Abstract
The relationship between venous thromboembolism (VTE) and cancer is supported by several pathogenetic factors, including circulating microparticles (MP) originating from different cells and often bearing tissue factor. Since VTE often complicates the clinical course of patients with glioblastoma multiforme (GBM; WHO grade IV astrocytoma) and the role of MPs in these patients population is still not clear, this prospective study was conducted to evaluate the procoagulant activity of circulating MP (MP activity) in GBM patients. We enrolled 61 GBM patients undergoing gross-total or subtotal surgical resection followed by combined radio-chemotherapy; 20 healthy volunteers were tested as controls. Blood samples for MP activity and hemostatic profiles were obtained before and then 1 week and 1, 4, and 7 months after surgery. GBM patients had significantly higher mean MP activity levels than healthy controls before and 7 days after surgery. During the follow-up, MP activity levels became significantly lower 1 and 4 months after surgery (P = 0.007 and P = 0.018, respectively) than prior to surgery, but this decrease was only seen in the subgroup achieving complete tumor resection. MP activity levels increased in 7 (63.6%) of 11 patients who developed VTE. The different incidence of the increase in MP activity levels between patients with and without VTE was statistically significant (χ 2 = 4.93, P = 0.026; relative risk 1.38, 95% CI 1.03–1.86). GBM patients may have an increase in MP-associated procoagulant activity that could contribute to any prothrombotic states and increases the likelihood of VTE complications; this procoagulant activity drops during control of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) may be the presenting symptom of occult cancers or complicate the clinical course of overt neoplasms. VTE in cancer has a reported prevalence ranging between 4 and 20%. It is a leading cause of morbidity and mortality, and a well-known prognostic factor associated with a worse survival [1, 2]. Major risk factors for VTE in cancer patients are older age, site of cancer (e.g., gastrointestinal, brain, pancreas), a history of VTE, major surgery, chemotherapy, metastatic disease, and the use of red cell growth factors [1, 3].
Structural and/or functional vascular damage and changes in the hemostatic process in cancer may lead to a local or systemic prothrombotic state, which has yet to be fully elucidated. A systemic inflammatory state mediated by cytokines and acute phase proteins is involved in the increased expression of tissue factor (TF), a transmembrane glycoprotein expressed in both tissue-specific and plasma circulating fashions. TF has several roles on blood coagulation, angiogenesis, tumor progression, and metastasis [4]. It can lead to VTE by complexing the active form of Factor VII (FVIIa) and thus activating the proteolytic cascade that generates thrombin. TF has been found to induce the expression of vascular endothelial growth factor by tumor cells, suggesting a role for it in cancer growth and angiogenesis [5]. TF is also associated with circulating microparticles (MP) deriving from leukocytes, platelets, endothelial cells, and tumor cells such as acute promyelocytic leukemia, epithelial solid tumors, glioma, and some types of meningioma [6]. MP are membrane vesicles less than 1 μm in diameter shed from the surface of normal and tumor cells to the cells’ surroundings and the blood circulation following their activation or apoptosis. The membrane proteins of these MP reflect those of the cell from which they originate. MP released from tumor and host cells after stimulation with chemotherapy, radiotherapy, and cytokines, for instance, seem to play a part in endothelial dysfunction, angiogenesis, immune suppression, reduced plasma levels of TF pathway inhibitor, and thrombogenicity [7]. MP have phospholipids and proteins such as TF exposed on their surface, thus potentially providing a substrate for the activation of clotting. TF-bearing MP may be an important link between cancer and thrombosis [8].
VTE is a major complication of glioblastoma multiforme (GBM), a grade IV malignant glioma affecting 3 in every 100,000 people a year [9] and associated with an extremely poor prognosis, the mean survival being 12–14 months [10] despite aggressive treatment. A literature review by Marras et al. [11] demonstrated that the incidence of VTE is high throughout the course of malignant glioma, suggesting the need of randomized controlled trial to evaluate the risk and costs to benefit ratio of a long-term anticoagulant prophylaxis. A recent epidemiological study by Semrad et al. [12] on 9,489 patients with malignant gliomas recorded a high 2-year cumulative incidence of VTE (7.5%), with a rate of 16.1 events per 100 person-years in the first 6 months. Risk factors for VTE included older age, a GBM histology, three or more chronic comorbidities, and neurosurgery within 61 days, which conferred a hazard ratio for VTE of 1.7 (95% CI 1.3–2.3). The authors also demonstrated that the onset of a VTE was associated with a 30% higher risk of death within 2 years.
Although a systemic procoagulant state has been demonstrated in patients with mainly solid neoplasms thanks to the detection of circulating thrombin–antithrombin complexes, prothrombin F1+2 fragment, increased clotting factors and D-dimer levels [13], similar findings in brain tumors are very scarce. Since GBM expresses the plasminogen activator inhibitor type 1 (PAI-1), it has been suggested that a dysregulation of fibrinolytic activity might contribute to the prothrombotic state associated with this tumor [14]. Studies on GBM have demonstrated that the loss of PTEN tumor suppressor gene and the enhanced expression of epidermal growth factor receptor (EGFR), be it the wild-type or the deletion-mutant form EGFRvIII, may induce TF expression and procoagulant activity in the tumor cells [15].
In the present prospective study, we aimed to evaluate the procoagulant activity of circulating MP (MP activity) in patients with GBM (WHO grade IV astrocytoma) and their potential contribution to prothrombotic states.
Materials and methods
Patients
Patients with GBM (WHO grade IV astrocytoma) consecutively admitted to the Neurosurgery Department of Padua University Hospital between September 2007 and December 2008 were prospectively assessed. Inclusion criteria were: age >18 years, Karnofsky performance status ≥70%, and brain MR images compatible with and histological confirmation of GBM (WHO grade IV astrocytoma). Exclusion criteria were: age <18 years, refusal of informed consent, pregnancy, severe liver or renal failure, and inoperable patients. Written informed consent in accordance with the Helsinki Declaration was obtained from all patients before their inclusion in the study. Twenty healthy age- and sex-matched volunteers were tested as controls. The study design conforms to the currently applicable standards in the country of origin.
All patients underwent craniotomy and brain tumor resection. Based on the MRI findings obtained within 48 h of surgery, patients were divided into two groups by extent of tumor resection. The first group included patients achieving a gross-total resection, defined as no residual enhancement; the second group consisted of those with a subtotal resection, with residual nodular enhancement. Within 6 weeks after surgery, all patients received fractionated radiation at a dose of 60 Gy (2 Gy for cycles of 5 days/week for 6 weeks) and concomitant chemotherapy with oral temozolomide, 75 mg/m2 body surface a day for 7 weeks, followed by adjuvant chemotherapy with temozolomide given orally at a dose of 150–200 mg/m2 body surface a day for 5 days every 28 days for 6–12 months. Dexamethasone for cerebral edema, antiepileptic drugs for seizures, and anti-emetic drugs were given as necessary.
During their hospital stay, all patients received antithrombotic prophylaxis with graduated elastic stockings and low-molecular-weight heparin (sc. nadroparin 3,800 U or enoxaparin 4,000 U or dalteparin 5,000 daily), which was replaced with intermittent pneumatic compression during the surgical procedure and for 48–72 h afterwards. VTE was clinically suspected on the basis of the Wells pretest probability score for deep vein thrombosis (DVT) and pulmonary embolism (PE) [16, 17]. In addition, since all patients were at high risk of VTE but often had severe neurological impairments, consciousness disorders, and limb edema due to plegia probably masking the clinical signs of VTE, as well as high D-dimer levels soon after surgery, D-dimer levels were monitored every 48–72 h during the first 7–14 post-operative days following an internal protocol for this specific subpopulation. In fact, any progressive rise in D-dimer levels respect to those observed at first control after surgery, and not the single increased D-dimer value itself, was usually considered an additional laboratory indication to search for VTE in this particular patient population. The results of clinical examinations and any episodes of VTE were recorded at each outpatient visit during the follow-up. DVT was confirmed by compression ultrasound, and PE by computed tomography angiography or perfusion lung scintigraphy.
Determination of disease status
Patient assessments included a preoperative and a monthly postoperative examination of clinical conditions. Patients underwent radiological investigations (MRI) at scheduled times, i.e., pre-operatively (range 1 ± 5 days before surgery), post-operatively (<48 h after surgery), 1 month after surgery (before radio-chemotherapy began), then 3-monthly (the first of these being after stopping radiotherapy). MRI was performed with the 1.5-T GE scanner (Achieva; Philips, Best, Netherlands). All MRI studies included fluid-attenuated inversion recovery, and T2- and T1-weighting before and after administering a gadolinium contrast agent (gadopentetate dimeglumine).
Blood samples and laboratory assays
Venous blood samples were collected from each patient between 8.00 and 10.00 a.m. after overnight fasting and a 15-min period of supine rest.
The following tests were performed before and again 7 days after surgery: platelet count, PT, INR, aPTT, fibrinogen, D-dimer, PAI-1 antigen (PAI-1:Ag), and t-PA antigen (t-PA:Ag). In addition, the procoagulant activity of annexin V positive MP (MP-activity) was tested before surgery and then 7 days and 1, 4, and 7 months after surgery. For the clotting and fibrinolytic assays, 8.1 ml of whole blood were collected in a vacutainer containing trisodium citrate (0.109 M) as an anticoagulant. After centrifugation at 3,000g for 15 min, plasma aliquots were stored at −40°C and tested within a month. To assay MP activity, platelet-poor plasma was further centrifuged at 14,000g for 2 min and stored at –80°C.
Platelet count (n.r. 150–450 × 109/l), PT (n.r. 75–112%), INR (n.r. 0.88–1.13), and aPTT (n.r. 23–37 s) were determined using standard procedures. Fibrinogen (n.r. 0.2–0.4 g/l) was measured using the Clauss clotting time method with bovine thrombin (Biopool, Umeå, Sweden). D-dimer (n.r. <225 μg/l) was tested with an immunoturbidimetric method (AutoDimer; Biopool). PAI-1:Ag (n.r. 4–25 ng/ml) and t-PA:Ag (n.r. 2–12 ng/ml) were measured using ELISA methods (ZYmutest PAI-1 Antigen; HYPHEN BioMed, Neuville-sur-Oise, France; and TintElize t-PA Antigen; Trinity Biotech, Jamestown, NY, USA, respectively). MP activity was assayed with a functional chromogenic method on microELISA plates (ZYMUPHEN MP-activity; HYPHEN BioMed) according to the manufacturer’s instructions.
Statistical analysis
The statistical analysis was performed using the PASW Statistics 17.0.2 (SPSS) for Windows. The results were calculated as means ± standard deviations (SD). Student’s t test was used to compare the means. Pearson’s correlation analysis was used to detect significant univariate associations between the parameters analyzed. Frequencies were calculated using the χ 2 test or Fisher’s exact test. The relative risk (RR) was determined from contingency tables, and the 95% confidence intervals (95% CI) were calculated. A P value <0.05 was considered statistically significant.
Results
Sixty-one patients with GBM, WHO grade IV astrocytoma, were included in the study (Table 1). There were 36 males and 25 females with a mean age of 56.7 ± 14.2 years. Forty-one (67%) patients underwent gross-total GBM resection and 20 (33%) had a subtotal resection. The median clinical follow-up for the gross-totally resected cases was 445 days (range 281–650 days) and for the subtotally resected cases it was 227 days (range 154–515 days). The coagulation study 1, 4, and 7 months after surgery was completed for 34, 23, and 17 patients, respectively. The causes of dropout at follow-up were KPS worsening, death, continuation of adjuvant therapy in other centers, and withdrawal of consent.
Despite antithrombotic prophylaxis, 11 patients (18%) developed venous thromboembolic complications, including 9 of 41 patients (21.9%) with a gross-total resection and 2 of 20 (10%) with a subtotal resection (RR 1.28, 95% CI 0.90–1.81). Asymptomatic DVT of the lower limbs was diagnosed in ten cases (eight distal and two proximal DVT), while PE was confirmed in one. In particular, VTE was diagnosed while in hospital in three patients 8–22 days before surgery and in seven patients after 2–6 days from intervention; in one patient, DVT occurred 7 months post-operatively.
The mean platelet count, and clotting and fibrinolytic test findings before and 7 days after surgery are summarized in Table 2. Before surgery, the mean platelet count and PT, INR, aPTT, and fibrinogen levels were within normal range, whereas the mean D-dimer levels were higher than normal in 23 patients (38%). Seven days after surgery, the mean fibrinogen and D-dimer levels were significantly higher than at the baseline (P < 0.001 and P = 0.006, respectively); in addition, D-dimer levels were higher, but not significantly, in patients who developed VTE while in hospital than in patients with no VTE (1,131.8 ± 852.7 vs 684.7 ± 1,382.0 μg/l, P = n.s.).
In GBM patients, the mean levels of PAI-1:Ag and t-PA:Ag were within the normal range before surgery and they did not vary significantly 7 days after surgery. There were also no significant differences in mean PAI-1:Ag levels between patients with and without VTE, or between patients achieving gross-total versus subtotal tumor resections (data not shown).
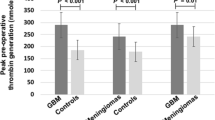
As shown in Fig. 1, the mean MP activity levels were significantly higher in GBM patients than in healthy controls, both before and 7 days after surgery (P < 0.05), whereas they dropped after 1 and 4 months, when they were no longer significantly different from those of the healthy controls. Another increase in MP activity levels was recorded after 7 months, but the difference was not significant.
Mean ± SD levels of procoagulant activity of circulating MP (MP activity) in patients with GBM (gray bars) before and then 7 days and 1, 4, and 7 months after surgery, and in healthy controls (white bars). *P < 0.05 GBM patients versus healthy controls • P = 0.018, •• P = 0.007 post-surgery versus basal levels in GBM patients
As compared to their baseline values, the drop in the GBM patients’ mean MP activity levels 1 and 4 months after surgery was statistically significant (P = 0.018 and P = 0.007, respectively), whereas no significant difference was apparent after 7 months (Fig. 1). As shown in Fig. 2, in the subgroup of 17 patients who completed the 7-month follow-up, 7 patients had neuroradiological demonstration of tumor progression at 7-month control and significantly higher MP activity levels with respect to patients without signs of disease progression (P = 0.05). MP activity levels were also increased as compared to baseline, although not significantly, in patients with signs of disease progression after 7 months.
When GBM patients were divided by extent of tumor resection (gross-total or subtotal), the mean MP activity levels did not differ between the two groups before or 7 days after surgery. On the other hand, these levels were found significantly lower than at the baseline when tested 1 and 4 months after surgery, but only in patients who had undergone gross-total resection (P = 0.001 and P = 0.043, respectively), not in those achieving a subtotal resection (Fig. 3).
Mean ± SD levels of procoagulant activity of circulating MP (MP activity) in patients with GBM divided by extent of tumor resection (black bars gross-total, gray bars subtotal) before and then 7 days and 1, 4, and 7 months after surgery, and in healthy controls (white bars). *P < 0.05 GBM patients versus healthy controls • P = 0.043, ••• P = 0.001 post-surgery versus basal levels in GBM patients
No significant correlation was observed between MP activity levels and coagulation or fibrinolytic parameters, in particular D-dimer levels, either before or 7 days after surgery (data not shown).
Among the 11 patients who developed VTE, an increase in MP activity levels beyond the upper normal range (10 nmol/ml) was seen in 7 cases (63.6%), i.e., 5 of 9 patients who had a gross-total resection and both the 2 patients who had a subtotal resection. Twenty-four of 50 GBM patients (48%) with no thromboembolic complications had increased MP activity levels. The different incidence of the increase in MP activity between patients with and without VTE was statistically significant (χ 2 = 4.93, P = 0.026; RR 1.38, 95% CI 1.03–1.86). In addition, four out of seven patients with VTE soon after surgery had an increase in baseline MP activity levels; in the other four patients (three with VTE before surgery and one after 7 months) MP activity levels were measured at the time of VTE and were increased in three cases.
Discussion
GBM is associated with a high risk of venous thromboembolic complications due to multifactorial pathogenetic mechanisms. Lower limb paresis or plegia, prolonged immobility, age over 75 years, steroid use and chemotherapy, glioma size, intraluminal thrombosis in the tumor specimen, and a subtotal rather than total resection are conditions known to predispose patients to thrombosis [11, 12, 18–20], whereas investigations on the presence of a systemic procoagulant state are scarce. In recent years, it has been suggested that a contribution to the prothrombotic state in cancer patients may come from circulating MP, which are small membrane vesicles shed from normal and tumor cells following activation or apoptosis. In particular, MP bearing TF (the main trigger of the clotting process) reportedly increase in patients with cancer of the breast, pancreas, colon, and ovary [21]. MP-associated TF activity has been found significantly greater in patients with metastatic breast cancer and pancreatic cancer than in healthy subjects [22].
In the present study, we prospectively evaluated the procoagulant activity of circulating MP in patients with GBM (WHO grade IV astrocytoma) before and for up to 7 months after treatment based on surgery followed by a standard combined radio-chemotherapy protocol. We found a higher MP activity in GBM patients than in healthy controls before and a week after surgery, which indicates that GBM patients may perioperatively period have a systemic procoagulant state due partly to a high circulating MP activity. No correlation emerged, however, between MP activity levels and both routine coagulation parameters (particularly PT, INR, aPTT, and D-dimer). In contrast, Hron et al. [23] demonstrated a twofold increase in the levels of TF-positive MP (mainly originating from platelets) in patients with advanced colorectal cancer by comparison with healthy controls—a result that correlated with D-dimer levels. We cannot rule out the possibility of the antithrombotic prophylaxis with low-molecular-weight heparin administered during the patients’ hospital stay influencing clotting and fibrinolytic activation, and consequently interfering with any correlation between MP activity and the coagulation test results.
During the follow-up, we observed a significant drop in MP activity levels 1 and 4 months after surgery by comparison with those recorded before surgery, and a subsequent rise by the 7th month, but only in the patients who had undergone gross-total resection, not in those with a subtotal resection of their tumor. In addition, at 7-month control, we observed a significant increase in MP activity levels in patients with neuroradiological evidence of tumor progression with respect to those without signs of disease progression. Our findings suggest that the presence of the tumor mass plays a part in the increase in procoagulant circulating MP activity. The cellular origin of these MP (from leukocytes, platelets, tumor, or endothelial cells) remains to be clarified. Circulating tumor-derived MP and exosomes (ranging from 50 to 500 nm in size) containing EGFRvIII mRNA have recently been demonstrated in the serum of patients with glioblastoma, and it has also been shown that adding these MP to brain endothelial cells in vitro induces an angiogenic phenotype [24].
Ours is the first description of a procoagulant activity of circulating MP in patients with GBM and of a transient reduction in their levels after radical surgery and chemo-radiotherapy, followed by a further increase probably due to relapsing or progressive disease. Similar findings were reported by Zwicker et al. [21] concerning three pancreatic cancer patients undergoing surgery with curative intent, whose levels of MP bearing TF (TF-bearing MP) coexpressing the epithelial tumor antigen MUC-1, as assayed by flow cytometry, dropped significantly after surgery.
A role for TF-bearing MP has also been suggested in the onset of VTE complications in cancer patients. High levels of such MP were found associated with VTE in patients with cancers at various sites, with an adjusted odds ratio of 3.72 [21]. Higher TF-bearing MP activity levels were also seen in cancer patients with VTE in comparison with those without VTE [25, 26], and in one of these studies [26], two patients with brain tumors were included among 53 cancer patients with VTE. A predictive role has recently been suggested for TF-bearing MP as a biomarker of the development of VTE [21, 27]. In our study, about 64% of GBM patients with VTE complications had higher than normal MP activity levels, as opposed to 48% of cases without VTE. These data are consistent with the above-mentioned publications; however, the number of our patients with VTE is too small to draw further conclusions dealing with predictive value of MP activity. It has to be said, in addition, that all our GBM patients received mechanical and pharmacological antithrombotic prophylaxis, which certainly had a crucial role in preventing VTE events in a substantial number of cases. Further studies on patients without any antithrombotic prophylaxis might better clarify the impact of procoagulant MP activity in predicting and/or causing VTE complications in GBM patients, but it would be unethical to design a randomized study involving a group of GBM patients not given heparin prophylaxis. Moreover, radiological examinations to verify the onset or absence of VTE were performed in GBM patients with clinical and laboratory suspicion of VTE. Our endpoint in this work, however, was mainly to investigate the presence of procoagulant activity associated with MP in GBM patients. On the basis of our results, a well-designed study is needed to evaluate the impact of MP in VTE development.
It has to be highlighted that the pathogenesis of VTE complications is complex and multifactorial in GBM patients; therefore, we can only surmise that high procoagulant MP activity may represent one of several factors predisposing to thrombosis. The characterization of circulating MP in terms of cellular origin and TF-bearing might help to clarify their role in the development of VTE.
Finally, our results do not seem to support any presence of a fibrinolytic dysregulation, and particularly of a hypofibrinolytic state due to any increase in PAI-1 levels, contributing to VTE in patients with GBM.
In conclusion, GBM patients have a higher than normal procoagulant activity associated with circulating MP, which may contribute to prothrombotic state and the likelihood of VTE complications, but this procoagulant activity level drops when the GBM is controlled.
Further studies are needed to evaluate the impact of MP levels among several risk factors for VTE already known in this population. Even more interesting will be to verify the role of circulating MP as a marker of disease progression in GBM patients.
References
Wun T, White RH (2009) Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest 27:63–74
Khorana AA (2010) Venous thromboembolism and prognosis in cancer. Thromb Res 125:490–493
Falanga A (2009) The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest 27:105–115
Milsom C, Rak J (2007) Tissue factor and cancer. Pathophysiol Haemost Thromb 36:160–176
Zhang J, Ding J, Zhang X, Shao X, Hao Z (2005) Regulation of vascular endothelial growth factor production and angiogenesis by tissue factor in SGC-7901 gastric cancer cells. Cancer Biol Ther 4:769–772
Zwicker JI, Furie BC, Furie B (2007) Cancer-associated thrombosis. Crit Rev Oncol Hematol 62:126–136
Aharon A, Brenner B (2009) Microparticles, thrombosis and cancer. Best Pract Res Clin Haematol 22:61–69
Davizon P, Lòpez JA (2009) Microparticles and thrombotic disease. Curr Opin Hematol 16:334–341
CBTRUS (2009) 2009–2010 CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in eighteen states in 2002–2006. Central Brain Tumor Registry of the United States, Hinsdale, IL. www.cbtrus.org
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma. Cancer 89:640–646
Semrad JT, O’Donnel R, Wun T et al (2007) Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 106:601–608
Franchini M, Montagnana M, Targher G, Manzato F, Lippi G (2007) Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis 24:29–38
Colin C, Voutsinos-Porche B, Nanni I et al (2009) High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol 118:745–754
Rong Y, Belozerov VE, Tucker-Burden C et al (2009) Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein 1 transcriptional activity. Cancer Res 69:2540–2549
Wells PS, Anderson DR, Bormanis J et al (1997) Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 350:1795–1798
Wells PS, Anderson DR, Rodger M et al (2000) Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 83:416–420
Simanek R, Vormittag R, Hassler M et al (2007) Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol 9:89–95
Rodas RA, Fenstermaker RA, McKeever PE et al (1998) Correlation of intraluminal thrombosis in brain tumor vessels with postoperative thrombotic complications: a preliminary report. J Neurosurg 89:200–205
Walsh DC, Kakkar AK (2001) Thromboembolism in brain tumors. Curr Opin Pulm Med 7:326–331
Zwicker JI, Liebman HA, Neuberg D et al (2009) Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res 15:6830–6840
Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S (2007) Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost 5:520–527
Hron G, Kollars M, Weber H et al (2007) Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost 97:119–123
Skog J, Wurdinger T, van Rijn S et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S (2009) Microparticle associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost 7:1421–1423
Manly DA, Wang J, Glover SL et al (2010) Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res 125:511–512
Khorana AA, Francis CW, Menzies KE et al (2008) Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost 6:1983–1985
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sartori, M.T., Della Puppa, A., Ballin, A. et al. Prothrombotic state in glioblastoma multiforme: an evaluation of the procoagulant activity of circulating microparticles. J Neurooncol 104, 225–231 (2011). https://doi.org/10.1007/s11060-010-0462-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0462-8