Abstract
Objective
The aim of this study was to evaluate the long-term outcomes of initial Gamma Knife radiosurgery (GKRS) for patients with nonfunctioning pituitary adenomas (NFPAs).
Design and methods
This was a single-center retrospective study. Eighty-one patients with NFPAs undergoing initial GKRS were enrolled. The median age was 44.9 years (range, 7.2–75.5 years). The median tumor volume was 2.3 cm3 (range, 0.1–31.3 cm3), and the median tumor margin dose was 13.0 Gy (range, 8–22 Gy).
Results
Tumor shrunk in 63 patients (77.8%), remained stable in 9 (11.1%), treatment failure in 9 (11.1%) during a median follow-up of 67.1 months (range, 11.5–263.9 months). The tumor control rates were 100%, 99%, 95%, and 84%, at 1, 3, 5, and 10 years, respectively. In multivariate analysis, tumor volume (≥4 cm3) and margin dose (<12 Gy) were associated with treatment failure (hazard ratio (HR) = 7.093, 95% confidence interval (CI) = 1.098–45.083, p = 0.040, and HR = 9.643, 95% CI = 1.108–83.927, p = 0.040, respectively). New apoplexy occurred in seven patients (8.6%) after GKRS with a median time of 39.9 months (range, 11.9–166.8 months). In multivariate analysis, tumor volume (≥10 cm3) was a significant risk factor (HR = 10.642, 95% CI = 2.121–53.398, p = 0.004). New hypopituitarism occurred in 14 patients (17.3%). No factors were associated with new hypopituitarism. Four patients (4.9%) developed new or worsening visual dysfunction. No new cranial neuropathy was noted.
Conclusions
In this study, initial GKRS can provide a high tumor control rate, as well as a low incidence rate of complications in NFPAs. GKRS may be an alternative initial treatment for selected NFPAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonfunctioning pituitary adenomas (NFPAs) are the most common varieties of pituitary adenomas, which represent almost 30% [1] of pituitary tumors and do not secrete biologically active hormones. Thus, NFPAs may be detected according to clinical symptoms, such as headache, visual impairment, and hypopituitarism, which are usually attributed to compression of surrounding structures. In these tumors, the goal of treatment is tumor growth control, decompression of visual system, preservation of pituitary function, and may be also histologic diagnosis.
Surgery and radiotherapy are the mainly therapeutic options for NFPAs. Transsphenoidal resection with minimal complications is widely recognized as an initial treatment for NFPAs [2]. However, initial surgical resection is not always appropriate for NFPA patients owing to comorbidities and an advanced age. Otherwise, complete surgical resection is difficult to achieve, especially in tumors with suprasellar and/or parasellar invasion. Publications reported the incidence of recurrent tumor was 10–20% following gross-total transsphenoidal resection for nonfunctioning macroadenomas [3,4,5,6,7]. The tumor progression following subtotal resection without adjuvant therapy ranged from 50 to 60% [4,5,6,7]. The results from a single institute by a single neurosurgeon showed that early surgical remission was achieved in 66.9% of 795 patients with NFPA. The recurrence free survival at 10 years was 75.3% in NFPAs [8]. Under the circumstance, Gamma Knife radiosurgery (GKRS) is usually recommended for recurrent or residual NFPA [6], which can provide 10-year tumor control rate of 85–92%, as well as a low risk of hypopituitarism varied from 9 to 32% [9,10,11,12,13,14,15]. In recent years, there are few studies have reported the results of initial GKRS for NFPAs [16]. There are more than 26 years’ experiences using Gamma Knife (Elekta, Stockholm, Sweden) for NFPAs at the Second Affiliated Hospital of Guangzhou Medical University since 1993. To evaluate the long-term outcomes of GKRS as the initial treatment for NFPA patients, we performed a large single-center retrospective study.
Methods
Patient population
Between December 1993 and December 2016, the medical records of 2557 patients that underwent GKRS for the treatment for pituitary adenoma were retrospectively reviewed at the Second Affiliated Hospital of Guangzhou Medical University. There were 751 patients had sufficient follow-up (>12 months). Among of them, 351 patients were diagnosed with NPFA. Two hundred and seventy patients were excluded according to the following inclusion criteria, leaving a final list of 81 patients. This retrospective study was approved by the institutional committee of the Second Affiliated Hospital of Guangzhou Medical University.
Study inclusion criteria: (1) no history of cancer; (2) magnetic resonance (MR) imaging findings suggested the diagnosis of pituitary adenoma, as interpreted by the treating clinicians and a radiologist; (3) no evidence of hormonal hypersecretion; (4) GKRS was the initial treatment, no prior surgical resection or radiotherapy for the tumor; (5) all patients had one or more clinical, imaging, and endocrinological evaluations with sufficient follow-up (>12 months).
Clinical and radiological evaluations
All NFPA patients were followed up with MR imaging of the sellar, clinical, and endocrinological evaluations. The follow-up evaluations were reviewed by the treating clinicians and a radiologist, and were compared with evaluations at the time of initial GKRS.
Tumor dimensions were obtained manually from MR imaging. A microadenoma was defined by a maximal tumor diameter of <10 mm, macroadenoma was >10 mm, large macroadenoma was >20 mm, and giant adenoma was >40 mm. The dimensional indices of the tumors were measured and recorded in three orthogonal planes: transverse (TR), anteroposterior (AP), and craniocaudal (CC). The tumor volumes were estimated using the following formula: V = (π × [TR × AP × CC])/6 [17]. Unfortunately, all the digital data from model B Leksell Gamma Knife (from 1993 to 2014) in Gamma plan were not available. So, we had to calculate tumor volume based on measurement of tumor diameters based on MR imaging kept in our record office. Considering the irregular shape of some pituitary tumors, tumor volume measurement was only a rough estimate of the actual volume. Tumor progression was defined as tumor enlargement at least 20% in tumor volume or tumor regrowth. Tumor shrinkage was defined as at least a 20% shrinkage in tumor volume. Stable tumor was defined as tumor volume change within 20%. Parasellar invasion was defined as the Knosp grade 3 or 4. Suprasellar extension was defined as the tumor close to optic structure (<2 mm).
Endocrinological evaluations included measurements of prolactin, growth hormone (GH), insulin-like growth factor–1, cortisol, adrenocorticotropic hormone (ACTH), free triiodothyronine, free thyroxine, thyrotropin-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, testosterone in men, and estradiol levels in premenopausal women. New hypopituitarism was defined by requiring hormone replacement or a new deficiency in any one of the hormonal axes after GKRS.
Gamma Knife radiosurgery technique
The procedure was performed using model B Leksell Gamma Knife Unit before April 2014 and Perfexion Unit (Elekta Instrument, Inc.) thereafter. Following stereotactic Leksell frame placement, thin-slice stereotactic MR imaging with contrast was performed through the sellar. Subsequently, GKRS treatment planning was made in consultation with a medical physicist, radiation oncologist, and neurosurgeon. Dose selection for GKRS was mainly depended on tumor volume, distance to the optic nerve, and chiasm. The maximal dose to the optic nerve and chiasm was maintained ≤9 Gy, and to the lateral wall of the cavernous sinus was ≤15 Gy. Small collimators of 4 mm and 8 mm were mainly adopted for better conformality.
Statistical analysis
Univariate and multivariate analyses were performed to confirm the prognostic value of different variables relative to tumor control and new hypopituitarism. Log-rank test statistics and a step forward likelihood ratio method of Cox proportional hazard models were used to perform univariate analysis and multivariate analysis, respectively. Kaplan–Meier curves were plotted for progression-free survival (PFS) and new hypopituitarism. Probability values <0.05 were defined as statistically significant. For statistical analysis, IBM’s SPSS (version 21.0) was used.
Results
Patient characteristics
Eighty-one patients who had undergone initial GKRS between December 1993 and December 2016 were selected from the single-center experience of GKRS treatment for 2557 pituitary adenomas. The patient population consisted of 38 male (46.9%) and 43 female (53.1%) patients with a median age of 44.9 years (range, 7.2–75.5 years). The median follow-up was 67.1 months (range, 11.5–263.9 months). The median tumor volume was 2.3 cm3 (range, 0.1–31.3 cm3). Based on the pre-GKRS MR imaging, the number of microadenomas, macroadenomas, large macroadenomas, and giant adenomas were 8 (9.9%), 32 (39.5%), 38 (46.9%), and 3 (3.7%), respectively. There were 48 patients (59.3%) with suprasellar extension and 14 patients (17.3%) with parasellar invasion. At the time of GKRS, 22 patient (27.2%) had visual dysfunction, including a visual field defect (n = 5), decreased visual acuity (n = 8), and both (n = 9). There are 4 patients presented with cranial nerve (CN) dysfunction, including CN III (n = 2) and CN V (n = 2). Thirty-six patients (44.4%) had hypopituitarism before GKRS, including hypogonadism (n = 31), hypothyroidism (n = 18), hypocortisolism (n = 12), and GH deficiency (n = 3) (Table 1).
The median tumor margin dose was 13 Gy (range, 8–22 Gy) at a median prescription isodose 40% (range, 25–52%). The median maximum dose was 33.3 Gy (range, 20–55 Gy) (Table 1).
Tumor control
Follow-up MR imaging studies confirmed tumor control in 72 patients (88.9%). The tumor shrunk in 63 patients (77.8%) and remained stable in 9 patients (11.1%). The median time to tumor shrinkage was 22.6 months (range, 5.5–175.5 months) after initial GKRS. Tumor treatment failures were confirmed in nine patients (11.1%), including tumor regrowth and tumor enlargement due to tumor apoplexy and progressive cystic enlargement (Fig. 1). The data were shown in Table 2. The median time of tumor treatment failure was 82.7 months (range, 16.8–166.8 months). There were seven and six patients developed new tumor apoplexy and tumor regrowth after GKRS, respectively. The median time of new tumor apoplexy and regrowth was 39.9 months (range, 11.5–166.8 months) and 93.4 months (range, 64.8–166.8 months), respectively. Of the seven patients with new apoplexy after GKRS, three patients who were under observation on account of no further signs of symptom progression were considered as tumor control, anther four patients who developed tumor enlargement due to apoplexy with or without progressive cystic enlargement or regrowth required surgical resection or GKRS were considered as treatment failure. Of the four patients, two patients with tumor enlargement due to apoplexy received surgical resection and repeat GKRS for the residual tumors at 45.1 and 51.4 months, respectively. One patient with progressive cystic enlargement and tumor apoplexy underwent additional surgical resection to relieve compression of surrounding structures at 16.8 months after initial GKRS. The last patient with apoplexy and tumor regrowth underwent surgery and repeat GKRS at 166.8 months. Five patients with tumor regrowth continued to receive repeat GKRS. The cumulative rates of tumor control for all patients after initial GKRS were 100%, 99%, 95%, and 84%, at 1, 3, 5, and 10 years, respectively (Fig. 2).
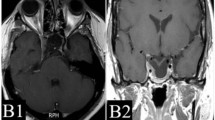
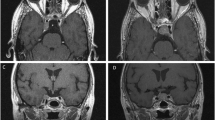
A 66-year-old male patient with nonfunctioning pituitary adenoma (13.7 cm3) received Gamma Knife radiosurgery (GKRS) (margin dose, 11.2 Gy; maximum radiation dose, 32 Gy) as initial treatment and underwent surgical resection for tumor progression due to progressive cystic enlargement and tumor apoplexy at 16.8 months after GKRS. a contrast-enhanced coronal T1-weighted magnetic resonance imaging (MRI) scans showed pituitary large macroadenoma in sellar area. b contrast-enhanced coronal T1-weighted MRI showed progressive cystic enlargement at 16.8 months after GKRS. c T1W MRI showed pituitary apoplexy at 16.8 months after GKRS. d contrast-enhanced coronal T1-weighted MRI showed pituitary adenoma had been resected at 37.8 months after surgery
In univariate analysis, factors associated with treatment failure included tumor volume ≥4 cm3 (p = 0.000) (Fig. 3), tumor margin dose <12 Gy (p = 0.000) (Fig. 4), parasellar invasion (p = 0.004), and suprasellar extension (p = 0.012). Factors associated with new apoplexy after GKRS included age ≥55 years (p = 0.003) and tumor volume ≥10 cm3 (p = 0.000) (Fig. 5). In multivariate analysis, only larger tumor volume (≥4 cm3) and tumor margin dose (<12 Gy) were significantly associated with treatment failure (hazard ratio (HR) = 7.093, 95% confidence interval (CI) = 1.098–45.083, p = 0.040 and HR = 9.643, 95% CI = 1.108–83.927, p = 0.040, respectively) (Table 3). Only tumor volume (≥10 cm3) was significantly associated with new apoplexy after GKRS (HR = 10.642, 95% CI = 2.121–53.398, p = 0.004).
Hormonal outcomes
The median endocrinological follow-up was 67.1 months (range 12.7–235.6 months). Of the 81 patients at the time of initial GKRS, 36 patients (44.4%) were presented with hypopituitarism, including hypogonadism (n = 31), hypothyroidism (n = 18), hypocortisolism (n = 12), and GH deficiency (n = 3). Of the 36 patients with baseline hypopituitarism, hypopituitarism resolved in four patients after initial GKRS, including hypogonadism (n = 1), hypothyroidism (n = 2), and hypocortisolism (n = 1). Of the 45 patients with normal endocrine function at the time of initial GKRS, five patients (11.1%) developed new hypopituitarism, including hypogonadism (n = 4) and hypothyroidism (n = 3). Of the five patients, one patient developed new hypopituitarism secondary to tumor progression, two patients developed new hypopituitarism secondary to initial GKRS, another two patients developed new hypopituitarism secondary to compression of residual tumor. Finally, of the 81 patients, 14 patients (17.3%) developed new hypopituitarism, including hypogonadism (n = 7), hypothyroidism (n = 8), and hypocortisolism (n = 7). The median time until detection of new hypopituitarism was 73.5 months (range, 12.7–187.3 months) after initial GKRS. The cumulative rates of developing new hypopituitarism at 1, 3, 5, 10, and 15 years were 0, 5.1, 10.2, 25.3, and 30.6% (Fig. 6 and Table 4).
In univariate analysis, there are no factors were significantly associated with new hypopituitarism (Table 3).
Clinical outcomes
After initial GKRS, clinical outcomes, including visual function and CN function, improved in seven patients and worsened in four patients (4.9%) (Table 5). Of the 22 patients presented with visual dysfunction before GKRS, five patients (22.7%) had improvement in visual function during follow-up, and three patients (13.6%) became worse in visual field or acuity due to tumor enlargement. One patient who presented with normal visual function showed a deterioration of visual acuity at 51.4 months after initial GKRS due to pituitary apoplexy. This patient underwent surgical resection and repeat GKRS. Among four patients presented with CN dysfunction at the time of initial GKRS, two patients had improvement in blepharoptosis. No patient suffered a new cranial neuropathy.
Discussion
Advantage and limitation of surgery
NPFAs are often detected due to symptoms and clinical signs relating to compression of surrounding structures. Transsphenoidal surgical resection is the recommended first-line treatment for most symptomatic patients with NFPAs [18]. It has the advantages of rapidly decompression of surrounding structure and pathological analysis; this histological information (such as P53 staining, Ki-67, and ACTH staining) is likely to be a helpful indicator for predict prognosis of NFPAs [19,20,21,22].
Published surgical outcomes with minimal complications were well described in previous studies [8, 23, 24]. In spite of the safety of surgical resection, initial surgical resection is not always appropriate for NFPA patients owing to significant comorbidities and an advanced age. For tumors with suprasellar extension and/or parasellar invasion, complete surgical resection is difficult to achieve. Furthermore, the risks of postoperative complications are inversely related to the experience of the surgeon, lack of surgical experience elevates the risks for residual tumors and complications. Given the high rate of recurrent or residual NFPAs after surgical resection, adjuvant radiotherapy seems to be unavoidable.
Radiosurgery for NFPAs
Adjuvant radiotherapy has been proved to control residual tumors and reduce the recurrence rate of tumors. Park et al. [7] performed a study of 176 NFPA patients treated with transsphenoidal resection and reported 10-year recurrence rates of 2.3% for patients treated with radiotherapy immediately after surgery compared with 50.5% in patients using a “wait and see” method. These results suggested that early adjuvant radiotherapy increased tumor control rates. Recently, Pomeraniec et al. [25] performed a multicenter retrospective study of comparing early and late radiosurgery after transsphenoidal surgery for 222 nonfunctioning pituitary macroadenomas. Early GKRS was associated with a lower risk of radiological progression of subtotally resected nonfunctioning pituitary macroadenomas compared with expectant management followed by late radiosurgery. Delaying radiosurgery may increase the risk for long-term adenoma progression.
In recent decades, previous reports had shown radiosurgery was a safe and effective treatment for residual or recurrent NFPAs after surgical resection. The data had shown that GKRS could provide 10-year tumor control rate of 85–92%, as well as a low risk of hypopituitarism varied from 9 to 30%. Nevertheless, there are few studies have reported the results of initial GKRS for NFPAs. The efficacy and safety of initial GKRS are still uncertain. Table 6 summarizes the available literature describing initial GKRS for NFPAs. In the study of Park et al. [26], tumor control was achieved in 13 (86.7%) of 15 patients with advanced age or significant surgical risks underwent initial GKRS. There was no significant difference in tumor control between unoperated NFPAs and residual or recurrent tumors (p = 0.59). The first report of initial GKRS for NFPAs was described by Lee et al. [16]. In this study, 41 NFPA patients underwent initial GKRS because of an advanced age, multiple comorbidities, or patient preference. The overall tumor control rate was 92.7%, and the actuarial tumor control rate was 94 and 85% at 5 and 10 years postradiosurgery, respectively. The rate of new or worsened hypopituitarism was 24%. However, the median neuroimaging follow-up was only 48 months, which was insufficient. In another retrospective study, Hasegawa et al. [27] described a small sample of 16 NFPA patients treated with initial GKRS. This study demonstrated that tumor regression occurred in 15 patients and stable tumor occurred in 1 patient. No patient developed tumor progression. One patient who had pituitary apoplexy before treatment required hormone replacements 2 years after GKRS. No patient developed CN injury or radiation-induced neoplasm.
In the current study, we described 81 NFPA patients treated with initial GKRS. Tumor control in 72 patients (88.9%), and treatment failure in 9 (11.1%) during a median of 67.1 months (range, 11.5–263.9 months) of imaging follow-up. The actuarial tumor control rates were 100%, 99%, 95%, and 84%, at 1, 3, 5 and 10 years, respectively. New hypopituitarism occurred in 14 of 81 patients (17.3%), which was lower than other reports [14, 15]. Four patients developed new or worsening visual dysfunction. No new cranial neuropathy was noted. In our experience, initial GKRS can provide a high tumor control rate with acceptable complications. To our knowledge, the current report is the largest retrospective study to evaluate the long-term outcomes of initial GKRS for NFPA patients.
Tumor control and related factors with GKRS
According to previous reports, tumor margin dose and tumor volume were significantly associated with tumor control. Gopalan et al. [28] reported that tumor control rate was significantly lower in those with tumor volumes greater than 5 ml based on 48 NFPA patients treated with GKRS. They also noted tumor margin doses less than 12 Gy significantly was associated with a lower tumor control rate (33%) relative to doses of 12 Gy or higher (80%). Park et al. [26] reported that, based on 125 NFPA patients undergoing GKRS, lower marginal dose (<14 Gy), larger tumor volume (≥4.5 ml), and ≥2 prior recurrences were associated with shorter PFS in univariate analysis; in multivariate analysis, larger (≥4.5 ml) tumor volume (p = 0.04; HR = 5.413), and ≥2 prior recurrences (p = 0.01; HR = 5.777) were associated with shorter PFS. In the first report of initial GKRS for 41 NFPA patients, Lee et al. [16] described a tumor increase in 3 patients (7.3%), there were no factors (including margin dose and tumor volume) significantly associated with tumor control. Perhaps small patient population and short-term follow-up were limitations for tumor control analysis.
In a systematic review and evidence-based guideline for the management of residual or recurrent NFPAs, radiosurgery with single doses of ≥12 Gy is recommended for greater local tumor control rate of ≥90% at 5 years [29]. In the first report of initial GKRS for NFPAs, the 5 and 10-year actual tumor control rate was 94% and 85%, respectively with median margin dose of 12 Gy and isodose level of 50% [16]. In the current study, the median margin dose and isodose level were 13 Gy and 40%, respectively. The tumor control rate was 95% and 84% at 5 and 10 years, respectively, which was similar with Lee et al. [16]. However, our study had a larger population and longer follow-up than study of Lee et al. [16]. In the nine patients with treatment failure in the current study, the median margin dose was 10 Gy (range, 9–12.8 Gy), the median isodose level was 35% (range, 30–40%). No patients occurred treatment failure with margin dose ≥13 Gy. We found tumor margin doses <12 Gy significantly was associated with a lower tumor control rate (72.4%) relative to margin doses ≥12 Gy (98.1%). In multivariate analysis, margin dose (<12 Gy) was significantly associated with treatment failure (HR = 9.643, 95% CI = 1.108–83.927, p = 0.040). The median tumor volume was 9.4 cm3 (range, 2.7–21.1 cm3) in the nine patients with treatment failure. All of them were suprasellar extension. Large tumor volume and suprasellar extension were the reasons of a relative low margin dose to these patients, which caused treatment failure. We found tumor volume ≥4 cm3 significantly was associated with a lower tumor control rate (74.1%) relative to tumor volume <4 cm3 (96.3%). In multivariate analysis, tumor volume (≥4 cm3) was significantly associated with treatment failure (HR = 7.093, 95% CI = 1.098–45.083, p = 0.040).
All the patients underwent single fraction GKRS in the study. For patients with large tumor volume, newer technologies, such as multisession GKRS, may be worthy to study. In this study, our results suggested initial GKRS were likely more engaging for smaller volume NFPAs. With the development of modern surgical techniques, for those patients with large tumor volume before surgical resection, and limited tumor volume after surgical resection may become more amenable to GKRS.
Pattern of treatment failure after initial GKRS for NFPAs
In the current study, tumor enlargement at least 20% in tumor volume or tumor regrowth was considered as GKRS treatment failure. As previous publications reported, tumor enlargement or tumor growth was the main pattern of GKRS treatment failure. Mostly, previous publications [16, 20, 28] did not analyze the reasons of tumor enlargement. As far as we know, progressive cystic enlargement, pituitary apoplexy, and tumor regrowth could contribute to tumor enlargement. Previous studies [15, 26, 30] reported the common cause of tumor enlargement after GKRS was tumor growth. Progressive cystic enlargement and pituitary apoplexy were rarely reported. Shen et al. [31] described 3 of 41 patient occurred in tumor progression. Among these three patients, two cases had progressive cystic enlargement at the follow-up of 6 months and 120 months from initial GKRS. Xu et al. [20] reported one of six patients treated with radiotherapy or repeat stereotactic radiosurgery (SRS) after initial failed SRS developed tumor progression and apoplexy 13 months afterward. However, apoplexy and cystic enlargement could coexist with tumor regrowth. Sometimes, it may be difficult to distinguish from each other.
In the current study, tumor regrowth and tumor enlargement due to pituitary apoplexy and progressive cystic enlargement were the main patterns of treatment failure after initial GKRS for NFPAs. Seven patients (8.6%) developed new apoplexy after GKRS, the median time to new apoplexy was 39.9 months (range, 11.9–166.8 months). Of the seven patients, only four patients were considered as treatment failure, because these patients with tumor enlargement ≥20% required surgical resection. The early pattern of initial GKRS treatment failure was pituitary apoplexy. Tumor regrowth commonly occurred in 5 years after initial GKRS, and was the late pattern of treatment failure with a median time of 93.4 months (range, 64.8–166.8 months). The median age of patients developed pituitary apoplexy after initial GKRS was 66 years (range, 31–69 years). All of patients with apoplexy in progression were macroadenomas. Previous reported pituitary apoplexy occurred in the fifth or sixth decade, accounted for 8–21% [32, 33] of NFPAs. Macroadenoma, especially NFPAs were most susceptible to apoplexy [34]. Risk factors included tumor volume, age, gender, suprasellar extension, parasellar invasion, and tumor margin dose were analyzed. In univariate analysis, factors associated with new apoplexy after GKRS included age ≥55 years (p = 0.003) and TV ≥ 10 cm3 (p = 0.000). In multivariate analysis, only tumor volume (≥10 cm3) was significantly associated with new apoplexy after GKRS (HR = 10.642, 95% CI = 2.121–53.398, p = 0.004). there was no correlation between tumor margin dose and apoplexy. In previous studies, increase in intracranial pressure, vasospasm, arterial hypertension, GnRH-agonists, dopamine agonists, angiography, major surgery, closed head trauma, anticoagulant therapy, and dynamic testing had been reported as precipitating factors of pituitary apoplexy [35]. To our knowledge, there were no studies reported the relation between radiosurgery and pituitary apoplexy. Is radiosurgery a precipitating factor of pituitary apoplexy? Perhaps, tumor infarction or necrosis after GKRS may precipitate apoplexy, which may contribute to treatment failure. Therefore, it is necessary to have a further study on the relation between radiosurgery and pituitary apoplexy by controlling potential confounders.
Complications associated with GKRS
According to postradiosurgery complications in previous studies [9,10,11,12,13,14,15], the most frequent complication was new or worsened hypopituitarism, which had been reported in 9–32% of NFPA patients. In the current study, the result was similar, with 17.3% of patients developing new hypopituitarism at a median of 67.1 months (range, 12.7–235.6 months) after initial GKRS. Age, gender, parasellar invasion, suprasellar extension, tumor margin dose, pituitary gland radiation dose, preexisting hypopituitarism, previous radiotherapy history, tumor response, as well as tumor volume were all potential factors for the development of new hypopituitarism. In our study, there were no factors correlated with new hypopituitarism. In a study by Pollock et al. [15], the risk of new hypopituitarism at 5 years after SRS was 18% for tumors ≤4 cm3, compared with 50% if the tumor volume was >4 cm3. In a study by Lee et al. [16], 24.4% of NFPA patients after initial GKRS experienced hypopituitarism at a median of 37 months. A prescribed tumor margin dose >18 Gy and a maximum dose >36 Gy were independent prognostic factors significantly associated with post-GKRS hypopituitarism. Graffeo et al. [36] found a higher mean pituitary gland dose (HR = 1.31, 95% CI = 1.16–1.47, p < 0.001) was correlated with post-SRS hypopituitarism. The rate of hypopituitarism for patients with a mean gland dose <11 Gy at 2 years and 5 years were 2 and 5%, whereas the rate of hypopituitarism for patients with a mean gland dose ≥11 Gy at 2 years and 5 years were 31 and 51%. Leenstra et al. [37] also found the pituitary gland radiation dose was correlated with the incidence of new hormonal deficits after SRS. In a study by Park et al. [26], a correlation was found between prior therapy and new hypopituitarism after SRS. From previous studies, patients receiving a lower dose to the normal pituitary gland, pituitary stalk, and hypothalamus may be the way to avoid or reduce the incidence of new hypopituitarism. As the incidence of new hypopituitarism after SRS seems to be higher than those for surgical resection, initial GKRS should be supported by evidence of MRI tumor growth in small NFPA. Otherwise the risk of hypopituitarism may be unwarranted. Although hypopituitarism is the most common complication after GKRS in pituitary adenomas, it is not difficult to manage, which can be treated with hormone replacement therapy. Furthermore, tumor progression poses a greater threat to the patients than hypopituitarism, and can lead to new or worsened hypopituitarism.
Neurological complications such as visual function and CN function were also reported after GKRS. The incidence of radiation-related neurological complication was reported to be 0 to 5% [16, 26, 28, 38,39,40]. In this study, there were four patients (4.9%) presented with worsened visual function after initial GKRS. No patient suffered a new cranial neuropathy. Of the 22 patients presented with visual dysfunction before GKRS, five patients had improvement in visual function. Among four patients presented with CN dysfunction before GKRS, two patients had improvement in blepharoptosis. Postradiosurgery improvement in neurological function has also been reported in previous publications [13, 26, 41]. These may be owed to decompression of the surrounding structure after tumor shrinkage.
Study limitations
Although the current study is large series of NFPA patients treated with initial GKRS, there are several limitations of this study that should be payed attention to. First, this was a single-center retrospective study and thereby reflected the treatment and selection biases. Second, all the patients did not accept surgical resection as the initial treatment, the histological information was unknown, which may predict unfavorable prognosis in patients. Third, the number of cases is relatively small that may limit statistical power. Fourth, considering the irregular shape of some pituitary tumors, tumor volume measurement in this study was only a rough estimate of the actual volume. Finally, there were differences in the GKRS instrument among the patients.
Conclusions
In this largest single-center retrospective study, we found that initial GKRS could afford a high tumor control rate and a low rate of hypopituitarism and neurological complications for NFPA patients. Tumor control rate occurred in 88.9%, hypopituitarism occurred in 17.3%, and visual function worsened in 4.9%. For patients are not candidate to surgery because of high comorbidity or in selected patients (such as small tumor, an advanced age, cavernous invasion, without visual impairment), GKRS may be an alternative initial treatment.
References
C. Hoybye, T. Rahn, Adjuvant Gamma Knife radiosurgery in nonfunctioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary 12(3), 211–216 (2009). https://doi.org/10.1007/s11102-008-0163-x
G. Frank, E. Pasquini, G. Farneti, D. Mazzatenta, V. Sciarretta, V. Grasso, M. Faustini Fustini, The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology 83(3–4), 240–248 (2006). https://doi.org/10.1159/000095534
E.F. Chang, G. Zada, S. Kim, K.R. Lamborn, A. Quinones-Hinojosa, J.B. Tyrrell, C.B. Wilson, S. Kunwar, Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J. Neurosurg. 108(4), 736–745 (2008). https://doi.org/10.3171/JNS/2008/108/4/0736
R.F. Dallapiazza, Y. Grober, R.M. Starke, E.R. Laws Jr., J.A. Jane Jr., Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery 76(1), 42–52 (2015). https://doi.org/10.1227/neu.0000000000000563.
E. Ferrante, M. Ferraroni, T. Castrignano, L. Menicatti, M. Anagni, G. Reimondo, P. Del Monte, D. Bernasconi, P. Loli, M. Faustini-Fustini, G. Borretta, M. Terzolo, M. Losa, A. Morabito, A. Spada, P. Beck-Peccoz, A.G. Lania, Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur. J. Endocrinol. 155(6), 823–829 (2006). https://doi.org/10.1530/eje.1.02298
P. Picozzi, M. Losa, P. Mortini, M.A. Valle, A. Franzin, L. Attuati, C. Ferrari da Passano, M. Giovanelli, Radiosurgery and the prevention of regrowth of incompletely removed nonfunctioning pituitary adenomas. J. Neurosurg. 102, 71–74 (2005).
P. Park, W.F. Chandler, A.L. Barkan, J.J. Orrego, J.A. Cowan, K.A. Griffith, C. Tsien, The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery 55(1), 100–106 (2004). https://doi.org/10.1227/01.neu.0000126885.71242.d7. discussion 106-107
P. Mortini, L.R. Barzaghi, L. Albano, P. Panni, M. Losa, Microsurgical therapy of pituitary adenomas. Endocrine 59(1), 72–81 (2018). https://doi.org/10.1007/s12020-017-1458-3
S. Sun, A. Liu, Y. Zhang, Long-term follow-up studies of Gamma Knife radiosurgery for postsurgical nonfunctioning pituitary adenomas. World Neurosurg. (2019). https://doi.org/10.1016/j.wneu.2019.01.009
S.C. Bir, R.D. Murray, S. Ambekar, P. Bollam, A. Nanda, Clinical and radiologic outcome of Gamma Knife radiosurgery on nonfunctioning pituitary adenomas. J. Neurol. Surg. Part B Skull Base 76(5), 351–357 (2015). https://doi.org/10.1055/s-0035-1549309
R.M. Starke, B.J. Williams, J.A. Jane Jr., J.P. Sheehan, Gamma Knife surgery for patients with nonfunctioning pituitary macroadenomas: predictors of tumor control, neurological deficits, and hypopituitarism. J. Neurosurg. 117(1), 129–135 (2012). https://doi.org/10.3171/2012.4.Jns112250
M. Losa, M. Valle, P. Mortini, A. Franzin, C.F. da Passano, M. Cenzato, S. Bianchi, P. Picozzi, M. Giovanelli, Gamma Knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J. Neurosurg. 100(3), 438–444 (2004). https://doi.org/10.3171/jns.2004.100.3.0438
J.P. Sheehan, D. Kondziolka, J. Flickinger, L.D. Lunsford, Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J. Neurosurg. 97(5 Suppl), 408–414 (2002). https://doi.org/10.3171/jns.2002.97.supplement
K. Zibar Tomsic, T. Dusek, I. Kraljevic, Z. Heinrich, M. Solak, A. Vucinovic, D. Ozretic, S. Mihailovic Marasanov, H. Hrsak, D. Kastelan, Hypopituitarism after Gamma Knife radiosurgery for pituitary adenoma. Endocr. Res. 42(4), 318–324 (2017). https://doi.org/10.1080/07435800.2017.1323913
B.E. Pollock, J. Cochran, N. Natt, P.D. Brown, D. Erickson, M.J. Link, Y.I. Garces, R.L. Foote, S.L. Stafford, P.J. Schomberg, Gamma Knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int. J. Radiat. Oncol. Biol. Phys. 70(5), 1325–1329 (2008). https://doi.org/10.1016/j.ijrobp.2007.08.018
C.C. Lee, H. Kano, H.C. Yang, Z. Xu, C.P. Yen, W.Y. Chung, D.H. Pan, L.D. Lunsford, J.P. Sheehan, Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. J. Neurosurg. 120(3), 647–654 (2014). https://doi.org/10.3171/2013.11.Jns131757
H.K. Mak, S.W. Lai, W. Qian, S. Xu, E. Tong, M.L. Vance, E. Oldfield, J. Jane Jr., J. Sheehan, K.K. Yau, M. Wintermark, Effective time window in reducing pituitary adenoma size by Gamma Knife radiosurgery. Pituitary 18(4), 509–517 (2015). https://doi.org/10.1007/s11102-014-0603-8
P.U. Freda, A.M. Beckers, L. Katznelson, M.E. Molitch, V.M. Montori, K.D. Post, M.L. Vance, S. Endocrine, Pituitary incidentaloma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(4), 894–904 (2011). https://doi.org/10.1210/jc.2010-1048
O. Cohen-Inbar, Z. Xu, C.C. Lee, C.C. Wu, T. Chytka, D. Silva, M. Sharma, H. Radwan, I.S. Grills, B. Nguyen, Z. Siddiqui, D. Mathieu, C. Iorio-Morin, A. Wolf, C.P. Cifarelli, D.T. Cifarelli, L.D. Lunsford, D. Kondziolka, J.P. Sheehan, Prognostic significance of corticotroph staining in radiosurgery for non-functioning pituitary adenomas: a multicenter study. J. Neuro-Oncol. 135(1), 67–74 (2017). https://doi.org/10.1007/s11060-017-2520-y
Z. Xu, S. Ellis, C.C. Lee, R.M. Starke, D. Schlesinger, M. Lee Vance, M.B. Lopes, J. Sheehan, Silent corticotroph adenomas after stereotactic radiosurgery: a case-control study. Int. J. Radiat. Oncol. Biol. Phys. 90(4), 903–910 (2014). https://doi.org/10.1016/j.ijrobp.2014.07.013
R. Hasanov, B.I. Aydogan, S. Kiremitci, E. Erden, S. Gullu, The prognostic roles of the Ki-67 proliferation index, P53 expression, mitotic index, and radiological tumor invasion in pituitary adenomas. Endocr. Pathol. 30(1), 49–55 (2019). https://doi.org/10.1007/s12022-018-9563-2
L. Mastronardi, Atypical pituitary adenomas: clinical characteristics and role of Ki-67 and p53 in prognostic and therapeutic evaluation. A series of 50 patients. Neurosurg. Rev. 40(2), 357–358 (2017). https://doi.org/10.1007/s10143-017-0818-z
M.H. Murad, M.M. Fernandez-Balsells, A. Barwise, J.F. Gallegos-Orozco, A. Paul, M.A. Lane, J.F. Lampropulos, I. Natividad, L. Perestelo-Perez, P.G. Ponce de Leon-Lovaton, F.N. Albuquerque, J. Carey, P.J. Erwin, V.M. Montori, Outcomes of surgical treatment for nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Clin. Endocrinol. 73(6), 777–791 (2010). https://doi.org/10.1111/j.1365-2265.2010.03875.x
J.H. Kim, J.H. Lee, J.H. Lee, A.R. Hong, Y.J. Kim, Y.H. Kim, Endoscopic transsphenoidal surgery outcomes in 331 nonfunctioning pituitary adenoma cases after a single surgeon learning curve. World Neurosurg. 109, e409–e416 (2018). https://doi.org/10.1016/j.wneu.2017.09.194
I.J. Pomeraniec, H. Kano, Z. Xu, B. Nguyen, Z.A. Siddiqui, D. Silva, M. Sharma, H. Radwan, J.A. Cohen, R.F. Dallapiazza, C. Iorio-Morin, A. Wolf, J.A. Jane, I.S. Grills, D. Mathieu, D. Kondziolka, C.C. Lee, C.C. Wu, C.P. Cifarelli, T. Chytka, G.H. Barnett, L.D. Lunsford, J.P. Sheehan, Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J. Neurosurg. 129(3), 648–657 (2018). https://doi.org/10.3171/2017.5.Jns163069
K.J. Park, H. Kano, P.V. Parry, A. Niranjan, J.C. Flickinger, L.D. Lunsford, D. Kondziolka, Long-term outcomes after Gamma Knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 69(6), 1188–1199 (2011). https://doi.org/10.1227/NEU.0b013e318222afed
T. Hasegawa, K. Shintai, T. Kato, H. Iizuka, Stereotactic radiosurgery as the initial treatment for patients with nonfunctioning pituitary adenomas. World Neurosurg. 83(6), 1173–1179 (2015). https://doi.org/10.1016/j.wneu.2015.01.054
R. Gopalan, D. Schlesinger, M.L. Vance, E. Laws, J. Sheehan, Long-term outcomes after Gamma Knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery 69(2), 284–293 (2011). https://doi.org/10.1227/NEU.0b013e31821bc44e
J. Sheehan, C.C. Lee, M.E. Bodach, L.M. Tumialan, N.M. Oyesiku, C.G. Patil, Z. Litvack, G. Zada, M.K. Aghi, Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery 79(4), E539–E540 (2016). https://doi.org/10.1227/neu.0000000000001385
J.P. Sheehan, R.M. Starke, D. Mathieu, B. Young, P.K. Sneed, V.L. Chiang, J.Y. Lee, H. Kano, K.J. Park, A. Niranjan, D. Kondziolka, G.H. Barnett, S. Rush, J.G. Golfinos, L.D. Lunsford, Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J. Neurosurg. 119(2), 446–456 (2013). https://doi.org/10.3171/2013.3.JNS12766
C.C. Shen, W.C. You, M.H. Sun, S.D. Lee, H.K. Tsou, Y.J. Chen, M.L. Sheu, J. Sheehan, H.C. Pan, Outcome of partially irradiated recurrent nonfunctioning pituitary macroadenoma by Gamma Knife radiosurgery. J. Neuro-Oncol. 139(3), 767–775 (2018). https://doi.org/10.1007/s11060-018-2925-2
G. Vargas, B. Gonzalez, C. Ramirez, A. Ferreira, E. Espinosa, V. Mendoza, G. Guinto, B. Lopez-Felix, E. Zepeda, M. Mercado, Clinical characteristics and treatment outcome of 485 patients with nonfunctioning pituitary macroadenomas. Int. J. Endocrinol. 2015, 756069 (2015). https://doi.org/10.1155/2015/756069
E.H. Nielsen, J. Lindholm, P. Bjerre, J.S. Christiansen, C. Hagen, S. Juul, J. Jorgensen, A. Kruse, P. Laurberg, Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin. Endocrinol. 64(3), 319–322 (2006). https://doi.org/10.1111/j.1365-2265.2006.02463.x
B.R. Randall, W.T. Couldwell, Apoplexy in pituitary microadenomas. Acta Neurochirurgica 152(10), 1737–1740 (2010). https://doi.org/10.1007/s00701-010-0706-6
C. Briet, S. Salenave, J.F. Bonneville, E.R. Laws, P. Chanson, Pituitary apoplexy. Endocr. Rev. 36(6), 622–645 (2015). https://doi.org/10.1210/er.2015-1042
C.S. Graffeo, M.J. Link, P.D. Brown, W.F. Young Jr., B.E. Pollock, Hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis based on patients treated using contemporary techniques. Int. J. Radiat. Oncol. Biol. Phys. 101(3), 618–623 (2018). https://doi.org/10.1016/j.ijrobp.2018.02.169
J.L. Leenstra, S. Tanaka, R.W. Kline, P.D. Brown, M.J. Link, T.B. Nippoldt, W.F. Young Jr., B.E. Pollock, Factors associated with endocrine deficits after stereotactic radiosurgery of pituitary adenomas. Neurosurgery 67(1), 27–32 (2010). https://doi.org/10.1227/01.Neu.0000370978.31405.A9. discussion 32-23
M. Yamamoto, H. Aiyama, T. Koiso, S. Watanabe, T. Kawabe, Y. Sato, Y. Higuchi, B.E. Barfod, H. Kasuya, Postsurgical salvage radiosurgery for nonfunctioning pituitary adenomas touching/compressing the optic chiasm: median 13-year postirradiation imaging follow-up results. Neurosurgery 85(4), 476–485 (2019). https://doi.org/10.1093/neuros/nyy357
F. Castinetti, J. Regis, H. Dufour, T. Brue, Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat. Rev. Endocrinol. 6(4), 214–223 (2010). https://doi.org/10.1038/nrendo.2010.4
R. Ove, S. Kelman, P.P. Amin, L.S. Chin, Preservation of visual fields after peri-sellar Gamma-knife radiosurgery. Int. J. Cancer 90(6), 343–350 (2000). https://doi.org/10.1002/1097-0215(20001220)90:6<343::aid-ijc6>3.0.co;2-h
T. Abe, M. Yamamoto, M. Taniyama, D. Tanioka, H. Izumiyama, K. Matsumoto, Early palliation of oculomotor nerve palsy following Gamma Knife radiosurgery for pituitary adenoma. Eur. Neurol. 47(1), 61–63 (2002). https://doi.org/10.1159/000047951
Funding
This work was supported by National Key Research and Development Project (grant number: 2017YFC0113700); National Natural Science Foundation of China (grant number: 81800682); the Medical Science and Technology Research Fund Project of Guangdong (grants number: A2018443); the Medical and Health Project of Guangdong (grant number: 20181A011069).
Author contributions
J.Y., Y.L., X.L., T.Q., C.P., J.Z., S.L., Y.H., and M.H. collected and analyszed the data; J.Y. and Y.L. wrote the paper; J.Y. and Y.D. conceived and designed all the experiments; J.Y. and Y.D. directed this work and edited the paper; and all the authors agreed on the final paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Li, Y., Quan, T. et al. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas: results from a 26-year experience. Endocrine 68, 399–410 (2020). https://doi.org/10.1007/s12020-020-02260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02260-1