Abstract
Purpose
Up to 30% of patients with glioblastoma (GBM) develop venous thromboembolism (VTE) over the course of the disease. Although not as high, the risk for VTE is also increased in patients with meningioma. Direct measurement of peak thrombin generation (TG) allows quantitative assessment of systemic coagulation activation in patients with GBM and meningioma. Our aim was to determine the extent of systemic coagulation activation induced by brain tumors, to measure the shift between pre- and post-operative peak TG in patients with GBM, and to assess the relationship between pre-surgical peak TG and pre-operative brain tumor volume on imaging.
Methods
Pre- and post-surgical plasma samples were obtained from successive patients with GBM and once from patients with meningioma and healthy age- and sex-matched blood donor controls. TG was measured using the calibrated automated thrombogram (CAT) assay, and tumor volumes were measured in pre-surgical MRI scans.
Results
Pre-surgical peak TG was higher in patients with GBM than in controls (288.6 ± 54.1 nM vs 187.1 ± 41.7 nM, respectively, P < 0.001), and, in the nine patients with GBM and paired data available, peak TG was significantly reduced after surgery (323 ± 38 nM vs 265 ± 52 nM, respectively, P = 0.007). Similarly, subjects with meningioma demonstrated higher peak TG compared to controls (242.2 ± 54.9 nM vs 177.7 ± 57.0 nM, respectively, P < 0.001). There was no association between peak TG and pre-operative tumor volume or overall survival.
Conclusion
Our results indicate that systemic coagulation activation occurs with both meningioma and GBM, but to a greater degree in the latter. Preoperative peak TG did not correlate with tumor volume, but removal of GBM caused a significant decrease in coagulation activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cancer are at increased risk for venous thromboembolism (VTE), and up to 30% of patients with malignant brain tumors, especially glioblastoma (GBM), may suffer a VTE event over the course of the disease [1,2,3,4,5,6,7]. While the incidence of VTE is lower in patients with meningioma than in patients with gliomas, it is nevertheless increased over the general population, particularly in the peri-operative period [8,9,10,11,12,13]. To date, however, information about activation of systemic coagulation in patients with meningioma has not been reported. While thromboprophylaxis effectively reduces VTE risk in cancer patients [14], this benefit occurs at the cost of an increased risk for bleeding, which may be catastrophic in patients with brain tumors [15, 16]. Therefore, identifying patients at highest risk for VTE is necessary to improve the therapeutic ratio of thromboprophylaxis [17, 18].
The prothrombotic state in cancer patients arises via numerous mechanisms [2, 19,20,21,22], and the ability to assess the net prothrombotic state by measuring blood biomarkers of coagulation activation (e.g., D-dimers, P-selectin) has allowed further refinement of VTE clinical prediction models to help stratify cancer patients into high and low VTE risk groups [23,24,25,26,27,28,29]. The Vienna Cancer and Thrombosis Study (CATS) identified peak plasma thrombin generation (TG) as a novel and reliable biomarker to predict VTE risk in cancer patients in general [30], but there is scant information about its value specifically in patients with brain tumors. One study measured peak TG in patients with high-grade gliomas after surgery and showed that TG was not an independent predictor of VTE [31]. In another study, patients with malignant brain tumors before surgery had higher levels of peak TG than healthy controls, but this was not confirmed in a follow up study [32, 33].
We undertook this study to characterize coagulation activation by measuring peak TG in patients with GBM and patients with meningioma before surgery. We hypothesized that both groups would have higher peak plasma TG compared to age- and sex-matched healthy donors. We further sought to assess the effect of tumor debulking on coagulation activation by measuring peak TG before and after surgical resection of brain tumors. Finally, we evaluated if brain tumor volume measured on pre-operative magnetic resonance imaging (MRI) correlated with systemic coagulation activation as assessed by plasma peak TG.
Materials and methods
Subjects
This study was approved by the Committee for the Protection of Human Subjects (CPHS) of Dartmouth College. Patients aged 18 years and above with MRI findings suggestive of a new GBM or meningioma and for whom surgical resection was planned were eligible. Patients with an additional active malignancy, history of unprovoked VTE at any time or provoked VTE within 5 years of entry, current treatment with an anticoagulant for any reason within the last 90 days or a surgical procedure other than a skin biopsy within the previous 30 days were excluded.
The research staff identified potentially eligible subjects after review of the neurosurgical operating room schedule. Medical records were reviewed for exclusion criteria, and eligible subjects were offered participation in the study and provided with a copy of the CPHS-approved informed consent form to review prior to surgery. Only subjects who signed the informed consent form were enrolled. Healthy control subjects were recruited from the Dartmouth-Hitchcock Medical Center Blood Donor Program at the time of a voluntary blood donation and provided separate informed consent for the collection of an additional 10 mL blood sample for this study.
Blood collection/thrombin generation
After informed consent was obtained, a total of 20 mL of blood was collected from study subjects (10 mL from control subjects) in 3.2% sodium citrate blood collection tubes prior to surgery at the time of a regularly scheduled venipuncture. Blood was immediately centrifuged at 4000×g for 10 min to prepare platelet poor plasma and divided into aliquots and cryopreserved at − 80 °C until analysis. An additional 20 mL of blood was collected from subjects with GBM 3 to 6 weeks after surgery at the time of a regularly scheduled follow up appointment and processed as previously described.
Thrombin generation was measured using the Calibrated Automated Thrombogram (CAT) assay (Thrombinoscope BV, Maastricht, Netherlands) according to the manufacturer’s instructions. In brief, eighty microliters of each plasma specimen was pipetted into each of six wells in a 96-well microliter plate. Twenty microliters of a mixture containing tissue factor (1 pM final concentration) and phospholipids (4 µM final concentration) was added to the first three wells, and 20 µL of thrombin calibrator (600 nM thrombin activity) was added to the remaining three wells for each plasma sample. Twenty microliters of a mixture containing a low-affinity fluorogenic substrate (Z-Gly-Gly-Arg-AMC) for thrombin (0.5 mM final concentration) and calcium chloride (20 mM final concentration) was added to each well, and fluorescence intensity was detected using the Fluoroscan Ascent (ThermoLabsystems, Helsinki, Finland) at an excitation wavelength of 390 nMand an emission wave length of 460 nM every 20 s over 60 min. Raw data were converted to thrombin activity over time and peak thrombin levels were calculated using Thrombinoscope software (Thrombinoscope BV, Maastricht, Netherlands). Measurements were performed in triplicate for each specimen and a commercially prepared, pooled normal plasma control (Precision BioLogic, Dartmouth, NS, Canada) was included in each run.
Tumor volumes
Tumor volumes were measured using the highest resolution available gadolinium-enhanced pre-operative MRI scan. The outer boundary of the enhancing tumor region was manually segmented by one of two trained segmenters using ITK-SNAP 3.6.0. A board-certified neuroradiologist reviewed and adjusted the segmentation boundaries as necessary to yield a final total tumor segmentation that included the enhancing tissue and any necrotic tissue contained inside the enhancing area. Next, using a coregistered T2-weighted scan for reference where available, necrotic voxels were segmented on the GBM scans, reviewed by the neuroradiologist, and subtracted from the total tumor region to yield a segmentation for non-necrotic tumor. The total and non-necrotic tumor volumes were calculated as the area of segmented voxels by slice thickness. Voxel volumes ranged from 0.39 to 4.64 cubic mm across scans depending on the resolution of the T1-contrast image.
Statistics
Each subject was assigned a unique patient number (UPN) and identifying data were removed.
Based on results from a previous study of circulating markers of coagulation activation in GBM [23] in which the results were normally distributed, we used t-tests to perform patient versus control comparisons of TG values for the GBM and meningioma groups separately. A paired t-test was used to evaluate the difference between pre- and post-surgical peak TG in patients with GBM. Further statistical analysis was performed in R using two linear regression models (separately for GBM and meningioma) with TG as the dependent variable and total pre-operative tumor volume as the independent variable with age, sex, and self-reported body weight at the time of scan as additional model terms. We also ran these models for pre-operative non-necrotic tumor volumes where available. There were too few patients with post-operative data to assess the relationship between tumor volume and TG post-operatively. We aimed to enroll 30 patients with GBM, 30 patients with meningioma and 60 healthy control subjects to detect a true difference in peak TG between experimental and control subjects of 20% with a power of 80% and a 2-sided type I error of 0.05.
Subject follow up
The electronic medical records were reviewed monthly after surgery to monitor for the development of VTE and for overall survival.
Results
Fifty-eight patients with newly diagnosed brain tumors consented to participate in the study. Fifteen subjects were excluded after final pathology review, leaving 20 with GBM and 23 with meningiomas for inclusion. The reason for exclusion in all cases was a final pathologic diagnosis inconsistent with either GBM or meningioma. Blood from one age and sex-matched healthy donor was obtained for each study subject during the time of a voluntary blood donation at the Dartmouth-Hitchcock Blood Donor Center.
Characteristics of the study participants are summarized in Table 1. The GBM cohort consisted of more males than females while the meningioma group was comprised predominantly of females. There were more former and current smokers than nonsmokers in the GBM group; however, the difference did not reach statistical significance.
The mean peak pre-operative TG was 288.6 ± 54.1 nM in the GBM cohort and 187.1 ± 41.7 nM in the GBM controls, a difference of 35.2% (P < 0.001). Within the meningioma group there were 18 WHO grade I and five grade II tumors, and peak TG was similar for both groups [244.8 ± 53.3 nM,vs 232.5 ± 66.1 nM, respectively (P = 0.667)]. Grade I and grade II meningiomas were, therefore, analyzed as a group. Peak TG was higher for the GBM group compared to the meningioma group by 16.1% (P = 0.009); however, the meningioma cohort demonstrated a 26.6% higher TG compared to controls (242.2 ± 54.9 nM vs 177.7 nM; P < 0.001). Comparisons between groups are depicted in Fig. 1.
Table 2 shows the total tumor volumes for the 19 patients with GBM and 21 patients with meningiomas who had evaluable in-house pre-operative scans. As expected, most of those with meningiomas had no necrotic components. The models correlating tumor volume and mean peak pre-operative TG did not show a statistically significant association between tumor volume and peak TG in GBM (6.8 × 10–4 mA/mm3, P = 0.32) or in meningioma (− 3.0 × 10–4 mA/mm3, P = 0.55).
To assess for the possibility that tumor volume effect was obscured by the inclusion of both viable and non-viable tumor tissue in our tumor segmentations, we also performed a follow up analysis on the subset of subjects for whom a T2-weighted image existed. Based on a second segmentation guided by simultaneously viewing the original T1-contrast scan and the T2-weighted image (reviewed and adjusted as necessary by a board-certified neuroradiologist) the non-viable areas of tumor were identified. This restriction reduced the number of available cases to 11 GBM and 18 meningiomas and again resulted in no significant effect of non-necrotic tumor volume on peak TG (GBM: − 4.5 × 10–4 mA/mm3, P = 0.78; meningioma: − 5.5 × 10–4 mA/mm3, P = 0.40).
Nine post-operative GBM blood samples (at 3–6 weeks after surgery) were available for analysis of TG. The most common reasons for failure to collect a post-operative blood specimen were transfer of care to a local hospital or to a hospice setting and absence of a concurrent clinical indication for a blood draw at the follow up office appointment. In the group with available post-operative blood specimens, the mean peak post-operative TG was 18% lower than the mean peak pre-operative TG (265 ± 52 nM vs 323 ± 38 nM, respectively, P = 0.007) (Fig. 2A) and the post-operative TG was lower than the pre-surgical TG in all nine cases (Fig. 2B). The study as originally configured also sought to assess post-surgical TG in meningioma patients, but this proved not feasible due to variations in local follow up protocols and the lack of an indication for post-operative blood sampling upon which to add study tests.
Pre/post-operative comparison of TG in GBM subjects (N = 9). Peak thrombin generation was assessed 3 to 6 weeks post-operatively in GBM patients. Mean peak thrombin generation was decreased post-operatively compared to pre-operative levels (A) and was lower post-operatively in each individual subject (B)
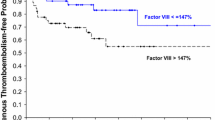
Study subjects were followed prospectively for the occurrence of thrombotic events and survival. Two patients in the GBM group developed symptomatic VTE with a mean peak TG of 364 nM compared to 290 nM for the remaining 18 patients without VTE (P = 0.04). The median overall survival for the 19 patients with GBM with complete outcome data available was 12 months. We identified no consistent relationship between pre-operative peak TG and overall survival.
Discussion
Coagulation activation leading to thrombin generation is increased in cancer via several mechanisms, including over expression of tissue factor (TF) by tumor cells and the presence of increased circulating TF-containing microparticles (TF-MP) [20, 22, 34,35,36]. Until recently, the consequence of increased TF-MP, namely, thrombin generation (TG), could be assayed in blood only indirectly by measuring surrogate biomarkers such as prothrombin fragment F1 + 2, the peptide released when coagulation factor Xa cleaves prothrombin to the active form thrombin, thrombin-antithrombin complexes and D-dimers [37]. With the advent of calibrated automated thrombogram technology, however, [38, 39] TG can be measured directly to provide a global view of the coagulation dynamic in cancer patients. Using this methodology, our study revealed that patients with GBM and meningioma have significantly higher TG in comparison to age and sex matched healthy controls. Moreover, surgical resection of GBM resulted in a significant reduction in peak TG although still higher than in healthy controls. The pre-operative peak TG did not correlate with tumor volume nor with survival in patients with GBM.
Tissue factor-driven coagulation activation is well described in malignant brain tumors, and may be directed by oncogenic mutations in genes that are otherwise not directly involved in coagulation activation such as EGFR and IDH1 [40,41,42,43,44,45,46,47]. For example, a study of 61 patients with GBM demonstrated increased concentration of circulating TF-MP compared to age- and sex-matched healthy controls [48]. This prospective study further demonstrated that the concentration of TF-MP in GBM patients decreased significantly after tumor resection, and there appeared to be trend toward an increase in TF-MP levels at the time of tumor progression. Eleven subjects in this study developed VTE, of whom 64% showed elevated TF-MP levels. These findings suggest that tumor activity as well as VTE risk in patients with malignant brain tumors may be correlated with circulating TF-MP, though to date, TF associated specifically with the primary tumors has not shown a similar association with VTE risk [49]. By measuring TG, we demonstrated significant pre-operative coagulation activation in patients with GBM and meningioma.
In our study, the peak TG, a measure of maximal thrombin production at a given time point, was significantly higher in patients with GBM compared to healthy donors. These results are in line with the findings of others, albeit under different study conditions. In a previously reported study, TG, assayed in fresh whole blood, was 20% higher in patients with GBM prior to treatment compared to age- and sex-matched healthy controls [32]. No association between TG and progression-free or overall survival was seen, and an association between TG and VTE risk was not investigated. The confounding aspect of this study is that TG was evaluated in whole blood rather than plasma as we have done, thus introducing inter-subject variability in red blood cell and platelet counts for which the analysis did not account. A subsequent study by the same investigators [33] assayed TG in plasma and, while they did not demonstrate a significant difference between GBM patients and healthy controls, they did show a decrease in TG after tumor debulking surgery similar to our findings.
Two of the 20 patients with GBM included in our study developed symptomatic venous thromboembolism. The mean peak pre-operative TG in these two subjects was significantly higher than in GBM subjects without VTE. Though this is a small sample size, our results are provocative in suggesting that pre-operative TG may be a useful biomarker for systemic coagulation activation, whereas post-operative TG does not seem to be [49]. This hypothesis warrants evaluation in future studies.
While it is known that patients with meningioma are at increased risk for VTE [8,9,10,11,12,13], to our knowledge, we are the first to report direct evidence for coagulation activation in these patients. We demonstrated that pre-operative peak TG was significantly higher in patients with meningioma compared to age- and sex-matched healthy controls, suggesting that systemic coagulation activation indeed occurs in association with these intracranial tumors. Compared to patients with GBM, however, patients with meningioma demonstrated lower peak TG, suggesting that systemic coagulation activation occurs to a greater degree in patients with intra-axial malignant gliomas compared to extra-axial less aggressive meningioma; likely reflecting differential regulation of tumor-associated procoagulants and anticoagulants by malignant and benign tumors [20, 42, 50].
Contrast-enhancing tumor volume appears to be a predictor of overall survival in patients with GBM, independent of age or treatment [51]. However, we were unable to confirm a correlation between pre-operative contrast enhancing tumor volume and pre-operative peak TG for either GBM or meningioma. While we cannot exclude the possibility that we missed such an association due to the small sample size, this finding may also reflect the molecular biologic heterogeneity of both malignant and benign tumors with respect to regulation of coagulation pathway genes and expression of procoagulant molecules that cannot be discerned radiographically. In fact, small, radiographically occult malignancies have often been observed to generate disproportionately large amounts of procoagulant activity resulting in systemic coagulation activation and thrombosis [52].
Our study, while yielding novel observations, has several shortcomings. First, the primary endpoint of the study was pre-operative peak TG, thus we enrolled potential subjects based on radiologic findings before a tissue diagnosis was confirmed. This led to exclusion of 26% of patients with non-GBM or non-meningioma diagnoses after surgery and a smaller than the intended sample size of 30 patients per group. Additionally, the validity of pre- and post-surgical TG comparisons is weak due to substantial drop out of GBM subjects after surgery. We were unable to perform pre- and post-surgical TG comparisons in patients with meningioma because these patients did not routinely have post-operative blood drawn as part of standard of care. As only two GBM subjects (10% of the entire cohort) developed VTE, evaluation of an association between pre-operative TG and VTE risk was not statistically reliable. Nevertheless, our finding that pre-operative peak TG in the participants who subsequently developed VTE was higher than the mean pre-operative peak TG in subjects who remained VTE-free warrants further investigation.
Thrombin generation has been studied as a predictive variable for VTE risk in patients with cancer in several studies [30,31,32,33]. TG is an attractive parameter for VTE prediction because it is a global measure of coagulation activation that provides summative information about the net status of the coagulation system, combining information about both procoagulant and anticoagulant forces. Several TG assays are commercially available, and though results within a single laboratory are generally consistent, there is substantial intra-laboratory variability, and comparisons among studies is difficult due to differences in reagents, methods and instrumentation. Moreover, the lack of a clinically validated, automated, high throughput assay system limits the clinical use of TG as a predictive marker presently.
In summary, despite the limitations of our study, we made some important observations regarding systemic coagulation activation in patients with brain tumors. We confirmed that systemic coagulation activation, as assessed by peak pre-operative TG, occurs in patients with GBM, and we demonstrated the role of the primary tumor in driving coagulation activation by showing a decrease in TG after surgical debulking. Additionally, we provide evidence that patients with meningioma also have systemic coagulation activation as demonstrated by elevated pre-operative TG compared to healthy donors. Finally, our results suggest that pre-operative TG warrants further investigation as a potential biomarker to predict the risk of VTE in patients with meningioma and GBM.
References
Czap AL, Becker A, Wen PY (2019) Thrombotic Complications in Gliomas. Semin Thromb Hemost 45:326–333. https://doi.org/10.1055/s-0039-1687892
Falanga A, Russo L, Milesi V (2014) The coagulopathy of cancer. Curr Opin Hematol 21:423–429. https://doi.org/10.1097/moh.0000000000000072
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 89:640–646
Muster V, Gary T (2020) Incidence, therapy, and bleeding risk-cancer- associated thrombosis in patients with glioblastoma. Cancers. https://doi.org/10.3390/cancers12061354
Rinaldo L, Brown DA, Bhargav AG, Rusheen AE, Naylor RM, Gilder HE, Monie DD, Youssef SJ, Parney IF (2019) Venous thromboembolic events in patients undergoing craniotomy for tumor resection: incidence, predictors, and review of literature. J Neurosurg 132:10–21. https://doi.org/10.3171/2018.7.Jns181175
Semrad TJ, O’Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH (2007) Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 106:601–608. https://doi.org/10.3171/jns.2007.106.4.601
Streiff MB (2016) Thrombosis in the setting of cancer. Hematol Am Soc Hematol Educ Program 2016:196–205. https://doi.org/10.1182/asheducation-2016.1.196
Cage TA, Lamborn KR, Ware ML, Frankfurt A, Chakalian L, Berger MS, McDermott MW (2009) Adjuvant enoxaparin therapy may decrease the incidence of postoperative thrombotic events though does not increase the incidence of postoperative intracranial hemorrhage in patients with meningiomas. J Neurooncol 93:151–156. https://doi.org/10.1007/s11060-009-9886-4
Carrabba G, Riva M, Conte V, Di Cristofori A, Caroli M, Locatelli M, Castellani M, Bucciarelli P, Artoni A, Stocchetti N, Martinelli I, Rampini P (2018) Risk of post-operative venous thromboembolism in patients with meningioma. J Neurooncol 138:401–406. https://doi.org/10.1007/s11060-018-2810-z
Eisenring CV, Neidert MC, Sabanes Bove D, Held L, Sarnthein J, Krayenbuhl N (2013) Reduction of thromboembolic events in meningioma surgery: a cohort study of 724 consecutive patients. PLoS ONE 8:e79170. https://doi.org/10.1371/journal.pone.0079170
Fluss R, Kobets AJ, Inocencio JF, Hamad M, Feigen C, Altschul DJ, Lasala P (2021) The incidence of venous thromboembolism following surgical resection of intracranial and intraspinal meningioma: a systematic review and retrospective study. Clin Neurol Neurosurg 201:106460. https://doi.org/10.1016/j.clineuro.2020.106460
Hoefnagel D, Kwee LE, van Putten EH, Kros JM, Dirven CM, Dammers R (2014) The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma: a retrospective study of a large single center patient cohort. Clin Neurol Neurosurg 123:150–154. https://doi.org/10.1016/j.clineuro.2014.06.001
Sughrue ME, Rutkowski MJ, Shangari G, Chang HQ, Parsa AT, Berger MS, McDermott MW (2011) Risk factors for the development of serious medical complications after resection of meningiomas. Clin Article J Neurosurg 114:697–704. https://doi.org/10.3171/2010.6.Jns091974
Di Nisio M, Porreca E, Otten HM, Rutjes AW (2014) Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008500.pub3
Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI (2017) Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood 129:3379–3385. https://doi.org/10.1182/blood-2017-02-767285
Riedl J, Ay C (2019) Venous thromboembolism in brain tumors: risk factors, molecular mechanisms, and clinical challenges. Semin Thromb Hemost 45:334–341. https://doi.org/10.1055/s-0039-1688493
Becattini C, Di Nisio M, Franco L, Lee A, Agnelli G, Mandalà M (2021) Treatment of venous thromboembolism in cancer patients: the dark side of the moon. Cancer Treat Rev 96:102190. https://doi.org/10.1016/j.ctrv.2021.102190
Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P (2021) American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 5:927–974. https://doi.org/10.1182/bloodadvances.2020003442
Dicke C, Langer F (2015) Pathophysiology of Trousseau’s syndrome. Hamostaseologie 35:52–59. https://doi.org/10.5482/hamo-14-08-0037
Donati MB, Lorenzet R (2012) Thrombosis and cancer: 40 years of research. Thromb Res 129:348–352. https://doi.org/10.1016/j.thromres.2011.12.022
Geddings JE, Mackman N (2013) Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 122:1873–1880. https://doi.org/10.1182/blood-2013-04-460139
Van Dreden P, Epsilonlalamy I, Gerotziafas GT (2017) The role of tissue factor in cancer-related hypercoagulability, tumor growth, angiogenesis and metastasis and future therapeutic strategies. Crit Rev Oncog 22:219–248. https://doi.org/10.1615/CritRevOncog.2018024859
Blix K, Gran OV, Severinsen MT, Cannegieter SC, Jensvoll H, Overvad K, Hammerstrom J, Tjonneland A, Naess IA, Braekkan SK, Rosendaal FR, Kristensen SR, Hansen JB (2018) Impact of time since diagnosis and mortality rate on cancer-associated venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) cohort. J Thromb Haemost. https://doi.org/10.1111/jth.14130
Khorana AA, Francis CW (2018) Risk prediction of cancer-associated thrombosis: appraising the first decade and developing the future. Thromb Res 164(Suppl 1):S70-s76. https://doi.org/10.1016/j.thromres.2018.01.036
Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902–4907. https://doi.org/10.1182/blood-2007-10-116327
Konigsbrugge O, Pabinger I, Ay C (2014) Risk factors for venous thromboembolism in cancer: novel findings from the Vienna Cancer and Thrombosis Study (CATS). Thromb Res 133(Suppl 2):S39-43. https://doi.org/10.1016/s0049-3848(14)50007-2
Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, Di Nisio M, Cesarman-Maus G, Kraaijpoel N, Zielinski CC, Buller HR, Ay C (2018) A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 5:e289–e298. https://doi.org/10.1016/s2352-3026(18)30063-2
Patell R, Rybicki L, McCrae KR, Khorana AA (2017) Predicting risk of venous thromboembolism in hospitalized cancer patients: Utility of a risk assessment tool. Am J Hematol 92:501–507. https://doi.org/10.1002/ajh.24700
Shi S, Cheng J, Chen H, Zhang Y, Zhao Y, Wang B (2020) Preoperative and intraoperative predictors of deep venous thrombosis in adult patients undergoing craniotomy for brain tumors: a Chinese single-center, retrospective study. Thromb Res 196:245–250. https://doi.org/10.1016/j.thromres.2020.09.005
Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, Zielinski C, Pabinger I (2011) Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 29:2099–2103. https://doi.org/10.1200/jco.2010.32.8294
Thaler J, Ay C, Kaider A, Reitter EM, Haselbock J, Mannhalter C, Zielinski C, Marosi C, Pabinger I (2014) Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high-grade gliomas. Neuro Oncol 16:1645–1651. https://doi.org/10.1093/neuonc/nou106
Reynes G, Vila V, Martin M, Parada A, Fleitas T, Reganon E, Martinez-Sales V (2011) Circulating markers of angiogenesis, inflammation, and coagulation in patients with glioblastoma. J Neurooncol 102:35–41. https://doi.org/10.1007/s11060-010-0290-x
Reynes G, Vila V, Fleitas T, Reganon E, Font de Mora J, Jorda M, Martinez-Sales V (2013) Circulating endothelial cells and procoagulant microparticles in patients with glioblastoma: prognostic value. PLoS ONE 8:e69034. https://doi.org/10.1371/journal.pone.0069034
Macey MG, Enniks N, Bevan S (2011) Flow cytometric analysis of microparticle phenotype and their role in thrombin generation. Cytometry B 80:57–63. https://doi.org/10.1002/cyto.b.20551
Passamonti SM, Artoni A, Carrabba G, Merati G, Abbattista M, Capecchi M, Castellani M, Marenghi C, Trombetta E, Giammattei L, Caroli M, Bucciarelli P, Scalambrino E, Peyvandi F, Martinelli I (2021) Plasma levels of extracellular vesicles and the risk of post-operative pulmonary embolism in patients with primary brain tumors: a prospective study. J Thromb Thrombolys. https://doi.org/10.1007/s11239-021-02441-3
Ruf W, Mueller BM (2006) Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost 32(Suppl 1):61–68. https://doi.org/10.1055/s-2006-939555
Ay C, Pabinger I (2010) Tests predictive of thrombosis in cancer. Thromb Res 125(Suppl 2):S12-15. https://doi.org/10.1016/s0049-3848(10)70005-0
Debaugnies F, Azerad MA, Noubouossie D, Rozen L, Hemker HC, Corazza F, Efira A, Demulder A (2010) Evaluation of the procoagulant activity in the plasma of cancer patients using a thrombin generation assay. Thromb Res 126:531–535. https://doi.org/10.1016/j.thromres.2010.09.002
Hemker HC, Giesen PL, Ramjee M, Wagenvoord R, Beguin S (2000) The thrombogram: monitoring thrombin generation in platelet-rich plasma. Thromb Haemost 83:589–591
Fadul CE, Zacharski LR (2005) Coagulation biology in glioma pathogenesis: a missing link? J Thromb Haemost 3:1915–1916. https://doi.org/10.1111/j.1538-7836.2005.01511.x
Haciyakupoğlu E, Yilmaz DM, Walter J, Erdoğan Ş, Haciyakupoğlu S, Kuhn SA (2018) Immunohistochemical evaluation of hemostatic changes in glioblastoma multiforme and low-grade astrocytoma. Turk Neurosurg. https://doi.org/10.5137/1019-5149.Jtn.22739-18.3
Magnus N, D’Asti E, Garnier D, Meehan B, Rak J (2013) Brain neoplasms and coagulation. Semin Thromb Hemost 39:881–895. https://doi.org/10.1055/s-0033-1357483
Navone SE, Guarnaccia L, Locatelli M, Rampini P, Caroli M, La Verde N, Gaudino C, Bettinardi N, Riboni L, Marfia G, Campanella R (2019) Significance and prognostic value of the coagulation profile in patients with glioblastoma: implications for personalized therapy. World Neurosurg 121:e621–e629. https://doi.org/10.1016/j.wneu.2018.09.177
Ornstein DL, Meehan KR, Zacharski LR (2002) The coagulation system as a target for the treatment of human gliomas. Semin Thromb Hemost 28:19–28. https://doi.org/10.1055/s-2002-20561
Sartori MT, Della Puppa A, Ballin A, Campello E, Radu CM, Saggiorato G, d’Avella D, Scienza R, Cella G, Simioni P (2013) Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role. Thromb Haemost 110:378–385. https://doi.org/10.1160/th12-12-0957
Wojtukiewicz MZ, Mysliwiec M, Matuszewska E, Sulkowski S, Zimnoch L, Politynska B, Wojtukiewicz AM, Tucker SC, Honn KV (2021) Imbalance in coagulation/fibrinolysis inhibitors resulting in extravascular thrombin generation in gliomas of varying levels of malignancy. Biomolecules. https://doi.org/10.3390/biom11050663
Tawil N, Spinelli C, Bassawon R, Rak J (2020) Genetic and epigenetic regulation of cancer coagulome: lessons from heterogeneity of cancer cell populations. Thromb Res 191(Suppl 1):S99-s105. https://doi.org/10.1016/s0049-3848(20)30405-9
Sartori MT, Della Puppa A, Ballin A, Saggiorato G, Bernardi D, Padoan A, Scienza R, d’Avella D, Cella G (2011) Prothrombotic state in glioblastoma multiforme: an evaluation of the procoagulant activity of circulating microparticles. Journal Neurooncol 104:225–231. https://doi.org/10.1007/s11060-010-0462-8
Thaler J, Preusser M, Ay C, Kaider A, Marosi C, Zielinski C, Pabinger I, Hainfellner JA (2013) Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res 131:162–165. https://doi.org/10.1016/j.thromres.2012.09.020
Falanga A, Russo L, Milesi V, Vignoli A (2017) Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol 118:79–83. https://doi.org/10.1016/j.critrevonc.2017.08.003
Ellingson BM, Harris RJ, Woodworth DC, Leu K, Zaw O, Mason WP, Sahebjam S, Abrey LE, Aftab DT, Schwab GM, Hessel C, Lai A, Nghiemphu PL, Pope WB, Wen PY, Cloughesy TF (2017) Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol 19:89–98. https://doi.org/10.1093/neuonc/now187
Ornstein DL (2008) A nickel’s worth of cancer. Ann Intern Med 149:350–352. https://doi.org/10.7326/0003-4819-149-5-200809020-00010
Acknowledgements
This study was supported by grants from the Northern New England Clinical Oncology Society (NNECOS), the Brain Tumor Research Fund at Dartmouth-Hitchcock Medical Center, and by the Imaging Sciences Group of the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the funding agencies.
Funding
This study was supported by grants from the Northern New England Clinical Oncology Society (NNECOS), the Brain Tumor Research Fund at Dartmouth-Hitchcock Medical Center, and by the Imaging Sciences Group of the Dartmouth Clinical and Translational Science Institute, under Award Number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
BLG, CEF and DLO conceived, designed and conducted the study and analyzed thrombin generation data. SY enrolled subjects, collected and analyzed study data. SY, HAW, CEF and DLO provided critical writing of the manuscript. JCF, SRA, SJG, MP and HAW performed tumor volume analyses. MSE contributed to design of the study and provided laboratory support for specimen collection and processing. All authors have reviewed, revised and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Camilo E. Fadul MD is a member of the Editorial Board of the Journal of Neuro-Oncology. Otherwise, the authors declare they have no relevant conflicts of interest or competing interests.
Ethical approval
This study was approved by the Committee for the Protection of Human Subjects (CPHS) of Dartmouth College (study #D12096).
Consent to participate
All study subjects provided written informed consent for participation in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yerrabothala, S., Gourley, B.L., Ford, J.C. et al. Systemic coagulation is activated in patients with meningioma and glioblastoma. J Neurooncol 155, 173–180 (2021). https://doi.org/10.1007/s11060-021-03865-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03865-w