Abstract
This study investigated a single institution’s experience with volumetric modulated arc therapy (VMAT) directed stereotactic ablative body radiotherapy (SABR) for vertebral metastases. From 2010 to 2014, 95 lesions of spinal metastases in 73 patients were treated with SABR using VMAT. Clinical local control, pain level, and use of steroid medication were employed to evaluate treatment responses. The majority (79%) of patients were treated with a radiation dose of 20 Gy in a single fraction. However, when normal tissue constraints could not be achieved, the dose was reduced to 18 Gy (11%) or 16 Gy (8%) in 1 fraction. At the median follow up of 12.7 months (mean 18.0, range 1–56 months), clinical local control was 97% (92 out of 95). There was a mean 81% (median 100%, range 28–100%) decrease in subjective pain score. Seventy-seven percent of patients had a decrease in narcotic pain medication use. Pain was completely resolved at the treatment site for 69% (66/95) of patients. Prior to the SABR treatment, 33% (31/95) of patients had epidural extension of tumor. Among patients with epidural involvement, 45% (14/31) exhibited neurologic impairment prior to treatment. Twenty-three percent (7/31) experienced spinal cord compression. Prior to treatment, 34 patients experienced some form of neurologic impairment. Of these patients, 24% (8/34) experienced improved motor functioning; the remaining 76% (26/34) of patients’ neurological dysfunction were stable. Our results indicate the SABR regimen using VMAT technique is clinically effective in achieving clinical local control and palliation. This is the first publication reporting clinical outcomes of VMAT directed SABR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertebrae are among the most common sites of osseous metastatic disease [1]. Palliation of painful vertebral metastases or high risk epidural extension can be achieved with surgery, traditional fractionated external beam radiation therapy (EBRT), stereotactic ablative body radiation (SABR), or a combination of these modalities [2, 3]. Complete pain response rates with EBRT are reported as approximately 30%, and partial response is approximately 60% [4]. Single and multiple institutional experiences have demonstrated improved outcomes with SABR when compared with conventionally fractionated EBRT [5–7]. Increased accuracy of radiation delivery with higher dose rates have resulted in a potential for higher prescription dose delivery to the target. As a result, pain response rates of 80–90% have been reported in patients with spinal metastases who have undergone treatment with SABR [5–7]. Currently, the radiation therapy oncology group (RTOG) is assessing the clinical efficacy of SABR versus EBRT in a randomized prospective trial to determine whether there is a clinical benefit in the application for SABR application compared with EBRT. The protocol compares SABR delivered in a single fraction 16 or 18 Gy versus EBRT of 8 Gy in a single fraction [8].
There are multiple radiotherapy beam delivery methods available to treat spinal metastases using SABR. Earlier SABR efforts have employed static-field intensity modulated radiation therapy (IMRT) using conventional linear accelerator (linac) or a cone-based robotic radiosurgery system such as CyberKnife [9, 10]. These methods use multiple coplanar/non-coplanar static-field beam arrangements to deliver highly conformal radioablative doses to the tumor while sparing adjacent organs at risk (OAR), such as the spinal cord [11, 12].
Further technological advancements in radiation therapy have led to the advent of volumetrically modulated arc therapy (VMAT), which generally employs 1–3 rotational arc beams with intensity modulations and static/variable dose rates throughout the arc movements. There are numerous documented, advantageous clinical benefits to the use of VMAT over static field IMRT including higher attainable target radiation doses, greater conformity indices, spinal cord sparing, decreased treatment times, and reduced low-dose integral volume spread [13–18].
At the time of initiating a spine SABR program at our institution, VMAT was chosen as the sole means of radiation delivery. The purpose of our study is to report our experience with this technique in this large cohort of spine SABR patients.
Materials and methods
Patients
Our Institutional Review Board approved review of patients treated from May 2010 through February 2015 with histologically confirmed malignancy and limited vertebral column metastatic disease. Patients were not offered SABR if more than three contiguous vertebral bodies were involved, or they had paraspinal mass components greater than 5 cm in size. All patients had either baseline pain secondary to the metastases or epidural extension warranting intervention. All patients were evaluated by a multidisciplinary team, including neurosurgery, and underwent surgery or were deemed inoperable/poor operative candidates for medical or oncologic reasons.
Patient set-up
All patients underwent computerized tomography (CT)-based simulation using a radiation oncology CT simulator (Model: Optima CT580 RT, GE Healthcare,Waukesha, WI). Patients treated prior to 2014 underwent immobilization with thermoplastic mould (Model: Aquaplast RT™, Qfix, Avondale, PA), while patients treated after 2014 were immobilized with BodyFIX system (Elekta, Stockholm, Sweden). The CT images were acquired with 1 mm slice thickness and fused with magnetic resonance imaging (MRI) of T1-wieghted post-contrast and T2-weighted images with the voxel resolution of 1 mm3 through the target. All planning was completed using Eclipse treatment planning software (Varian Medical Systems, Palo Alto, CA, USA). When the cervical spine was treated, a 6-point restraint thermoplast mask was employed (Model: Aquaplast RT™, Qfix, Avondale, PA). ExacTrac infrared markers (BrainLAB AG, Feldkirchen, Germany) were utilized for automated isocenter localization.
Target delineation
The RTOG 0631 served as a guide for delineation of normal tissue and target contours. Patients underwent a stereotactic MRI, as well as CT simulation, for planning. The dose was prescribed to the planning target volume (PTV). If involved, 1–3 involved vertebral bodies were included in the target clinical target volume (CTV). If only the vertebral body was diseased, the anterior elements (vertebral body and pedicles) were contoured (Fig. 1a). The anterior and posterior elements of the entire vertebra was contoured if the pedicles were involved (Fig. 1b). If the spinous process or laminae appeared to be involved with tumor, the posterior elements were contoured (Fig. 1c). Postoperative patient CTV included the surgical fixation devices in the post-operative bed. No PTV expansion was added to the CTV volume. Adjacent OAR, including the spinal cord from 6 mm superior and inferior to the CTV, were also contoured. Other OAR were contoured according to the RTOG 0631 protocol [8]. Post-operative patients had fusion of the pre/post-operative MRI for target delineation. Post-operative targets included spinal stabilization devices within the involved region.
VMAT treatment planning
All VMAT treatment plans were generated by using RapidArc® technology in Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA) with 6 MeV photon stereotactic radiosurgery (SRS) mode. Dependent upon dosimetric constraints, one to three rotational arc beams with the arc angle of 360° were placed. Inverse planning algorithm (Progressive Resolution Optimizer, Varian Medical Systems, Palo Alto, CA) was employed to optimize the VMAT treatment plans. The PTV was prescribed 14–24 Gy in 1 fraction (median dose of 20 Gy). Based upon patient specific clinical characteristics, i.e., normal OAR constraints, radio-resistant tumor histology and tumor size, the treating physician determined the single fraction dose choice. The majority of patients were treated to a dose of 20 Gy in a single fraction. Maximum doses were restricted to below 140% of the prescription dose. The OAR dose constraints for single fraction VMAT SABR followed RTOG 0631 protocol [8]. Cord constraints for a single fraction were 0.35 cm3 and <10% of the volume of the cord <10 Gy. The cord point dose (0.035 cm3) also was restricted to <14 Gy.
Treatment
All treatment was administered using a Novalis Tx (BrainLAB AG, Feldkirchen, Germany) linac with high definition multi-leaf collimators measuring 2.5 mm in width. Multiple rotational arc beams of 6 MV photons in SRS mode with 1000 MU/min dose rate were delivered to the target. A 6-dimension robotic couch ExacTrac system was used to localize the isocenter of the treatment. Prior to each treatment session, a Winston-Lutz test [19] was performed to ensure that the radiation isocenter matched the mechanical isocenter of the linac system within less than 0.8 mm in 3 dimensions. After the initial ExacTrac localization, the CBCT imaging was operated to verify the accuracy of the Exactrac localization and determined the final shifts.
Monitoring
Patients were evaluated 2 weeks after radiation therapy and attended follow-up visits every 3–6 months to assess responses and side effects of radiation therapy. To avoid radiation sensitization, patients did not receive chemotherapy cycles 48 h before or after each treatment. Pain response was assessed using the Verbal Numerical Rating Score of subjective pain prior to and following treatment. Verbal Numerical Rating Scores are implemented at every clinical encounter per our institutional protocol, and thus were assessed at every follow-up appointment for the area of treatment. Reported pain scores were reported at the site of treatment for the last follow up encounter on file. Epidural extension of tumor was graded according to validated criteria based on T2 weighted MRI characteristics [20]. Spinal Instability Neoplastic Score (SINS) was calculated for patients. Parameters used to evaluate treatment response included patients’ use, dose, type, and frequency of steroid and narcotic pain medications. Staff radiation and/or medical oncologists assessed the neurological status on follow-up. Local control was determined clinically with confirmation radiographically after progression of symptoms. Routine post-treatment imaging was not completed. Repeat imaging was ordered if patients suffered from neurologic decline or an increase in subjective pain scores occurred at the site of treatment. Progression within the radiation field was confirmed based upon imaging without necessitating biopsy confirmation.
Statistical analysis
Descriptive statistics were reported for all demographic and baseline characteristics, as well as outcomes. Outcome response measures included pre- and post-treatment objective neurologic function, pain response rates, and local control. Pre- and post-treatment response rates were compared for evaluation of treatment effect. Continuous variables were reported as mean (standard deviation) or median (range), as appropriate. Categorical variables were reported as counts (percentages).
Results
Treatment/tumor characteristics
Ninety-five lesions were treated with SRS in a total of 73 patients with spinal metastases. The most utilized dosing regimen was 20 Gy in 1 fraction (79%), 18 Gy in 1 fraction (11%), and 16 Gy in 1 fraction (8%). A summary of SRS fractionation specifics is shown in Table 1. Non-small cell lung cancer (n = 30) was the most common primary malignancy treated in this study. A distribution of histology is shown in Table 2. Radiation was delivered with a median of two arcs (range 1–4). Median beam on time was 8.56 min (mean 8.51, range 4–12.5). Only the anterior elements were treated in 54% of patients, both the anterior and posterior elements were treated in 38% of patients, while only the posterior elements were treated in 8% of patients. Post-operative patients were treated to the tumor bed with hardware included in the region of the involved vertebral body based on the pre-operative MRI study.
Patient outcomes
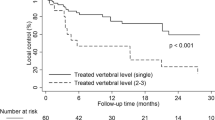
Tumor sites included 44, 36, and 15 in the lumbar, thoracic, and cervical spine, respectively. Clinical local control was 97% at a median follow-up of 12.7 months (mean 18.0, range 1–56 months). There were no geographical misses. Of the three patients with recurrences, two underwent salvage EBRT, and one patient underwent salvage laminectomy. None of the patients with failure had epidural extension of tumor. The histology of the three recurrences included breast, renal cell, and colon primaries treated with 16, 20, and 18 Gy respectively. Time to failure occurred at 11, 4, and 3 months post-treatment in patients with metastatic breast, renal cell and colon cancer respectively. All patients completed a pre- and post-Verbal Numerical Rating Score of subjective pain. There was a median 81% reduction in subjective pain scores. Pain was completely resolved at the treatment site for 69% (66/95) of patients, while 15% of patients’ pain scores remained stable. Findings also indicated that 77% of patients reduced narcotic pain medication use. Of patients who were taking steroids prior to SABR, 80% reported decreased total steroid dose after therapy.
Although it is not the primary goal of this study to examine patients with epidural extension, 33% (31/95) of patients exhibited epidural extension of tumor prior to treatment. Epidural extension grading with the epidural spinal cord compression scale Grade 1a, 1b, 1c, 2, and 3 in 10% (3/31), 45% (14/31), 26% (8/31), 16% (5/31), and 3% (1/31) patients, respectively. In patients with epidural extension, the median distance from PTV to the spinal cord was 1.0 mm (mean 1.14 mm, range 0–3.8 mm). Among these patients, 45% (14/31) exhibited neurologic impairment prior to treatment. Only 3 of these 31 patients were considered neuro-surgical candidates and underwent surgical decompression prior to SABR. The remaining patients were considered medically and/or oncologically non-surgical candidates. The median SINS for neurologically impaired patients was 8.0 (median 8.9, range 5–15). All patients with SINS greater than ten underwent surgical intervention. One patient with grade threecord compression and SINS of six underwent decompression. Of the patients with epidural extension and neurologic compromise, 36% (5/14) had neurologic function improvement. The remaining 64% (9/14) of patients had stable neurologic function after treatment. Of all patients treated, 34 patients exhibited neurologic impairment prior to treatment, 24% (8 patients) had improved motor functioning after treatment; the remaining 76% (26 patients) demonstrated no further progression of neurological deficits.
Patients with pain and more than 1/3 of the vertebral body replaced by tumor on imaging determined whether patients underwent percutaneous fixation prior to or after radiosurgery. Eighteen percent (17/95) of patients were treated with percutaneous fixation for vertebral fracture. Fifteen patients underwent vertebroplasty and two underwent kyphoplasty. The procedure was completed following therapy for 65% (11/17) of the patients (median time length after treatment: 1.53 months, range 0.13–9.7 months). The remaining 35% of patients (6/17) completed percutaneous fixation prior to SABR (median time prior to fixation: 1 month, range 0.7–2.6 months). Three of 78 patients (4%) who had not undergone percutaneous fixation suffered from vertebral collapse. Of the patients who experienced vertebral collapse/fracture one patient was prescribed 18 Gy to a mixed lytic/blastic NSCLC. The vertebral collapse occurred 4 months after treatment in this patient. Two other patients with metastatic prostate and NSCLC experienced fracture having each received a dose of 20 Gy. Fractures occurred at 4 and 2 months post treatment respectively. These doses were well within our standard maximum allowed doses of 140% of the prescription dose and did not deviate from our institutional protocol of prophylactic vertebroplasty for >1/3rd vertebral body involvement. We have now instituted routine use of the Spine Instability Neoplastic Score (SINS) score to recommend evaluation for percutaneous fixation for those patients with potentially unstable or unstable scores. No acute or late toxicities > grade 2 were reported in relation to the radiation therapy. The only grade 2 toxicity that did occur was skin desquamation in one patient.
Discussion
A multi-disciplinary approach is essential to effective management of spinal metastases. Specifically, close consultation with neurosurgery prior to and following radiation can optimize patient outcomes [21]. This study describes our experience for patients treated with VMAT technique in spinal SABR. The present study demonstrates that VMAT is a viable clinical approach for spine SABR in a single fraction.
Our results compare favorably with previously published studies utilizing static field IMRT for spine SABR (Table 3). Gerszten et al. published their experience of 500 patients with metastatic involvement of the spine treated with SABR utilizing static field IMRT in 2007 [5]. There was an 86% long term improvement in pain with 12.5–25 Gy per fraction and a 90% local control of the tumor [5]. There were no long term grade 3 or higher toxicities reported. Ryu et al. also utilized SABR with static field IMRT in 61 vertebral lesions [22]. That study demonstrated lower levels of pain control compared with our study with complete responses in 46% of patients and partial pain relief in 19% of patients [22]. This compares unfavorably with our study which has reported 97% clinical local control and 81% decrease in pain score. Notably, lower doses per fraction of 10–16 Gy may have led to the reduced pain control. Chang et al. also had phase I/II data in 63 patients who underwent fractionated spinal SABR. They reported local control of 84% at a follow up of 21 month without any late toxicity. There were two primary mechanism of failures in their series including recurrence in the bone directly adjacent to the site of treatment and recurrence in the epidural space. The authors recommended wider margins to incorporate adjacent osseous structures to decrease rates of local failure [30]. Yamada et al. published their experience with 103 patients using 18–24 Gy in a single fraction regimen utilizing static field IMRT. They reported a local control rate of 90% with 15 month follow up. There was a statistically significant improvement in local control for patients receiving greater than 23 Gy [24].
Traditionally, spinal metastases have been treated with fractionated EBRT regimens which offer pain relief in approximately 30–60% of patients [4]. Complete pain response rates for patients treated with EBRT are low and duration of pain response is often limited to <6 months [4, 17]. This study reveals a 69% complete resolution of pain at the treatment site for the date of last follow up utilizing the Verbal Numerical Rating Score. Pain and steroid medication use are more difficult to attribute to the SABR treatment. These medications were not assessed continually, and instead were tabulated from the last follow up encounter information to reflect the long-term pain control. Due to the nature of metastatic disease, many patients had other concomitant sites of pain, and the details regarding pain medication use specifically for each site of pain was not assessable in the chart. As a result, most patients did not experience complete independence from narcotic pain and steroid medications. However, treatment significantly decreased absolute quantities of both with a 77% reduction in narcotic medication use and 80% decrease in steroid intake. Reductions in these medications can greatly affect a patient’s quality of life. Reductions in opioid pain medication intake are associated with increases in overall health related quality of life measures and decreases in fatigue, sleep, and distress parameters [23].
When presenting with spinal cord compression, patients are often considered poor surgical candidates due to comorbidities, suboptimal performance status, or a large extent of disease. Unfortunately, this often results in a frustrating reality for both patients and radiation oncologists, as radiation may become the last option for palliation. Ablative therapy offers the best hope of maintaining adequate motor function and quality of life for these non-surgical patients [23]. Due to the close proximity of these tumors to the spinal cord, advances in radiation delivery systems are paramount [24, 25]. Interestingly, neurological decline was halted in the majority of patients and neurologic function was improved in nearly one-third of those with epidural extension and neurological compromise. As EBRT is associated with lower historical local control and palliation rates, SABR is a reasonable approach for patients who meet dosimetric tolerances. There was no evidence of increased clinical toxicity or compromise in tumor control based on this study of 95 tumors. This study has important clinical implications for patients with neurological impairment who are not surgical candidates. In cases of poor surgical candidacy or patient refusal of surgery, this study illustrates that VMAT directed SABR might deliver clinically effective doses in a reasonably safe manner to patients with favorable low-grade spinal cord compression. This hypothesis-generating result warrants further study.
There may be clinical benefits to the use of VMAT over other applied spine SABR technologies. Other technologies utilized for spine SABR include CyberKnife, Tomotherapy, static field IMRT, and different forms of VMAT such as VMAT with flattening filter free (FFF) mode [26–28]. Beam on time is of particular importance when choosing a technology for spine SABR. Patients with painful spinal metastases often experience difficulty with being immobilized over long periods. Increasing treatment delivery time is associated with greater intra-fraction patient movement which has the potential to be increased in patients with pain [13–15]. Greater intra-fractional movements reduce the accuracy of radiation dose delivery to the target and may increase the probability of spinal cord overdose. Beam on times for various technologies are presented in Table 4. Resulting in the shortest beam on time of approximately 8.5 min, VMAT beam on time is approximately 15% the beam on time of CyberKnife and 30% of the beam on time of Tomotherapy. With the utilization of a FFF mode, VMAT beam on time is further reduced by about 50% [27].
For radiation centers, considering Linac-based SABR spine treatment like this has important implications for machine throughput, as well as a potential clinical impact. Comparatively, static field IMRT consumes a significant amount of treatment time due to the large number of beams needed to achieve highly conformal dose distributions. Not including image guidance and patient set up, typical beam on time is approximately 15 min per treatment [13]. Whereas VMAT reduces the total treatment time by approximately 50% compared with static field IMRT [13–15]. In addition, dosimetric planning studies have demonstrated superior dosimetric parameters for VMAT versus static field IMRT. The findings include greater conformity indices and spinal cord sparing along with reduced low-dose integral volume spread [15–17].
There is a growing interest in the use of VMAT directed SABR for other sites of disease [28]. The DESTROY-2 trial is a phase I study that explored VMAT directed SABR to various oligometastatic sites with dose escalation. Dose escalation resulted in excellent local control of approximately 90% at 12 months, with no dose limiting toxicity greater than grade 2 (seen in only 2 patients out of 65). The maximum tolerated dose limit was not reached [29, 31, 32]. The trial is ongoing, but preliminary reports are promising and a phase II trial is planned.
In conclusion, our single institution experience offers valuable insight into the growing application of this new technology. There are inherent limitations in our study including its retrospective nature, lack of data concerning previous systemic therapy received, and the heterogeneous nature of malignant histology. A major limitation of the study is our lack of radiologic follow up. The reported 97% clinical local control rates are applicable to a patient population with vigorous clinical follow up. However, the rate of local control with both routine clinical examination and MRI is likely lower than reported in our study. Nonetheless, rates of clinical pain relief and maintenance of motor function compare favorably with previously published data. Our study provides proof of principle that VMAT based SABR is a feasible treatment approach for patients with spinal metastases, but further study is warranted.
References
Ammori MB, Panchani S, Gregory JJ, Wylie J, Paul A (2015) Survival rates following skeletal metastases—a twenty-year analysis. Open J Orthop 5:288–296
Expert Panel on Radiation Oncology-Bone Metastases, Lo SM, Ryu S, Chang EL, Galanopoulos N, Jones J, Kim EY, Kubicky CD, Lee CP, Rose PS, Sahgal A, Sloan AE, Teh BS, Traughber BJ, Van Poznak C, Vassil AD (2015) ACR appropriateness criteria® metastatic epidural spinal cord compression and recurrent spinal metastasis. J Palliat Med 18:573–584. doi:10.1089/jpm.2015.28999.sml
Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, von Guten C, Mendel E, Vassil A, Bruner DW, Hartsell W, American Society for Radiation Oncology (ASTO) (2011) Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 79:965–976. doi:10.1016/j.ijrobp.2010.11.026
Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD (2003) Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy–a systematic review of randomised trials. Clin Oncol (R Coll Radiol) 15:345–352
Gerszten PC, Burton SA, Ozhasoglu C, Welch WC (2007) Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 32:193–199
Yamada Y, Lovelock DM, Yenice KM, Bilsky MH, Hunt MA, Zatcky J, Leibel SA (2005) Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys 62:53–61
Bishop AJ, Tao R, Rebueno NC, Christensen EN, Allen PK, Wang XA, Amini B, Tannir NM, Tatsui CE, Rhines LD, Li J, Chang EL, Brown PD, Ghia AJ (2015) Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys 92:1016–1026. doi:10.1016/j.ijrobp.2015.03.037
Ryu S, Pugh SL, Gerszten PC, Yin FF, Timmerman RD, Hitchcock YJ, Movsas B, Kanner AA, Berk LB, Followill DS, Kachnic LA (2014) RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol 4:76–81. doi:10.1016/j.prro.2013.05.001
Kavanagh BD, Timmerman RD (2006) Stereotactic radiosurgery and stereotactic body radiation therapy: an overview of technical considerations and clinical applications. Hematol Oncol Clin North Am 20:87–95
Wang Z, Kong QT, Li J, Wu XH, Li B, Shen ZT, Zhu XX, Song Y (2015) Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis 7:407–412. doi:10.3978/j.issn.2072-1439.2015.01.09
Yin FF, Ryu S, Ajlouni M, Zhu J, Yan H, Guan H, Faber K, Rock J, Abdalhak M, Rogers L, Rosenblum M, Kim JH (2002) A technique of intensity-modulated radiosurgery (IMRS) for spinal tumors. Med Phys 29:2815–2822
Chin LS, Regine WF (2008) Principles and practice of stereotactic radiosurgery. Springer, New York
Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D, Yin FF (2009) Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys 75:1596–1604. doi:10.1016/j.ijrobp.2009.05.005
Amoush A, Dalton A, Rabatic B et al (2015) Volumetric modulated arc therapy for spine SBRT patients to reduce treatment time and intrafractional motion. Int J Cancer Ther Oncol 3:03026. doi:10.14319/ijcto.0302.6
Matuszak MM, Yan D, Grills I, Martinez A (2010) Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 77:608–616. doi:10.1016/j.ijrobp.2009.08.032
Chae SM, Lee GW, Son SH (2014) The effect of multileaf collimator leaf width on the radiosurgery planning for spine lesion treatment in terms of the modulated techniques and target complexity. Radiat Oncol 9:72. doi:10.1186/1748-717X-9-72
Zach L, Tsvang L, Alezra D, Ben Ayun M, Harel R (2016) Volumetric modulated arc therapy for spine radiosurgery: superior treatment planning and delivery compared to static beam intensity modulated radiotherapy. Biomed Res Int 2016:6805979. doi:10.1155/2016/6805979
Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A (2011) Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 84:967–996. doi:10.1259/bjr/22373346
Lutz W, Winston KR, Maleki N (1988) A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys 14:373–381
Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y, Bilsky MH (2013) The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 18:744–751. doi:10.1634/theoncologist.2012-0293
Claus EB (2012) Neurosurgical management of metastases in the central nervous system. Nat Rev Clin Oncol 9:79–86. doi:10.1038/nrclinonc.2011.179
Ryu S, Jin R, Jin JY, Chen Q, Rock J, Anderson J, Movsas B (2008) Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manag 35:292–298. doi:10.1016/j.jpainsymman.2007.04.020
Ahmed KA, Stauder MC, Miller RC, Bauer HJ, Rose PS, Olivier KR, Brown PD, Brinkmann DH, Laack NN (2012) Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys 82:e803–e809. doi:10.1016/j.ijrobp.2011.11.036
Oh SA, Kang MK, Kim SK, Yea JW (2013) Comparison of IMRT and VMAT techniques in spine stereotactic radiosurgery with international spine radiosurgery consortium consensus guidelines. Prog Med Phys 24:145–153. doi:10.14316/pmp.2013.24.3.145
Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I, Liu P, Allen PK, Azeem SS, Brown PD, Sharp HJ, Weksberg DC, Cleeland CS, Chang EL (2012) Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1–2 trial. Lancet Oncol 13:395–402. doi:10.1016/S1470-2045(11)70384-9
Ong CL, Verbakel WF, Dahele M, Cuijpers JP, Slotman BJ, Senan S (2012) Fast arc delivery for stereotactic body radiotherapy of vertebral and lung tumors. Int J Radiat Oncol Biol Phys 83:e137–e143. doi:10.1016/j.ijrobp.2011.12.014
Kuijper IT, Dahele M, Senan S, Verbakel WF (2010) Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: a planning study and early clinical data. Radiother Oncol 94:224–228. doi:10.1016/j.radonc.2009.12.027
Gallo JJ, Kaufman I, Powell R, Pandya S, Somnay A, Bossenberger T, Ramirez E, Reynolds R, Solberg T, Burmeister J (2015) Single-fraction spine SBRT end-to-end testing on TomoTherapy, Vero, TrueBeam, and CyberKnife treatment platforms using a novel anthropomorphic phantom. J Appl Clin Med Phys 16(1):5120
Yamada Y, Bilsky MH, Lovelock DM et al (2008) High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 71:484–490
Chang EL, Shiu AS, Mendel E et al (2007) Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 7:151–160
Sapkaroski D, Osborne C, Knight KA (2015) A review of stereotactic body radiotherapy—is volumetric modulated arc therapy the answer? J Med Radiat Sci 62:142–151. doi:10.1002/jmrs.108
Deodato F, Cilla S, Macchia G, Torre G, Caravatta L, Mariano G, Mignogna S, Ferro M, Mattiucci GC, Balducci M, Frascino V, Piermattei A, Ferrandina G, Valentini V, Morganti AG (2014) Stereotactic radiosurgery (SRS) with volumetric modulated arc therapy (VMAT): interim results of a multi-arm phase I trial (DESTROY-2). Clin Oncol (R Coll Radiol) 26:748–756. doi:10.1016/j.clon.2014.08.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript have no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Gestaut, M.M., Thawani, N., Kim, S. et al. Single fraction spine stereotactic ablative body radiotherapy with volumetric modulated arc therapy. J Neurooncol 133, 165–172 (2017). https://doi.org/10.1007/s11060-017-2428-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2428-6