Abstract
Background
The current standard of care for patients with a large brain metastasis and limited intracranial disease burden is surgical resection and post-operative single fraction stereotactic radiosurgery (SRS). However, post-operative SRS can still lead to substantial rates of local failure (LF), radiation necrosis (RN), and meningeal disease (MD). Pre-operative SRS may reduce the risk of RN and MD, while fractionated treatments may improve local control by allowing delivery of higher biological effective dose. We hypothesize that pre-operative fractionated stereotactic radiation therapy (FSRT) can minimize rates of LF, RN, and MD.
Methods
A retrospective, multi-institutional analysis was conducted and included patients who had pre-operative FSRT for a large or symptomatic brain metastasis. Pertinent demographic, clinical, radiation, surgical, and follow up data were collected for each patient. A primary measurement was the rate of a composite endpoint of (1) LF, (2) MD, and/or (3) Grade 2 or higher (symptomatic) RN.
Results
53 patients with 55 lesions were eligible for analysis. FSRT was prescribed to a dose of 24–25 Gy in 3–5 fractions. There were 0 LFs, 3 Grade 2–3 RN events, and 1 MD occurrence, which corresponded to an 8% per-patient composite endpoint event rate.
Conclusions
In this study, the composite endpoint of 8% for pre-operative FSRT was improved compared to previously reported rates with post-operative SRS of 49–60% (N107C, Mahajan etal. JCOG0504) and pre-operative SRS endpoints of 20.6% (PROPS-BM). Pre-operative FSRT appears to be safe, effective, and may decrease the incidence of adverse outcomes. Prospective validation is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are a frequent cause of morbidity and mortality for cancer patients. Historically, whole brain radiation therapy (WBRT) plus surgery was a standard of care treatment to improve overall survival (OS) and local control for patients with brain metastases [1]. One early study showed that compared to surgery alone, adjuvant WBRT reduced the risk of intracranial recurrence and neurological death, albeit without an improvement in OS [2]. However, multiple randomized studies have shown the poor neurological and cognitive effects of WBRT in this patient population [3, 4]. Prospective trials have demonstrated that post-operative stereotactic radiosurgery (SRS) is an acceptable alternative to WBRT that decreases the risk of cognitive dysfunction without negatively affecting overall survival (OS) [5, 6]. However, post-operative SRS is complicated by patterns of potential local failure (LF), radiation necrosis (RN), and meningeal disease (MD).
Pre-operative radiation therapy is theorized to improve outcomes compared to post-operative SRS for a variety of reasons. Radiating brain metastases prior to surgery exposes less brain tissue to high doses of radiation and may minimize the risk of RN. In addition, pre-operative SRS reduces the risk of tumor spillage and subsequent MD which is often seen when radiation was delivered after surgery. Also, up to 30% of patients do not receive their prescribed course of post-operative radiation therapy [6]. However, although the PROPS-BM cohort of patients treated with pre-operative SRS showed a minimized risk of RN and LMD, the one year incidence of LF rate was still 15% [7]. Fractionation may facilitate safer delivery of higher biological effective dose (BED) treatments which could improve the disappointing local control rates seen in N107C, Mahajan et al. and the PROPS-BM cohort [5, 7, 8]. However, outcomes for pre-operative fractionated stereotactic radiation therapy (FSRT) have not been previously reported. We hypothesize that pre-operative FSRT can maintain the improved rates of RN and MD seen in the PROPS-BM study while also minimizing the incidence of LF.
Methods
This retrospective study was approved by our institutional review board. Because this study was retrospective, IRB-approved, and did not involve an additional intervention, patient consent was not obtained. Data was pooled from two institutions for analysis. At both institutions, patients are eligible for pre-operative fractionated stereotactic radiation therapy (FSRT) if they have a new dominant brain metastasis and limited intracranial disease. Our centers now use this as the standard pre-operative option for all patients who do not have significant symptoms or are not responding to steroids. At both institutions, pre-operative FSRT was scheduled 1–2 weeks after radiation oncology and neurosurgery evaluation for a diagnosis of new or progressive brain metastases, with surgery being performed on the same day or soon after the last radiation treatment.
A systematic query was used to identify patients who had surgical resection with a pre- or post-operative treatment for a brain metastasis. Patients with single fraction radiosurgery or post-operative treatment were excluded. Pertinent demographic, clinical, radiation, surgical, and follow up data were collected for each patient.
Patients were defined as having uncontrolled extracranial disease if they had progressive extracranial disease or were treatment naïve at the time of their brain metastasis diagnosis. A patient was defined as having absent extracranial metastases if there was no extracranial disease outside of the primary tumor and regional lymph nodes at the time of their brain metastasis diagnosis. Karnofsky performance status (KPS) was documented at the time of radiation oncology consultation. Patients with more than one lesion removed at the time of surgery were still eligible for analysis. Radionecrosis (RN) was defined as any radiographic post-treatment change felt by a multidisciplinary team (radiation oncology, neurosurgery, neuro-oncology, neuro-radiology) to be consistent with treatment effect rather than disease progression. RN was graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (Grade 1: asymptomatic; Grade 2: moderate symptoms, corticosteroids indicated; Grade 3: severe symptoms, medical intervention indicated; Grade 4: life-threatening; urgent intervention; and Grade 5: death). A per-patient composite endpoint was measured; this composite endpoint was defined as patients with either: (1) LF, (2) MD, or (3) Grade 2 or higher (symptomatic) RN. This endpoint is a modified version of the composite endpoint published by PROPS-BM [7]. This endpoint was collected because of the low event rate of each individual adverse outcome. A patient was positive for this composite endpoint if they met any of these three criteria.
The treatment planning for this patient population was uniform. Radiation therapy was planned and delivered uniformly using intensity modulated radiation therapy (IMRT) based planning and delivered with daily image guidance using cone beam CT. T1 post contrast MRI imaging was used in all patients and was fused to each radiation planning CT scan. Gross tumor volume (GTV) was defined as the contrast enhanced tumor and adjacent abutting meninges. A clinical treatment volume (CTV) of 2 mm was used and an optional 1 mm planning treatment volume (PTV) was used for all lesions. Patients were followed every 2–3 months for the first year, 3–4 months for the second year, and every 6 months after 2 years.
Statistics
Descriptive statistics summarized baseline demographic, clinical, and treatment variables. The median and interquartile range (IQR) were used for continuous variables and the frequency and percent for categorical variables. Differences between groups were summarized using Wilcoxon’s rank sum test or Fisher’s exact test. Overall survival was defined as the date of onset of treatment to the date of death and censored at the date of last follow up for those still alive. The Kaplan–Meier estimate of survival and it’s 95% confidence interval (CI) are presented. The cumulative incidence of the composite endpoint was estimated treating death as a competing risk. Univariable Cox regression models were constructed to estimate the hazard ratios (HR) for baseline variables. Complete case analysis was used for all summaries and reported p-values and CIs are unadjusted for multiplicity. Statistical analyses were performed with R version 4.1.2 using the survival (version 3.2-13) and cmprsk (version 2.2-11) packages.
Results
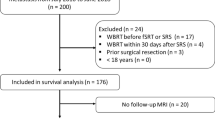
Between the dates of 1/1/2016 and 12/31/2020, 273 patients were identified with surgical resection for brain metastases, and 53 patients with 55 brain metastases of these met our criteria for pre-operative treatment and were included in the final analysis. For both patients with two resected lesions, each patient had both lesions resected during the same surgery. The median follow-up time through last follow-up or time of death was 9 months (IQR: 4, 16). During the follow up period, 18 deaths (34%) were observed. 55% of patients were male (Table 1). The majority of patients (51%) had metastatic lung cancer, with genitourinary (17%) and breast (11%) forming a smaller proportion of patients. Fewer than half of patients (43%) had extracranial disease control at the time of their pre-operative radiation treatment, and 55% of patients had extracranial metastases present during treatment. 69.8% of patients had a KPS of > 70. The median number of treated brain metastases was 2 (IQR 1,3; range 1–11).
Two patients (3.8%) had multiple lesions resected (Table 1). Nearly all patients (98.1%) had a gross total resection. In this study, the two most common locations of resected lesions were the frontal lobe (36%) and cerebellum (23%). All patients in this study were treated with a linear accelerator. Almost half (47%) of patients had single isocenter multi-target (SIMT) radiosurgery, and 15% had five or more lesions treated during the radiation treatment course. The median GTV volume was 12 ccs (IQR: 7, 19), and the median PTV volume was 19 ccs (IQR: 12, 28). The median radiation dose was 24 Gy (range of 24–25 Gy). All patients either received 24 Gy in 3 fractions or 25 Gy in 5 fractions. 92% of patients were prescribed three fractions with the remainder receiving five fraction treatment. The median time from last radiation fraction to surgery was 2 days (IQR: 1, 4.5). Only 3 patients had their surgery more than 1 week after the last radiation treatment. The 12 month survival probability was 70% (95% CI 0.58, 0.84) (Fig. 1). Patients with extracranial metastases present at time of their radiation course had a worse overall survival (HR: 3.65; 95% CI 1.28, 10.4, p = 0.01).
Three patients (6%) experienced serious post-surgical complications. One patient had an acute right subdural hemorrhage after surgery and was brought back to the operating room for evacuation. A second patient experienced a Methicillin-resistant Staphylococcus Aureus (MRSA) infection post-operatively. Another patient had a clot in the resection cavity 5 days after surgery and developed hydrocephalus. The patient was then taken to the operating room for clot removal.
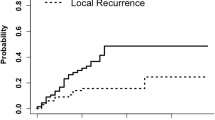
None of the eligible patients experienced local progression (Table 2). 12% of patients experienced radiation necrosis of any grade. Of those, 1 patient experienced Grade 2 radiation necrosis for which steroids were started. Two patients experienced Grade 3 radiation necrosis, with 1 patient started on bevacizumab, and the other having surgical resection of the necrotic area. Only 1 patient experienced leptomeningeal disease, which was categorized as nodular MD. Overall, 8% of eligible patients were positive for the composite endpoint. The cumulative incidence for experiencing the composite endpoint with death as a competing event is illustrated in Fig. 2. At 12 months, the cumulative probability of the composite endpoint was 7% (95% CI 0.02, 0.18).
Discussion
This study represents the largest report of outcomes for patients treated with pre-operative FSRT for brain metastases. These data support that pre-operative fractionated treatment is safe and effective. There were no incidences of LF in this cohort. This treatment was well tolerated, with only 3 patients (6%) experiencing symptomatic RN and 1 patient (2%) experiencing MD. Although post-operative SRS or FSRT is the current standard-of-care, these data suggest that pre-operative FSRT should be evaluated prospectively.
Radiation therapy is indicated after resection of brain metastases to decrease the risk of local recurrence [1, 2, 8]. Prospective trials have demonstrated that SRS is an acceptable alternative to WBRT, and that focal radiation decreases the risk of cognitive dysfunction without negatively affecting OS [5, 6]. Because postoperative SRS was comprehensively studied in a prospective setting [5, 6, 8], it is considered to be standard of care [9]. However, rates of radiation necrosis, leptomeningeal disease, and 1-year local failure rates of 0–3%, 7–28%, and approximately 25–50% respectively with postoperative SRS in modern, phase III trials are not optimal [5, 6, 8]. A smaller, phase II study of postoperative SRS had a lower 1-year LF of 15% but noted a much higher rate of pathologically-proven RN at 18% [10] that more accurately reflects the elevated rates of RN after postoperative SRS seen in large retrospective studies including meta-analyses [11]. Elevated LF rates are believed to be a consequence of the dose de-escalation required to safely administer single fraction SRS to large postoperative cavities without creating an unacceptably high risk of RN [11]. Consequently, interest in FSRT is increasing, as the radiobiologic advantage of smaller fractions may allow safe delivery of a higher biological effective dose (BED) and result in improved LC [11]. A recent meta-analysis found a trend towards improved 1-year LC (86.8% vs 68.0%, p = 0.08) with postoperative FSRT versus SRS without an increase in RN (7% vs 10%, p = 0.46) [11], and a multi-institution, randomized, phase III trial is accruing patients to definitively evaluate this potential benefit (NCT04114981).
Recently, preoperative SRS has been proposed as an alternative to postoperative SRS. Preoperative SRS may decrease the risk of RN by minimizing irradiation of normal brain tissues through the use of smaller target volumes [7, 12]. Postoperative cavities are typically larger than intact lesions, and pre-operative therapy eliminates the need to cover surgically manipulated tissues [7, 12]. Additionally, preoperative SRS may decrease the risk of MD by reducing the risk of surgical spillage through preoperative surgical field sterilization [7, 12]. However, prospective data evaluating the safety and efficacy of pre-operative SRS is limited. An unpublished, prospective, phase I, dose escalation trial of preoperative SRS in 27 patients presented at the American Society of Radiation Oncology (ASTRO) 2019 annual meeting reported a 28% rate of 1-year LF [13], which was comparable to LC with postoperative SRS. The 1-year rate of MD was just 4%, and RN rates were not reported. An unpublished, 24-patient, single arm, phase II trial presented at ASTRO 2021 reported a 1-year LF rate of just 10% with preoperative SRS [14]. RN and MD rates were not presented. A large, retrospective, multi-center cohort noted 6% and 15% rates of 1-year MD, and LF, respectively [7]. Rates of RN were not provided. Smaller studies, some with mixed prospective and retrospective cohorts, have reported symptomatic RN, MD, and 1 year LF rates of 0–5%, 0–17%, and 14–50% with pre-operative SRS [15,16,17,18]. A multi-institution, retrospective analysis compared outcomes for preoperative versus postoperative SRS and found significantly decreased rates of 2-year symptomatic RN (5% vs 16%, p = 0.02) and MD (3% vs 17%, p = 0.01) with no difference in 2-year LF (23% vs 16%, p = 0.33) [12]. These findings suggest that preoperative SRS may offer improved rates of RN and MD without compromising cancer control. To our knowledge, prospective or retrospective outcomes with preoperative FSRT have not been previously reported.
Pre-operative FSRT can safely deliver a higher BED to improve local control while also minimizing treatment volume and reducing tumor spillage to decrease the risk of RN or MD. Mahajan etal. in their study examining post-operative SRS versus observation, showed a 0% rate of RN, a 24% rate of LF, and a 28% rate of MD, showing a composite endpoint of over 50% in the SRS arm [8]. In N107C, the seminal study establishing post-operative SRS as a standard treatment, there was a 4% rate of Grade 2 or higher RN, a 38% rate of LF, and a 7% rate of MD in the SRS arm, leading to a composite endpoint of up to 49% in the SRS arm [5]. In JCOG0504, another study evaluating post-operative SRS, there was a 3% rate of RN, a 51% incidence of local failure, and a 7% rate of MD, leading to a composite endpoint of 61% in the SRS arm, although it was noted that over 30% of patients enrolled to the SRS arm did not complete radiation treatment [6]. In the PROPS-BM cohort examining single fraction pre-operative SRS, they published a similar composite endpoint to this current study which had an incidence rate of 20.6% at one year and 24.8% at two years [7]. Of note, the PROPS-BM had a lower proportion of gross total resections (93.7% vs. 98.1%) and a higher proportion of melanoma patients (12.8% vs. 7.5%) compared to our study, both which were associated with LR in their analyses. In our study, the composite endpoint of 8% for pre-operative FSRT was improved compared to post-operative SRS endpoints of 49–60% and pre-operative SRS endpoints of 20.6% in two studies.
To our knowledge, this is the largest study evaluating the efficacy of pre-operative FSRT. In this multi-institutional study, pre-operative FSRT treatment is safe and effective when compared to previously published prospective and retrospective data. Pre-operative treatment is more likely to be completed, especially with JCOG0504 showing that over 30% of patients do not complete post-operative radiation treatment. Our composite endpoint has been previously published in other studies involving pre- and post-operative SRS. One weakness of this study is a relatively small sample size of 53 patients. In addition, radiation necrosis can be challenging to diagnose in both prospective and retrospective studies. Another limitation of this study was that information about quality of life or neurocognition was not collected. Pre-operative radiation approach for a large or symptomatic brain metastasis may not be suited for patients with an uncertain diagnosis or for emergent situations. This study is also limited by the inherent selection biases of retrospective analyses. A large, randomized controlled trial is needed to validate these results.
Conclusion
This study presents the largest report of outcomes for patients treated with pre-operative FSRT for brain metastases. Safety and efficacy data compare favorably to historical outcomes with pre- and post-operative SRS. Thus, pre-operative FSRT may be an excellent option for appropriately selected patients with resectable brain metastases. Prospective validation of pre-operative FSRT is warranted.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Patchell R, Tibbs P, Regine W et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060
Kayama T, Sato S, Sakurada K et al (2018) Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol 36:3282–3289
Prabhu RS, Dhakal R, Vaslow ZK et al (2021) Preoperative Radiosurgery for resected brain metastases: the PROPS-BM Multicenter Cohort Study. Int J Radiat Oncol Biol Phys 111:764–772
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048
Redmond KJ, De Salles AAF, Fariselli L et al (2021) Stereotactic radiosurgery for postoperative metastatic surgical cavities: a critical review and International Stereotactic Radiosurgery Society (ISRS) Practice Guidelines. Int J Radiat Oncol Biol Phys 111:68–80
Brennan C, Yang TJ, Hilden P et al (2014) A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys 88:130–136
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103:618–630
Patel RK, Burri HS, Asher LA et al (2016) Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery 79:279–285
Murphy ES, Yang K, Suh J et al (2019) Early results from a prospective phase ii dose escalation study for neoadjuvant radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 103:E5–E5
Iheagwara UK, Siddiqui Z, Holeva K et al (2021) A phase II study to determine the efficacy of pre-operative stereotactic radiosurgery followed by resection for brain metastasis. Int J Radiat Oncol Biol Phys 111:S25
Asher AL, Burri SH, Wiggins WF et al (2014) A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys 88:899–906
Patel AR, Nedzi L, Lau S et al (2018) Neoadjuvant stereotactic radiosurgery before surgical resection of cerebral metastases. World Neurosurg 120:e480-487
Vetlova E, Golbin DA, Golanov AV et al (2017) Preoperative stereotactic radiosurgery of brain metastases: preliminary results. Cureus 9:e1987
Prabhu RS, Miller KR, Asher AL et al (2018) Preoperative stereotactic radiosurgery before planned resection of brain metastases: updated analysis of efficacy and toxicity of a novel treatment paradigm. J Neurosurg 131:1–8
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HKP, JDP, JM, CH, BK, and RP. BK: responsible for Statistical Analysis. The first draft of the manuscript was written by HKP, JDP, JM, CH, BK, and RP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest related to this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Since this study is retrospective, informed consent was not obtained in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palmer, J.D., Perlow, H.K., Matsui, J.K. et al. Fractionated pre-operative stereotactic radiotherapy for patients with brain metastases: a multi-institutional analysis. J Neurooncol 159, 389–395 (2022). https://doi.org/10.1007/s11060-022-04073-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04073-w