Abstract
Purpose
To define the efficacy and complications of multisession Gamma Knife radiosurgery (MGKRS) delivered in three consecutive sessions for the treatment of residual or recurrent pituitary adenomas (PAs).
Methods
This was a retrospective study of data from the Neurosurgery and Gamma Knife Radiosurgery Department at San Raffaele Hospital between May 2008 and September 2017. We recruited 47 consecutive patients undergoing MGKRS in three consecutive fractions for residual or recurrent PA with a distance from the anterior optic pathway inferior to 2–3 mm.
Results
Thirty-eight (80.8%) patients had a nonfunctioning-PA (NFPA) while 9 (19.2%) had a hormone-secreting PA (HSPA). Tumor control was achieved in 100% of patients. Tumor shrinkage was seen in 33 out of 44 (75.0%) patients with a radiological follow-up. Mean tumor volume before MGKRS was 3.93 cm3. The mean tumor volume at last follow-up was 2.11 cm3, with a mean tumor shrinkage of 50.2%, as compared with baseline. One case of suspected radiation-induced optic neuropathy (RION) was documented while new-onset hypopituitarism for any axis occurred in 12 of the 31 (38.7%) patients at risk. The mean follow-up was 44.6 ± 4.0 months (range, 6–111 months).
Conclusions
MGKRS is a valid alternative to external fractionated radiotherapy and other types of stereotactic radiosurgery for the treatment of PAs, achieving a high tumor control rate with a low risk of visual deterioration. Moreover, the majority of patients showed a significant reduction of tumor size in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of pituitary adenomas (PAs) often requires a multimodal approach, including medical therapy, surgery, radiation therapy (RT), or stereotactic radiosurgery (SRS). RT and SRS are frequently employed as an adjuvant treatment in patients with residual or recurrent PAs following surgery or who fail to respond to medical therapy [1,2,3]. Nowadays, several radiation techniques are available for treating such tumors and great effort is put into maximizing the local tumor control rate while minimizing complications of treatment. Conventional RT has been limited by the inaccuracy of the treatment fields causing unpredictable irradiation of healthy tissues adjacent to the tumor. The introduction of SRS, thanks to its steep falloff dose outside the target volume, improved safety and efficacy of irradiation of the sellar region. Gamma Knife radiosurgery (GKRS) has gained widespread popularity as an adjuvant treatment for both nonfunctioning PA (NFPA) and hormone-secreting PA (HSPA) [4,5,6,7,8]. GKRS is traditionally delivered in a single session despite some limitations, such as the requirement of a distance between tumor margin and the anterior optic pathway of at least 2–3 mm to minimize the risk of radiation-induced optic neuropathy (RION). Indeed, visual preservation, together with tumor control, is a major concern following irradiation of PAs [9,10,11]. For this reason, fractionated stereotactic radiosurgery (FSRS) has been recently proposed for PA treatment in order to combine advantages of both conventional RT and SRS [12,13,14,15]. The principal aim of FSRS is to deliver more localized radiation, leading to a reduction of the volume of normal brain tissue that receives high radiation doses. The same principle may apply to GKRS as well when the optic pathway is too close to the tumor margin. Major studies on this technique for the treatment of tumors of the sellar region are lacking, probably because of its recent introduction [16]. Therefore, advantages, drawbacks, and indications of this fractionation scheme remain a matter of debate [17]. The aim of this report is to define the efficacy and complications of multisession GKRS (MGKRS) delivered in three consecutive sessions in the treatment of residual or recurrent PAs.
Materials and methods
Patients
We conducted a retrospective analysis of a prospectively maintained database of patients affected by PA and treated by GKRS in our department. Between May 2008 and September 2017, 47 consecutive patients affected by residual or recurrent pituitary adenoma located within 2–3 mm from the anterior optic pathway underwent MGKRS in three consecutive sessions at San Raffaele Hospital.
Standard informed consent relating to MGKRS procedure was obtained from all patients. Ethics committee approval was not sought because of the retrospective and observational nature of the study.
Clinical evaluation
Hormonal evaluation, ophthalmological evaluation, and magnetic resonance imaging (MRI) follow-up data were obtained at baseline, 6-month intervals after MGKRS for the first year and yearly thereafter for 5 years. After this period, clinical control, including neuroimaging, was scheduled at 2-year intervals or when clinically indicated. Whenever possible, patients underwent follow-up examination at our center. Otherwise, patients were invited to submit follow-up exams at our center. When available (30 patients), we imported the last follow-up MRI into the GammaPlan software (Elekta Inc., Atlanta, GA, USA) and co-registered it with the pretreatment stereotactic MRI in order to measure the variation of tumor volume. This was achieved through comparison of T1-weighted pre-contrast and post-contrast coronal sequences, axial ones or both.
Tumor volume was evaluated as the percentage change in comparison with baseline. A reduction of 10% or more of tumor volume was considered as significant decrease of tumor size, while an increase of 10% or more of tumor volume indicated progression of disease. Otherwise, tumor size was considered to be stable. In those cases, in which we failed to obtain the last follow-up MRI, we inferred tumor behavior based on MRI reports.
Endocrinological status was investigated as previously detailed [4, 5]. Briefly, baseline hormone values and need of hormonal substitution therapy prior to MGKRS were evaluated at each follow-up. Radiation-induced hypopituitarism was diagnosed when a new onset pituitary deficit occurred during follow-up.
Neuro-ophthalmological examination included visual acuity testing, computerized perimetry and oculomotor function. Visual function was considered improved, unchanged, or worsened as compared with baseline function. RION was diagnosed as a decrease in visual function in the absence of evidence of tumor recurrence on MRI and after exclusion of other potential causes of visual deterioration (e.g., glaucoma).
Radiosurgical treatment
The phase of stereotactic headframe application and brain imaging scans acquisition using MRI were previously described [18]. Subsequently, a multidisciplinary team, including a neurosurgeon, a medical physicist, and a radiation oncologist supervised and approved the treatment plan. Initially, gross tumor volume (GTV) and organs at risk (OARs) were delineated. The GTV was represented by the lesion visible on MRI. The entire residual or recurrent PA was covered within the 50% isodose line (prescription isodose). The clinical target volume (CTV) includes microscopic disease and it is usually used for malignant tumors. For this reason, additional margin expansion from GTV to CTV was unnecessary in PAs. The OARs are the anterior optic apparatus and pituitary stalk. As suggested by the International Leksell Gamma Knife Society, right optic nerve (RON), left optic nerve (LON), and chiasm were outlined as separate structures so as to eliminate inadequacies in GammaPlan software, which may have led to difficulties in rendering a three-dimensional volume as complex as the optic apparatus [19]. Pituitary gland tissue (excluding the adenoma) was not delineated because of low signal contrast compared with surrounding tissues, even though a correlation was established between doses to the pituitary gland and risk for radiation-induced hypopituitarism [20].
When the distance between the tumor and the optic apparatus were <2–3 mm, GKRS was thus split into three fractions, with an interval between each session of ~24 h. This appNroach was supposed to allow enough recovery of healthy tissue while maintaining efficacy on the target lesion. A prescription dose around 7 Gy (range, 6.5–8 Gy) and 10 Gy (range, 7–13 Gy) per fraction was selected for NFPAs and HSPAs, respectively. Multiple isocenters were distributed throughout the GTV to conform the dose to the tumor margins. We did not accept percentage of GTV coverage inferior than 97%. Radiation physicists performed the conversion of the total fractionated dose to the equivalent single session dose to the tumor margin.
Radiation doses to OARs were monitored through the analyses of dose–volume histograms. In general, when maximum doses to the optic apparatus could not be kept below 20 Gy in three fractions (empirically chosen as the threshold for a high risk for RION), the prescription dose was slightly reduced, or dose conformation was changed in order to prevent potential damages. Evidently, a compromise between an optimal coverage of the GTV and an acceptable risk for RION was necessary.
Once the treatment plan was complete, MGKRS was performed using the Leksell Gamma Knife Perfexion model (Elekta Instruments AB, Stockholm, Sweden).
The frame was maintained until the last dose fraction was delivered.
On the third day, after the entire prescription dose had been delivered, the frame was removed, and the patient could be discharged if no early complications occurred.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics package (IBM Corporation, SPSS Statistics for Macintosh, Version 20, Armonk, New York, USA). Continuous data was expressed as mean ± SE. Student’s t-test was used to compare continuous variables. Categorical variables were compared with Pearson’s chi-square test or the Fisher exact test. Bivariate analysis of the independent variable was performed using the Spearman Rank test. Overall survival, radiological progression-free survival and recurrence-free survival was calculated using the Kaplan–Meier method. A probability value <0.05 was considered indicative of statistical significance.
Results
Patient’s characteristics
The patient population consisted of 47 patients, including 21 women (44.7%), and 26 men (55.3%) with a mean age of 58.9 ± 1.8 years (range, 26–79 years). Thirty-eight patients (80.8%) were affected by NFPA, while the remaining 9 patients (19.2%) were affected by HSPA. Patients with NFPA were older than patients with HSPA (p < 0.01). Forty-six patients (97.9%) had previously undergone at least one surgical intervention. Only one patient (2.1%) received MGKRS as primary treatment because of refusal to undergo surgery.
Mean tumor volume before MGKRS was 3.93 ± 0.46 cm3 (range, 0.32–16.25 cm3).
Two patients (4.3%) with NFPA had previously received radiation treatment (single session GKRS in one case and conventional RT in the other case). The first one had an “out of field” tumor recurrence nearby the optic pathway 6 years after GKRS, while the other one had an “in field” tumor recurrence 3 years after an external beam RT.
In the group of HSPA, one patient had Cushing’s disease, two patients had a prolactin (PRL)-secreting adenoma, and six patients had acromegaly. Evaluation of pituitary function before MGKRS disclosed a normal gonadal function in 18 of 47 patients (38.3%), 28 of 47 patients (59.6%) had normal thyroid function, while 22 of 47 patients (46.8%) had normal adrenal function.
Visual function before MGKRS was impaired in 26 patients (55.4%) and normal in 18 patients (38.2%). Three patients (6.4%) did not had a formal recent neuroophthalmological examination but did not complain of subjective visual disturbances.
Patients’ demographic, tumor characteristics, and MGKRS variables are summarized in Table 1.
Clinical and radiological outcome
The mean follow-up after MGKRS in all patients was 44.6 ± 4.0 months (range, 6–111 months). Patients with NFPA had a mean follow-up of 40.9 ± 4.0 months (range, 6–111 months) while patients with HSPA had a mean follow-up of 70.8 ± 10.1 months (range, 25–96, months). Three patients (6.4%), one with NFPA, one with prolactinoma, and one with acromegaly, were lost to follow-up. Among the 44 patients (93.6%) with radiological follow-up, quantitative tumor volume data, performed by direct measurement of the tumor volume on MRI before and at the last follow-up, could be obtained in 30 patients (68.2%, 26 NFPAs and 4 HSPAs). Qualitative information on the remaining 14 patients (31.8%) was inferred by MRI reports.
Forty-four patients (93.6%) were alive at the last follow-up, whereas one patient (2.1%) with NFPA died for unrelated reasons; data is missing for 2 patients (4.3%).
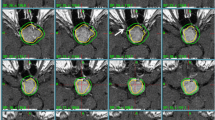
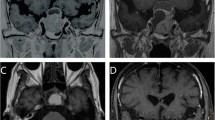
Tumor size remained stable over the period of radiological follow-up in 11 of the 44 patients (25.0%) and decreased in 33 of the remaining 44 patients (75.0%). Therefore, no patients experienced recurrence of disease. Notably, when we had the possibility to directly measure tumor volume on the last available MRI and compared it with the baseline MRI (Fig. 1), we found a significant tumor reduction (>10% decrease in tumor volume) in 29 of the 30 cases available for this analysis (96.7%). GTV before MGKRS was 3.93 ± 0.46 cm3 (range, 0.32–16.25 cm3) and significantly decreased after MGKSR to 2.11 ± 0.48 cm3 (range, 0.15–12.38 cm3; p < 0.01). The mean percentage of tumor shrinkage was 50.2 ± 3.8% (range, 18.7–92.1%). Tumor volume reduction was first observed 13.0 ± 2.3 months after MGKRS (range, 5–43 months). Table 2 summarizes data on tumor control while Fig. 2 depicts a practical example of tumor shrinkage in one of our patients.
a and b Pre-MGKRS axial and coronal T1-weighted MR images with contrast, respectively, showing the postoperative residual of a NFPA with suprasellar extension; c and d Follow-up axial and coronal T1-weighted MR images with contrast, respectively, obtained after 72 months from MGKRS surgery demonstrating the significant reduction in tumor volume of PA
Reversal of hormone hypersecretion in patients with HSPA occurred in only one of six patients (16.7%) with available hormonal data during follow-up (one patient with Cushing’s disease and five patients with acromegaly). The patient with Cushing’s disease had gradual symptomatic and hormonal improvement after MGKRS but still had a mildly elevated urinary-free cortisol level 8 years after MGKRS and a stable tumor size. Four acromegalic patients were still on medical therapy with GH-receptor antagonist and/or somatostatin analog and, therefore, could not be considered in remission of disease, even though IGF-1 levels were normalized during medical treatment. One of these patients could reduce the octreotide LAR dosage from 20 mg monthly to 10 mg monthly after MGKRS. He also had a concomitant reduction on tumor volume on MRI. Another patient, who was assuming both pegvisomant and octreotide LAR before MGKRS, could stop octreotide treatment without experiencing re-expansion of the tumor on the last MRI. One patient with complete resistance to somatostatin analogs achieved remission of disease 40 months after MGKRS. At last follow-up, 6 years after treatment, GH and IGF-1 levels had a reduction from 9.7 μg/L and 743 ng/mL (reference value adjusted for sex and age <227 ng/mL) to 1.0 μg/L and 101 ng/mL, respectively.
Complications of MGKRS
No serious side effects occurred after MGKRS, except for transient headache and local pain where the head-frame was fixed to skull.
At the last follow-up examination, 7 of the 28 patients at risk (25.0%) had a new onset hypothyroidism, 3 of 18 (16.7%) developed a new onset hypogonadism, and 5 of 22 patients (22.7%) developed hypoadrenalism (Table 3).
Of the 26 patients with visual dysfunction, we are not able to have an ophthalmological follow-up in one patient. Of the remaining 25 patients, 9 (36.0%) had an improvement in visual function after MGKRS (Table 4). One patient had deterioration of vision because of glaucoma but his visual field and visual acuity were stable before the development of the disease. Lastly, one patient (2.3%) had an asymptomatic worsening of the left visual field, despite a reduction of tumor volume. This patient was thus considered to have a suspected RION. Dosimetric analysis of the treatment plan revealed that the RON absorbed a maximum dose of 6.7 Gy per fraction, the LON absorbed a maximum dose of 2.8 Gy per fraction, and the optic chiasm received a maximum dose of 2 Gy per fraction. The radiation doses to the optics apparatus are well within the widely accepted safe limit of 20 Gy in three fractions. It is, therefore, uncertain whether the patient suffered a RION caused by MGKRS.
Discussion
Our study includes 47 PAs (38 NFPAs and 9 HSPAs) treated with MGKRS and constitutes, according to current medical literature, the largest series described so far and with the longest follow-up period.
Given its recent introduction, data assessing FSRS efficacy and complication rate are still lacking and confusing. We only found four studies regarding fractionated GKRS for PAs in medical literature, among which the largest number of patients recruited was 11 [16]; in addition, none of them examined PAs as a separate entity, since they included other perioptic tumors [16, 21,22,23]. Moreover, there are studies reporting the results of other FSRS techniques, such as LINAC-based systems, for the treatment of perioptic tumors [12, 21].
Tumor control rate in our series of patients was 100% with a mean follow-up period of 44.6 ± 4.0 months (range, 6–111). This result is comparable to other MGKRS series where local control rate was found to be 100%, if one extracts data separately for PAs. Kim et al. reported a local control rate of 95.4% but the only tumor relapsed was a craniopharyngioma (CPH). Median prescription dose was 20 Gy (range, 15–20) delivered in 3–4 sessions, with a mean follow-up of 29 months (range, 14–44) [21]. The same can be said for the study by the same group published in 2014: a local control rate of 94.6% was due to recurrence of two CPHs; in this case the median prescription dose was 20 Gy (range, 16–20) with a mean follow-up of 38.2 months (range, 6–81). Of note, these studies included only three and six PAs, respectively [22].
Among our 44 patients with a radiological follow-up, tumor volume was either stable (25%) or reduced (75%). Remarkably, reduction rate was significantly higher (96.7%) when we manually compared pre-treatment MRI with the last follow-up MRI. Although there is usually some margin of error in comparing thin-slice (1 mm) MRI sequences obtained for treatment plan with the standard ones submitted by the patients, choosing a cut-off variation of 10% from the baseline to diagnose a reduction in tumor volume should minimize false positive cases [24]. Of note, the minimum percentage of volume shrinkage was 18.7% and only 2 of 30 patients (6.7%) had values below 20%. These findings suggest that the overall 75% reduction rate reported in our series could be an underestimation because progressive changes of tumor volume in official radiological reports may be unrecognized. Nevertheless, the overall tumor shrinkage rate in our study is clearly higher than in other reports. No tumor size reduction was reported in the two patients with PA, one NFPA and one GH-secreting PA, 7.1 and 6.6 months after MGKRS, respectively, in the study of Nguyen and coworkers [23]. Given that our study found a mean reduction interval of 13.0 ± 2.3 months (range, 5–43 months), it is likely that the short follow-up precluded the possibility to observe tumor shrinkage. In the report by Jee and coworkers, 2 of 6 PAs (33.3%) decreased in size [22]. Of note, they considered significant a 20% variation from baseline; if this cut-off value was reduced to 10% (as in our study), the reduction rate would rise to 3 of 6 PAs (50%). The last study reported a decrease in tumor size in 2 of 3 (66.7%) PAs [21].
With regards to the Cyber Knife system (CK), Puataweepong and coworkers reported a tumor control rate of 97.5% in the treatment of 40 PAs with fractionated CK. The tumor reduction rate was 20% [25]. Using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [25], Iwata and coworkers in their series of 100 NFPAs with a median follow-up of 33 months (range, 18–118.5 months) reported one case (1%) of complete response (defined as disappearance of the target lesion), 29 cases (29%) with partial response (defined as a decrease of at least 30% in the sum of diameters of the target lesion), 65 cases (65%) with stable disease, while the remaining 5 patients (5%) had tumor recurrence, for an overall tumor control rate of 95% [15]. All of the studies regarding fractionated LINAC treatment for PAs reported a mean isodose line of around 80%. Unlike LINAC-based systems, in our study all patients were treated with a prescription isodose of 50%. This different setup allows GKRS to deliver a higher maximum dose and greater dose heterogeneity to the tumor volume with the same prescription dose compared to CK [26]. Nevertheless, further research is needed to investigate the biological differences between GKRS and LINAC-based systems, especially in the setting of fractionated treatments.
New-onset hypopituitarism during follow-up occurred between 16.7% and 25.0% of patients at risk, depending on the pituitary axis considered. These results compare favorably with those in the SRS and FSRS literature, even though the huge heterogeneity of the reported data (from 0% to 68%) must be underscored [12, 15, 22, 25, 27,28,29,30,31]. Several studies regarding FSRS did not report endocrine data [13, 14, 16, 21, 23]. However, hypopituitarism is a late effect of radiotherapy and may emerge several years after treatment, especially when lower radiation doses or fractionated therapy is used [3]. Some studies have documented up to an 80% incidence of newly arising hypopituitarism 10–15 years after conventional radiotherapy for sellar neoplasms [32, 33]. Therefore, a continuous follow-up of our patients is needed to fully evaluate the long-term risk of new-onset hypopituitarism after MGKRS.
RION is a major concern for irradiation of the sellar region. Tolerance dose for the optic pathway has recently been well established for single fraction SRS and multisession SRS, except for patients with prior radiotherapy [34]. In particular, Milano and coworkers recommend an optic apparatus maximum dose limit associated with a clinically reasonable RION risk of 10 Gy in one fraction, 20 Gy in three fractions, and 25 Gy in five fractions [34]. In our series, we used a median prescription dose per fraction of 7 Gy (range, 6.5–13 Gy). According to Milano and coworkers, whenever possible, we tried to keep the maximum dose to the optic pathway below 20 Gy in three fractions. We paid great attention to the maximum doses to the optic apparatus for patients who had previous radiation treatment. In both cases the dose to the optic pathway was below 17 Gy in three fractions.
Visual outcome in our patients was excellent. Only one patient (2.3%) presented a new-onset visual dysfunction of LON, but it is uncertain if it was due to MGKRS given the low radiation dose received by the optic apparatus.
Out of six PAs that underwent MGKRS in the study by Jee and coworkers, one patient experienced a mild deterioration of visual function but the radiation doses to the optic pathway were not reported [22]. Nevertheless, other studies using MGKRS reported no visual deterioration in patients treated for PA [16, 21, 23].
Interestingly, a study that included 100 patients with NFPA treated by fractionated CK reported only one case of visual deterioration, which was not clearly caused by radiation toxicity [15].
These data support the hypothesis that the α/β ratio of the optic nerves and chiasm is lower than that of PA [9]. If this were the case, fractionation schemes would be reasonable even on a biological basis. Unfortunately, there are no definite data about α/β ratio of PA and further research is needed to address this issue.
Limitations of this study include its retrospective nature, which exposes it to errors of bias and confounding, and the relatively short follow-up because some of the side effects of MGKRS may develop in the long-term only. Gamma Knife Icon model (frameless) was not available at our department when we started to treat recurrent and residual PAs with MGKRS. Our study results, therefore, arising from the only use of Leksell Gamma Knife Perfexion model. Extended follow-up times and prospective studies are thus needed to better characterize the virtues and limitations of MGKRS for PAs.
Conclusion
Our results in a large series of patients show that MGKRS is a very effective adjuvant treatment for recurrent or residual PA. We achieved tumor control in all cases and most patients experienced a significant reduction of tumor size. The safety profile of MGKRS is also very encouraging as we found only one case (2.3%) of possible RION despite the fact that patients selected for MGKRS are those with tumor rest very near or in contact with the optic pathway. New-onset hypopituitarism remains a concern of MGKRS, as well as of all other types of fractionated radiotherapy in the sellar region. Our study suggests that the main limitation of GKRS in patients with PA, i.e., a distance of the tumor margin from the optic pathway of at least 3 mm, can be overcome by MGKRS.
References
G. Minniti, D.C. Gilbert, M. Brada, Modern techniques for pituitary radiotherapy. Rev. Endocr. Metab. Disord. 10(2), 135–144 (2009). https://doi.org/10.1007/s11154-008-9106-0
J.P. Sheehan, A. Niranjan, J.M. Sheehan, J.A. Jane Jr., E.R. Laws, D. Kondziolka, J. Flickinger, A.M. Landolt, J.S. Loeffler, L.D. Lunsford, Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J. Neurosurg. 102(4), 678–691 (2005). https://doi.org/10.3171/jns.2005.102.4.0678
J.S. Loeffler, H.A. Shih, Radiation therapy in the management of pituitary adenomas. J. Clin. Endocrinol. Metab. 96(7), 1992–2003 (2011). https://doi.org/10.1210/jc.2011-0251
M. Losa, M. Valle, P. Mortini, A. Franzin, C.F. da Passano, M. Cenzato, S. Bianchi, P. Picozzi, M. Giovanelli, Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J. Neurosurg. 100(3), 438–444 (2004). https://doi.org/10.3171/jns.2004.100.3.0438
M. Losa, L. Gioia, P. Picozzi, A. Franzin, M. Valle, M. Giovanelli, P. Mortini, The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J. Clin. Endocrinol. Metab. 93(7), 2546–2552 (2008). https://doi.org/10.1210/jc.2008-0135
J.P. Sheehan, R.M. Starke, D. Mathieu, B. Young, P.K. Sneed, V.L. Chiang, J.Y. Lee, H. Kano, K.J. Park, A. Niranjan, D. Kondziolka, G.H. Barnett, S. Rush, J.G. Golfinos, L.D. Lunsford, Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J. Neurosurg. 119(2), 446–456 (2013). https://doi.org/10.3171/2013.3.JNS12766
B.E. Pollock, P.D. Brown, T.B. Nippoldt, W.F. Young Jr., Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery 62(6), 1271–1276 (2008). https://doi.org/10.1227/01.neu.0000333298.49436.0e. discussion 1276–1278
F. Castinetti, M. Nagai, I. Morange, H. Dufour, P. Caron, P. Chanson, C. Cortet-Rudelli, J.M. Kuhn, B. Conte-Devolx, J. Regis, T. Brue, Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J. Clin. Endocrinol. Metab. 94(9), 3400–3407 (2009). https://doi.org/10.1210/jc.2008-2772
C.A. Girkin, C.H. Comey, L.D. Lunsford, M.L. Goodman, L.B. Kline, Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology 104(10), 1634–1643 (1997). https://doi.org/10.1016/s0161-6420(97)30084-0
J.A. Leavitt, S.L. Stafford, M.J. Link, B.E. Pollock, Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 87(3), 524–527 (2013). https://doi.org/10.1016/j.ijrobp.2013.06.2047
P. Doroslovacki, M.A. Tamhankar, G.T. Liu, K.S. Shindler, G.S. Ying, M. Alonso-Basanta, Factors associated with occurrence of radiation-induced optic neuropathy at “safe” radiation dosage. Semin. Ophthalmol. 33(4), 581–588 (2018). https://doi.org/10.1080/08820538.2017.1346133
P. Puataweepong, M. Dhanachai, A. Hansasuta, S. Dangprasert, C. Sitathanee, R. Ruangkanchanasetr, P. Yongvithisatid, Clinical outcomes of perioptic tumors treated with hypofractionated stereotactic radiotherapy using CyberKnife(R) stereotactic radiosurgery. J. Neurooncol. 139(3), 679–688 (2018). https://doi.org/10.1007/s11060-018-2913-6
H.I. Liao, C.C. Wang, K.C. Wei, C.N. Chang, Y.H. Hsu, S.T. Lee, Y.C. Huang, H.C. Chen, P.W. Hsu, Fractionated stereotactic radiosurgery using the Novalis system for the management of pituitary adenomas close to the optic apparatus. J. Clin. Neurosci. 21(1), 111–115 (2014). https://doi.org/10.1016/j.jocn.2013.03.024
B.D. Killory, J.J. Kresl, S.D. Wait, F.A. Ponce, R. Porter, W.L. White, Hypofractionated CyberKnife radiosurgery for perichiasmatic pituitary adenomas: early results. Neurosurgery 64(2 Suppl.), A19–A25 (2009). https://doi.org/10.1227/01.NEU.0000341630.42160.18
H. Iwata, K. Sato, K. Tatewaki, N. Yokota, M. Inoue, Y. Baba, Y. Shibamoto, Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro. Oncol. 13(8), 916–922 (2011). https://doi.org/10.1093/neuonc/nor055
E. McTyre, C.A. Helis, M. Farris, L. Wilkins, D. Sloan, W.H. Hinson, J.D. Bourland, W.A. Dezarn, M.T. Munley, K. Watabe, F. Xing, A.W. Laxton, S.B. Tatter, M.D. Chan, Emerging indications for fractionated gamma knife radiosurgery. Neurosurgery 80(2), 210–216 (2017). https://doi.org/10.1227/NEU.0000000000001227
G. Minniti, J. Flickinger, B. Tolu, S. Paolini, Management of nonfunctioning pituitary tumors: radiotherapy. Pituitary 21(2), 154–161 (2018). https://doi.org/10.1007/s11102-018-0868-4
M. Losa, G. Spatola, L. Albano, A. Gandolfi, A. Del Vecchio, A. Bolognesi, P. Mortini, Frequency, pattern, and outcome of recurrences after gamma knife radiosurgery for pituitary adenomas. Endocrine 56(3), 595–602 (2017). https://doi.org/10.1007/s12020-016-1081-8
M. Torrens, C. Chung, H.T. Chung, P. Hanssens, D. Jaffray, A. Kemeny, D. Larson, M. Levivier, C. Lindquist, B. Lippitz, J. Novotny Jr., I. Paddick, D. Prasad, C.P. Yu, Standardization of terminology in stereotactic radiosurgery: Report from the Standardization Committee of the International Leksell Gamma Knife Society: special topic. J. Neurosurg. 121(Suppl.), 2–15 (2014). https://doi.org/10.3171/2014.7.GKS141199
G. Sicignano, M. Losa, A. del Vecchio, G.M. Cattaneo, P. Picozzi, A. Bolognesi, P. Mortini, R. Calandrino, Dosimetric factors associated with pituitary function after Gamma Knife Surgery (GKS) of pituitary adenomas. Radiother. Oncol. 104(1), 119–124 (2012). https://doi.org/10.1016/j.radonc.2012.03.021
J.W. Kim, Y.S. Im, D.H. Nam, K. Park, J.H. Kim, J.I. Lee, Preliminary report of multisession gamma knife radiosurgery for benign perioptic lesions: visual outcome in 22 patients. J. Korean Neurosurg. Soc. 44(2), 67–71 (2008). https://doi.org/10.3340/jkns.2008.44.2.67
T.K. Jee, H.J. Seol, Y.S. Im, D.S. Kong, D.H. Nam, K. Park, H.J. Shin, J.I. Lee, Fractionated gamma knife radiosurgery for benign perioptic tumors: outcomes of 38 patients in a single institute. Brain Tumor Res. Treat. 2(2), 56–61 (2014). https://doi.org/10.14791/btrt.2014.2.2.56
J.H. Nguyen, C.J. Chen, C.C. Lee, C.P. Yen, Z. Xu, D. Schlesinger, J.P. Sheehan, Multisession gamma knife radiosurgery: a preliminary experience with a noninvasive, relocatable frame. World Neurosurg. 82(6), 1256–1263 (2014). https://doi.org/10.1016/j.wneu.2014.07.042
J.W. Snell, J. Sheehan, M. Stroila, L. Steiner, Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Tech. Note J. Neurosurg. 104(1), 157–162 (2006). https://doi.org/10.3171/jns.2006.104.1.157
P. Puataweepong, M. Dhanachai, A. Hansasuta, S. Dangprasert, T. Swangsilpa, C. Sitathanee, C. Jiarpinitnun, P. Vitoonpanich, P. Yongvithisatid, The clinical outcome of hypofractionated stereotactic radiotherapy with cyberknife robotic radiosurgery for perioptic pituitary adenoma. Technol. Cancer Res. Treat. 15(6), NP10–NP15 (2016). https://doi.org/10.1177/1533034615607113
D. Kondziolka, S. Somaza, C. Comey, L.D. Lunsford, D. Claassen, S. Pandalai, A. Maitz, J.C. Flickinger, Radiosurgery and fractionated radiation therapy: comparison of different techniques in an in vivo rat glioma model. J. Neurosurg. 84(6), 1033–1038 (1996). https://doi.org/10.3171/jns.1996.84.6.1033
R.M. Starke, B.J. Williams, J.A. Jane Jr., J.P. Sheehan, Gamma Knife surgery for patients with nonfunctioning pituitary macroadenomas: predictors of tumor control, neurological deficits, and hypopituitarism. J. Neurosurg. 117(1), 129–135 (2012). https://doi.org/10.3171/2012.4.JNS112250
R. Attanasio, P. Epaminonda, E. Motti, E. Giugni, L. Ventrella, R. Cozzi, M. Farabola, P. Loli, P. Beck-Peccoz, M. Arosio, Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J. Clin. Endocrinol. Metab. 88(7), 3105–3112 (2003). https://doi.org/10.1210/jc.2002-021663
G.C. Feigl, C.M. Bonelli, A. Berghold, M. Mokry, Effects of gamma knife radiosurgery of pituitary adenomas on pituitary function. J. Neurosurg. 97(5 Suppl.), 415–421 (2002). https://doi.org/10.3171/jns.2002.97.supplement
C. Hoybye, E. Grenback, T. Rahn, M. Degerblad, M. Thoren, A.L. Hulting, Adrenocorticotropic hormone-producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery 49(2), 284–291 (2001). discussion 291–282
K.J. Park, H. Kano, P.V. Parry, A. Niranjan, J.C. Flickinger, L.D. Lunsford, D. Kondziolka, Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 69(6), 1188–1199 (2011). https://doi.org/10.1227/NEU.0b013e318222afed
A.C. van den Bergh, G. van den Berg, M.A. Schoorl, W.J. Sluiter, A.M. van der Vliet, E.W. Hoving, B.G. Szabo, J.A. Langendijk, B.H. Wolffenbuttel, R.P. Dullaart, Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int. J. Radiat. Oncol. Biol. Phys. 67(3), 863–869 (2007). https://doi.org/10.1016/j.ijrobp.2006.09.049
G. Minniti, M. Osti, M.L. Jaffrain-Rea, V. Esposito, G. Cantore, R. Maurizi Enrici, Long-term follow-up results of postoperative radiation therapy for Cushing’s disease. J. Neurooncol. 84(1), 79–84 (2007). https://doi.org/10.1007/s11060-007-9344-0
M.T. Milano, J. Grimm, S.G. Soltys, E. Yorke, V. Moiseenko, W.A. Tome, A. Sahgal, J. Xue, L. Ma, T.D. Solberg, J.P. Kirkpatrick, L.S. Constine, J.C. Flickinger, L.B. Marks, I. El Naqa, Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int. J. Radiat. Oncol. Biol. Phys. (2018). https://doi.org/10.1016/j.ijrobp.2018.01.053
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Standard informed consent was obtained from each patient undergoing gamma knife treatment.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albano, L., Losa, M., Nadin, F. et al. Safety and efficacy of multisession gamma knife radiosurgery for residual or recurrent pituitary adenomas. Endocrine 64, 639–647 (2019). https://doi.org/10.1007/s12020-019-01876-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01876-2