 to 68% [61,62,63]. Differences in reported rates may be due to how pain flare was defined and recorded (i.e., pain diary vs. patient reported outcomes). In the CCTG SC.24 trial, incidence of pain flare was found to be 43% in the SBRT arm compared to 34% in the cEBRT arm, which was not significantly different [54]. Thus, SBRT is not perceived to increase the risk of pain flare over cEBRT. In terms of risk factors, two studies showed that pain flare was more common in patients with a higher performance status and postulated that these individuals were taking less baseline analgesics, leading to greater perceived pain [61, 63]. In regards to corticosteroid prophylaxis, Khan et al. reported pain flare in 19% of patients treated with dexamethasone, with no difference between 4 and 8 mg dosing [64]. Potential benefits of steroid supplementation should be balanced with toxicities of its use and risk of post-dexamethasone pain flare.
Vertebral compression fracture
VCF is a complication in patients with spine metastases that has significant potential of morbidity. Following radiotherapy there is appreciable risk of VCF, particularly with single-fraction spine SBRT. Memorial Sloan-Kettering Cancer Center reviewed 62 patients with 71 spinal segments and showed a 39% rate of VCF following single-fraction SBRT to a dose of 18–24 Gy [13].
Two-fraction dosing appears to be associated with a lower risk of VCF. With 24 Gy in 2 fractions, Tseng et al. showed a cumulative risk of VCF of 8.5% and 13.8% at 1- and 2-years post SBRT in 145 patients with 279 metastases [56]. Lytic tumors and spinal malalignment were found to be predictive of VCF. In the SC.24 trial, VCF rate was 11% in the SBRT arm compared to 17% in the cEBRT arm. The majority were grade 1 events with only one grade 3 VCF in SBRT arm. Furthermore, Zeng et al. analyzed long term outcomes and found that in 79 patients surviving 3 years or more post-SBRT, VCF rates were 10.4% and 14.4% at 3- and 5-years, respectively.
The largest and most comprehensive systematic review was conducted by Faruqi et al. [65]. This included 11 studies with a total of 2911 spinal segments and reported a crude VCF rate of 13.9%. Lytic disease, baseline VCF, spinal deformity, older age and > 40–50% vertebral body involved with tumor were predictive of VCF. Intervention was required in 37% of VCF events, most commonly cement augmentation.
Plexopathy and radiculopathy
As long term data matures regarding the delayed adverse events of spine SBRT, there is increasing awareness of radiation-induced plexopathy and radiculopathy as the associated symptoms can be debilitating [66, 67]. Recently, the Hypofractionation Treatment Effects in the Clinic (HyTEC) report summarized the risk of radiation-induced brachial plexopathy following SBRT [68]. Based on published data, an inferior brachial plexus Dmax of 32 Gy in 5 fractions and 25 Gy in 3 fractions were associated with a 10% risk of brachial plexopathy. Similarly, Lindberg et al. reported a rate of 13% of brachial plexopathy following apical lung SBRT, with a median biological effective dose (BED) of 381 Gy (α/β ratio of 3) in those with plexopathy and authors suggested keeping the Dmax of the plexus ≤ 130 Gy BED3 [69]. In terms of spine data, Ito et al. reported 2 cases of upper extremity radiculopathy following spine reirradiation with SBRT [16]. One patient had a slight decline in grip strength and numbness, while the other experienced muscle weakness in the triceps.
Data on lumbosacral plexopathy is limited but is thought to be more common than brachial plexopathy following spine SBRT. In patients living 3 years or more after spine SBRT, Zeng et al. reported 6 total cases of plexopathy (2.2%), 5 in the lumbosacral region [20]. These occurred at a median of 35.7 months (range 10.9–41.9 months) post-SBRT, and most commonly in patients who had multiple courses of radiotherapy. Compared to the brachial plexus, the lumbosacral plexus descends more vertically as it exits the spinal column which can result in a higher volume of nerve tissue being irradiated. The combination of serial and parallel components of toxicity may contribute to the higher risk of plexopathy in this region, and thus, contouring and appropriate dose limitation should be employed to the lumbosacral plexus and nerve roots.
Radiation myelopathy
RM is a devastating late toxicity of radiotherapy that is exceptionally rare with conventional fractionation schemes. With the advent of spine SBRT, there were concerns of this complication re-emerging, particularly with the inhomogeneity of dose adjacent to the spinal cord and uncertainties in regards to cord response to extreme hypofractionation. However, with proper techniques and quality assurance, the risk of RM is very low and modern studies have shown this to be a non-issue with appropriate patient selection.
In the Japanese study, there were four reported cases of RM following reirradiation to the spine, all experiencing complete paraplegia [16]. Two patients underwent intraoperative radiotherapy and one had carbon-ion therapy which are techniques which may ultimately deliver more dose to the spinal cord than calculated.
A dosimetric analysis was conducted on 9 patients who developed RM following spine SBRT, and these data were compared to patients who did not have RM [49]. From the dose-volume histogram (DVH) analysis and logistic regression model, a thecal sac Dmax of 17 Gy was shown to be associated with < 5% probability of developing RM with 2-fraction SBRT. This was the dose constraint on which the SC.24 trial was based in which there were no cases of RM, but was limited to a follow-up of 6 months post treatment. In the mature outcome analyses of the institutional cohort randomized on SC.24, there were no cases of RM with a median follow-up of 11.3 months [55].
Recently, a HyTEC report provided recommendations for spinal cord and thecal sac constraints based on the past decade of spine SBRT experience [50]. This analysis showed that the risk of RM with 2-fraction SBRT was 1–5% when spinal cord and thecal sac Dmax were limited to 17–19.3 Gy in de novo disease [50]. The same dose constraints are suggested to be used in the postoperative setting.
SBRT in the reirradiation setting has also been shown to be safe when following recommended constraints. Sahgal et al. reviewed 5 patients who developed RM following SBRT reirradiation and determined a retreatment thecal sac point maximum normalized biologically equivalent dose (nBED) of 20–25 Gy2/2 with the retreatment nBED comprising no more than 50% of the total nBED [51]. The total point maximum nBED for all treatment courses should not exceed 70 Gy2/2. Following conventional palliative radiotherapy, a minimum of 5 months should have elapsed prior to reirradiation SBRT.
Alternative SBRT fractionation schedules
Ultimately, there is no consensus on the optimal dose-fractionation schedule for spine SBRT. Single-fraction regimens using doses of 16–24 Gy continue to be used in many centers, as well as 24–27 Gy in 3 fractions and 30–35 Gy in 4–5 fractions [70,71,72,73]. At this time, there is no high-level evidence that supports one fractionation schedule over another. Some data suggests that single-fraction SBRT results in a higher rate of local control though this may come with a higher rate of VCF [56, 74, 75].
Dose escalation is being actively investigated within the realm of 2-fraction prescriptions in order to maximize local control and clinical outcomes. Recently, a report on a large institutional database of patients treated with spine SBRT was published, comparing 301 spinal segments receiving 28 Gy in 2 fractions to 646 spinal segments treated with 24 Gy in 2 fractions [76]. In total, 11.6% of segments had local progression in the 28 Gy cohort, compared to 21.7% in the 24 Gy cohort. In the 28 Gy cohort, the cumulative incidence of local failure at 6-, 12- and 24-months were 3.5%, 5.4% and 11.1%, respectively, while local failure rates in the 24 Gy cohort were 6.0%, 12.5% and 17.6%, respectively (p = 0.008). There was no significant difference in VCF or plexopathy rates between the two comparator groups. This suggests that spine metastases may be safely dose escalated to 28 Gy in 2 fractions resulting in improvements in local control without an increase in VCF rates, and these data will help to inform a prospective dose escalation randomized trial.
Conclusion
Spine SBRT is an effective treatment modality to maximize oncologic outcomes in patients with metastatic cancer. With appropriate patient selection and modern imaging/immobilization techniques, it can be safely delivered with low rates of clinically significant toxicity. The dose-fractionation of 24 Gy in 2 fractions is well-supported by published literature and is an ideal starting point for centers looking to establish a spine SBRT program. Prospective data on alternative fractionation schedules and dose escalation will help to further evolve this approach.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on request.
References
Jacobs WB, Perrin RG (2001) Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus FOC 11:1–11. https://doi.org/10.3171/foc.2001.11.6.11
Schiff D, O’Neill BP, Suman VJ (1997) Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology 49:452–456. https://doi.org/10.1212/WNL.49.2.452
Conti A, Acker G, Kluge A et al (2019) Decision making in patients with metastatic spine. The role of minimally invasive treatment modalities. Front Oncol 9:915
Klimo P, Thompson CJ, Kestle JRW, Schmidt MH (2005) A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 7:64–76. https://doi.org/10.1215/S1152851704000262
Lutz S, Balboni T, Jones J et al (2017) Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based Guideline. Pract Radiat Oncol 7:4–12. https://doi.org/10.1016/j.prro.2016.08.001
Chow E, Zeng L, Salvo N et al (2012) Update on the systematic review of Palliative Radiotherapy trials for bone metastases. Clin Oncol 24:112–124. https://doi.org/10.1016/j.clon.2011.11.004
Sprave T, Verma V, Förster R et al (2018) Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol 128:274–282. https://doi.org/10.1016/j.radonc.2018.04.030
Foro Arnalot P, Fontanals AV, Galcerán JC et al (2008) Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 gy in 10 fractions compared with 8 gy in single fraction. Radiother Oncol J Eur Soc Ther Radiol Oncol 89:150–155. https://doi.org/10.1016/j.radonc.2008.05.018
Husain ZA, Sahgal A, De Salles A et al (2017) Stereotactic body radiotherapy for de novo spinal metastases: systematic review International Stereotactic Radiosurgery Society practice guidelines. J Neurosurg Spine 27:295–302. https://doi.org/10.3171/2017.1.SPINE16684
Mizumoto M, Harada H, Asakura H et al (2011) Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys 79:208–213. https://doi.org/10.1016/j.ijrobp.2009.10.056
Sahgal A, Myrehaug S, Dennis K et al (2017) A randomized phase II/III study comparing stereotactic body radiotherapy (SBRT) versus conventional palliative radiotherapy (CRT) for patients with spinal metastases (NCT02512965). J Clin Oncol 35:TPS10129. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS10129
Sahgal A, Atenafu EG, Chao S et al (2013) Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 31:3426–3431. https://doi.org/10.1200/JCO.2013.50.1411
Rose PS, Laufer I, Boland PJ et al (2009) Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 27:5075–5079. https://doi.org/10.1200/JCO.2008.19.3508
Chang JH, Gandhidasan S, Finnigan R et al (2017) Stereotactic ablative body radiotherapy for the treatment of spinal oligometastases. Clin Oncol (R Coll Radiol) 29:e119–e125. https://doi.org/10.1016/j.clon.2017.02.004
Ito K, Ogawa H, Shimizuguchi T et al (2018) Stereotactic body radiotherapy for spinal metastases: clinical experience in 134 cases from a single Japanese Institution. Technol Cancer Res Treat 17:1533033818806472. https://doi.org/10.1177/1533033818806472
Ito K, Ogawa H, Nakajima Y (2021) Efficacy and toxicity of re-irradiation spine stereotactic body radiotherapy with respect to irradiation dose history. Jpn J Clin Oncol 51:264–270. https://doi.org/10.1093/jjco/hyaa178
Alghamdi M, Sahgal A, Soliman H et al (2019) Postoperative stereotactic body radiotherapy for spinal metastases and the impact of Epidural Disease Grade. Neurosurgery 85:E1111–E1118. https://doi.org/10.1093/neuros/nyz349
Ogawa H, Ito K, Shimizuguchi T et al (2018) Re-irradiation for painful bone metastases using stereotactic body radiotherapy. Acta Oncol 57:1700–1704. https://doi.org/10.1080/0284186X.2018.1503712
Kim H, Rajagopalan MS, Beriwal S et al (2015) Cost-effectiveness analysis of single fraction of stereotactic body radiation therapy compared with single fraction of external beam radiation therapy for palliation of vertebral bone metastases. Int J Radiat Oncol Biol Phys 91:556–563
Zeng KL, Sahgal A, Tseng C-L et al (2021) Prognostic factors Associated with surviving less than 3 months vs greater than 3 years specific to spine stereotactic body radiotherapy and late adverse events. Neurosurgery 88:971–979. https://doi.org/10.1093/neuros/nyaa583
Tseng CL, Eppinga W, Charest-Morin R et al (2017) Spine stereotactic body radiotherapy: indications, outcomes, and points of caution. Glob Spine J 7:179–197. https://doi.org/10.1177/2192568217694016
Jabbari S, Gerszten PC, Ruschin M et al (2016) Stereotactic body radiotherapy for spinal metastases: practice guidelines, outcomes, and risks. Cancer J 22:280–289. https://doi.org/10.1097/PPO.0000000000000205
Laufer I, Rubin DG, Lis E et al (2013) The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 18:744–751. https://doi.org/10.1634/theoncologist.2012-0293
Fisher CG, DiPaola CP, Ryken TC et al (2010) A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the spine oncology Study Group. Spine (Phila Pa 1976) 35:E1221–E1229. https://doi.org/10.1097/BRS.0b013e3181e16ae2
Wardak Z, Bland R, Ahn C et al (2019) A phase 2 clinical trial of SABR followed by Immediate Vertebroplasty for Spine Metastases. Int J Radiat Oncol Biol Phys 104:83–89. https://doi.org/10.1016/j.ijrobp.2019.01.072
Fisher C, Ali Z, Detsky J et al (2019) Photodynamic therapy for the treatment of vertebral metastases: a phase I clinical trial. Clin cancer Res 25:5766–5776. https://doi.org/10.1158/1078-0432.CCR-19-0673
Bilsky MH, Laufer I, Fourney DR et al (2010) Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 13:324–328. https://doi.org/10.3171/2010.3.SPINE09459
Redmond KJ, Lo SS, Fisher C, Sahgal A (2016) Postoperative stereotactic body Radiation Therapy (SBRT) for spine metastases: a critical review to Guide Practice. Int J Radiat Oncol Biol Phys 95:1414–1428. https://doi.org/10.1016/j.ijrobp.2016.03.027
Di Perna G, Cofano F, Mantovani C et al (2020) Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol 25:100320. https://doi.org/10.1016/j.jbo.2020.100320
Turel MK, Kerolus MG, O’Toole JE (2017) Minimally invasive “separation surgery” plus adjuvant stereotactic radiotherapy in the management of spinal epidural metastases. J Craniovertebral Junction Spine 8:119–126. https://doi.org/10.4103/jcvjs.JCVJS_13_17
Furuya T, Lee YK, Archibald-Heeren BR et al (2020) Evaluation of multi-institutional end-to-end testing for post-operative spine stereotactic body radiation therapy. Phys Imaging Radiat Oncol 16:61–68. https://doi.org/10.1016/j.phro.2020.09.005
Dunne EM, Sahgal A, Lo SS et al (2020) International consensus recommendations for target volume delineation specific to sacral metastases and spinal stereotactic body radiation therapy (SBRT). Radiother Oncol 145:21–29. https://doi.org/10.1016/j.radonc.2019.11.026
Redmond KJ, Robertson S, Lo SS et al (2017) Consensus Contouring Guidelines for postoperative stereotactic body Radiation Therapy for Metastatic Solid Tumor Malignancies to the spine. Int J Radiat Oncol Biol Phys 97:64–74. https://doi.org/10.1016/j.ijrobp.2016.09.014
Cox BW, Spratt DE, Lovelock M et al (2012) International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 83:e597–605. https://doi.org/10.1016/j.ijrobp.2012.03.009
Chen X, LeCompte MC, Gui C et al (2022) Deviation from consensus contouring guidelines predicts inferior local control after spine stereotactic body radiotherapy. Radiother Oncol 173:215–222. https://doi.org/10.1016/j.radonc.2022.05.035
Pontoriero A, Iatì G, Cacciola A et al (2020) Stereotactic body Radiation Therapy with Simultaneous Integrated Boost in patients with spinal metastases. Technol Cancer Res Treat 19:1533033820904447. https://doi.org/10.1177/1533033820904447
Lucido JJ, Mullikin TC, Abraha F et al (2022) Single and multifraction spine stereotactic body radiation therapy and the risk of radiation induced myelopathy. Adv Radiat Oncol 7:101047. https://doi.org/10.1016/j.adro.2022.101047
van der Velden JM, Hes J, Sahgal A et al (2018) The use of a simultaneous integrated boost in spinal stereotactic body radiotherapy to reduce the risk of vertebral compression fractures: a treatment planning study. Acta Oncol (Madr) 57:1271–1274. https://doi.org/10.1080/0284186X.2018.1468089
Chan MW, Thibault I, Atenafu EG et al (2016) Patterns of epidural progression following postoperative spine stereotactic body radiotherapy: implications for clinical target volume delineation. J Neurosurg Spine 24:652–659. https://doi.org/10.3171/2015.6.SPINE15294
Faruqi S, Chen H, Fariselli L et al (2022) Stereotactic radiosurgery for postoperative spine malignancy: a systematic review and International Stereotactic Radiosurgery Society Practice Guidelines. Pract Radiat Oncol 12:e65–e78. https://doi.org/10.1016/j.prro.2021.10.004
Li W, Sahgal A, Foote M et al (2012) Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys 84:520–526. https://doi.org/10.1016/j.ijrobp.2011.12.039
Hyde D, Lochray F, Korol R et al (2012) Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys 82:e555–e562. https://doi.org/10.1016/j.ijrobp.2011.06.1980
Chang JH, Sangha A, Hyde D et al (2017) Positional accuracy of treating multiple Versus single vertebral metastases with stereotactic body radiotherapy. Technol Cancer Res Treat 16:231–237. https://doi.org/10.1177/1533034616681674
Thibault I, Chang EL, Sheehan J et al (2015) Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol 16:e595–603. https://doi.org/10.1016/S1470-2045(15)00166-7
Mir R, Kelly SM, Xiao Y et al (2020) Organ at risk delineation for radiation therapy clinical trials: global harmonization group consensus guidelines. Radiother Oncol J Eur Soc Ther Radiol Oncol 150:30–39. https://doi.org/10.1016/j.radonc.2020.05.038
Dunne EM, Lo SS, Liu MC et al (2022) Thecal Sac Contouring as a surrogate for the Cauda Equina and Intracanal spinal nerve roots for spine stereotactic body Radiation Therapy (SBRT): Contour Variability and Recommendations for Safe Practice. Int J Radiat Oncol Biol Phys 112:114–120. https://doi.org/10.1016/j.ijrobp.2021.08.023
Tseng C-L, Sussman MS, Atenafu EG et al (2015) Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 91:995–1002. https://doi.org/10.1016/j.ijrobp.2014.12.037
Oztek MA, Mayr NA, Mossa-Basha M et al (2020) The dancing cord: inherent spinal cord motion and its effect on cord dose in spine stereotactic body radiation therapy. Neurosurgery 87:1157–1166. https://doi.org/10.1093/neuros/nyaa202
Sahgal A, Weinberg V, Ma L et al (2013) Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys 85:341–347. https://doi.org/10.1016/j.ijrobp.2012.05.007
Sahgal A, Chang JH, Ma L et al (2021) Spinal cord dose tolerance to stereotactic body Radiation Therapy. Int J Radiat Oncol Biol Phys 110:124–136. https://doi.org/10.1016/j.ijrobp.2019.09.038
Sahgal A, Ma L, Weinberg V et al (2012) Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 82:107–116. https://doi.org/10.1016/j.ijrobp.2010.08.021
Hashmi A, Guckenberger M, Kersh R et al (2016) Re-irradiation stereotactic body radiotherapy for spinal metastases: a multi-institutional outcome analysis. J Neurosurg Spine 25:646–653. https://doi.org/10.3171/2016.4.SPINE151523
Sangha A, Korol R, Sahgal A (2013) Stereotactic body radiotherapy for the treatment of spinal metastases: an overview of the University of Toronto, Sunnybrook Health Sciences Odette Cancer Centre, technique. J Med Imaging Radiat Sci 44:126–133. https://doi.org/10.1016/j.jmir.2013.04.002
Sahgal A, Myrehaug SD, Siva S et al (2021) Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol 22:1023–1033. https://doi.org/10.1016/S1470-2045(21)00196-0
Zeng KL, Myrehaug S, Soliman H et al (2022) Mature local control and reirradiation rates comparing spine stereotactic body radiotherapy to Conventional Palliative External Beam Radiotherapy. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.05.043
Tseng CL, Soliman H, Myrehaug S et al (2018) Imaging-based outcomes for 24 gy in 2 daily fractions for patients with de Novo spinal metastases treated with spine stereotactic body Radiation Therapy (SBRT). Int J Radiat Oncol Biol Phys 102:499–507. https://doi.org/10.1016/j.ijrobp.2018.06.047
Detsky JS, Nguyen TK, Lee Y et al (2020) Mature imaging-based outcomes supporting local control for Complex Reirradiation Salvage spine stereotactic body Radiotherapy. Neurosurgery 87:816–822. https://doi.org/10.1093/neuros/nyaa109
Thibault I, Campbell M, Tseng C-L et al (2015) Salvage stereotactic body Radiotherapy (SBRT) following In-Field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys 93:353–360. https://doi.org/10.1016/j.ijrobp.2015.03.029
Al-Omair A, Masucci L, Masson-Cote L et al (2013) Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol 15:1413–1419. https://doi.org/10.1093/neuonc/not101
Ito K, Nihei K, Shimizuguchi T et al (2018) Postoperative re-irradiation using stereotactic body radiotherapy for metastatic epidural spinal cord compression. J Neurosurg Spine 29:332–338. https://doi.org/10.3171/2018.1.SPINE171155
Balagamwala EH, Naik M, Reddy CA et al (2018) Pain flare after stereotactic radiosurgery for spine metastases. J Radiosurg SBRT 5:99–105
Pan HY, Allen PK, Wang XS et al (2014) Incidence and predictive factors of pain flare after spine stereotactic body radiation therapy: secondary analysis of phase 1/2 trials. Int J Radiat Oncol Biol Phys 90:870–876. https://doi.org/10.1016/j.ijrobp.2014.07.037
Chiang A, Zeng L, Zhang L et al (2013) Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys 86:638–642. https://doi.org/10.1016/j.ijrobp.2013.03.022
Khan L, Chiang A, Zhang L et al (2015) Prophylactic dexamethasone effectively reduces the incidence of pain flare following spine stereotactic body radiotherapy (SBRT): a prospective observational study. Support Care Cancer 23:2937–2943. https://doi.org/10.1007/s00520-015-2659-z
Faruqi S, Tseng CL, Whyne C et al (2018) Vertebral compression fracture after spine stereotactic body radiation therapy: a review of the pathophysiology and risk factors. Clin Neurosurg 83:314–322. https://doi.org/10.1093/neuros/nyx493
Tjong MC, Moraes FY, Yamada Y et al (2020) Radiation-induced lumbosacral plexopathy after spine stereotactic body radiotherapy 2013; should the lumbosacral plexi be contoured? Clin Oncol 32:884–886. https://doi.org/10.1016/j.clon.2020.10.001
Dahele M, Davey P, Reingold S, Shun Wong C (2006) Radiation-induced lumbo-sacral plexopathy (RILSP): an important enigma. Clin Oncol (R Coll Radiol) 18:427–428
Milano MT, Doucette C, Mavroidis P et al (2023) Hypofractionated stereotactic radiation therapy dosimetric tolerances for the inferior aspect of the Brachial Plexus: a systematic review. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.11.012
Lindberg K, Grozman V, Lindberg S et al (2019) Radiation-induced brachial plexus toxicity after SBRT of apically located lung lesions. Acta Oncol (Madr) 58:1178–1186. https://doi.org/10.1080/0284186X.2019.1601255
Gerszten PC, Chen S, Quader M et al (2012) Radiosurgery for benign tumors of the spine using the synergy S with cone-beam computed tomography image guidance. J Neurosurg 117 Suppl:197–202. https://doi.org/10.3171/2012.8.gks12981
Katsoulakis E, Jackson A, Cox B et al (2017) A detailed dosimetric analysis of spinal cord tolerance in high-dose spine radiosurgery. Int J Radiat Oncol 99:598–607. https://doi.org/10.1016/j.ijrobp.2017.05.053
Chang EL, Shiu AS, Mendel E et al (2007) Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 7:151–160. https://doi.org/10.3171/SPI-07/08/151
McClelland S III, Kim E, Passias PG et al (2017) Spinal stereotactic body radiotherapy in the United States: a decade-long nationwide analysis of patient demographics, practice patterns, and trends over time. J Clin Neurosci 46:109–112. https://doi.org/10.1016/j.jocn.2017.08.007
Folkert MR, Bilsky MH, Tom AK et al (2014) Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys 88:1085–1091. https://doi.org/10.1016/j.ijrobp.2013.12.042
Zelefsky MJ, Yamada Y, Greco C et al (2021) Phase 3 multi-center, prospective, randomized trial comparing single-dose 24 gy radiation therapy to a 3-fraction SBRT regimen in the treatment of oligometastatic cancer. Int J Radiat Oncol Biol Phys 110:672–679. https://doi.org/10.1016/j.ijrobp.2021.01.004
Zeng KL, Abugarib A, Soliman H et al (2022) Dose-escalated two-fraction spine stereotactic body radiotherapy: 28 gy vs. 24 gy in 2 daily fractions. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.09.076
Zeng KL, Myrehaug S, Soliman H et al (2019) Stereotactic body radiotherapy for spinal metastases at the extreme ends of the spine: imaging-based outcomes for cervical and sacral metastases. Neurosurgery 85:605–612. https://doi.org/10.1093/neuros/nyy393
Finnigan R, Burmeister B, Barry T et al (2015) Technique and early clinical outcomes for spinal and paraspinal tumours treated with stereotactic body radiotherapy. J Clin Neurosci Off J Neurosurg Soc Australas 22:1258–1263. https://doi.org/10.1016/j.jocn.2015.01.030
Thibault I, Al-Omair A, Masucci GL et al (2014) Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine 21:711–718. https://doi.org/10.3171/2014.7.SPINE13895
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search, article review, data abstraction and analysis were performed by EKN and CLT. The first draft of the manuscript was written by EKN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
MR owns intellectual property related to the image-guidance component on the Elekta Gamma Knife system. HS has travel and education grants from Elekta. SM has research support and honoraria from AAA/Novartis and Ipsen. AS is an advisor/consultant with Abbvie, Merck, Roche, Varian, Elekta, BrainLAB and VieCure, is a board member of the International Stereotactic Radiosurgery Society, has had past educational seminars with Elekta, Accuray Inc., Varian, BrainLAB, Medtronic Kyphon, received research grants with Elekta and Travel accommodations/expenses by Elekta, Varian, BrainLAB and belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. CLT is an advisor/consultant with Abbvie and Sanofi, has received travel accommodations/expenses & honoraria for past educational seminars by Elekta and belongs to the Elekta MR-Linac Research Consortium.
Ethical approval
This is a review article and no ethical approval is required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, E.K., Ruschin, M., Zhang, B. et al. Stereotactic body radiotherapy for spine metastases: a review of 24 Gy in 2 daily fractions. J Neurooncol 163, 15–27 (2023). https://doi.org/10.1007/s11060-023-04327-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04327-1