Abstract
Introduction
Tumor recurrence patterns after resection of intracranial low-grade gliomas (LGG) generally remain obscured. The objective of the present retrospective study was their multifaceted analysis, evaluation of associated factors, and assessment of impact on prognosis.
Methods
Study group comprised 81 consecutive adult patients (46 men and 35 women; median age, 37 years) with recurrent diffuse astrocytomas (DA; 51 cases) and oligodendrogliomas (OD; 30 cases). The median length of follow-up after primary surgery was 6.7 years.
Results
Early (within 2 years after primary surgery) and non-early (> 2 years after primary surgery) recurrence was noted in 23 (28%) and 58 (72%) cases, respectively. Fast (≤ 6 months) and slow ( > 6 months) radiological progression of relapse was noted in 31 (38%) and 48 (59%) cases, respectively. Tumor recurrence was local and non-local in 71 (88%) and 10 (12%) cases, respectively. Recurrence patterns have differed in OD, IDH1-mutant DA, and IDH wild-type DA. Early onset, fast radiological progression, and non-local site of relapse had statistically significant negative impact on overall survival of patients and were often associated with malignant transformation of the tumor (38 cases). However, in subgroup with extent of resection ≥ 90% (56 cases) no differences in recurrence characteristics were found between 3 molecularly defined groups of LGG.
Conclusions
Recurrence patterns after resection of LGG show significant variability, differ in distinct molecularly defined types of tumors, and demonstrate definitive impact on prognosis. Aggressive resection at the time of primary surgery may result in more favorable characteristics of recurrence at the time of its development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owed to invasive growth into brain parenchyma precluding radical surgical removal, and resistance to irradiation and chemotherapy the vast majority of gliomas are currently considered as incurable disease, thus relapse of the neoplasm can be always expected at some time point during the clinical course. High-grade gliomas recurrent after primary surgery, fractionated radiotherapy (FRT), and chemotherapy have been a subject of multiple investigations [1,2,3,4,5,6]. In contrast, much fewer studies dealt with recurrent low-grade gliomas (LGG), which may be particularly caused by their rarity and relatively favorable prognosis associated with prolonged progression-free survival (PFS) and overall survival (OS) of patients. Nevertheless, the clinical course of LGG may be unpredictable, as some of these tumors recur soon after primary treatment and/or undergo malignant transformation [7,8,9,10,11,12,13,14,15]. It is known, that approximately 80–90% of recurrences of LGG are local [7, 16], but other characteristics of relapse in such cases (e.g., time of onset, speed of progression, associations with molecular features of the tumor, extent of resection [EOR], and administration of postoperative FRT and chemotherapy) and their prognostic significance are mostly remain obscured, although it may have an important implication for choice of the optimal mode and timing of the adjuvant treatment after primary surgery. The objective of the present retrospective study was multifaceted analysis of recurrence patterns after resection of intracranial LGG along with evaluation of associated factors and assessment of the general impact on the outcome.
Materials and methods
From January 2000 till June 2013, 227 consecutive adult patients (age ≥ 18 years) underwent surgical resection of newly diagnosed intracranial LGG in the Department of Neurosurgery of the Tokyo Women’s Medical University. Out of the total cohort, 81 patients (36%) experienced tumor recurrence during follow-up and comprised study group (Table 1). During the same time span in 15 other patients histopathological diagnosis of LGG was established after tumor biopsy, but these cases were excluded from the present analysis. Study protocol was approved by the ethics committee and institutional review board of the Tokyo Women’s Medical University (approval #3540-R03).
Surgical treatment
In all cases tumor resection was performed according to the concept of information-guided surgery based on the integrated analysis of various intraoperative data reflecting anatomical, functional, and histopathological characteristics of the clinical case, which presumes routine use of low-field-strength intraoperative MRI (AIRIS IITM; Hitachi Medical Corporation, Tokyo, Japan), updated neuronavigation, comprehensive intraoperative neurophysiological techniques, and histopathological monitoring of the resected tissue [17, 18]. In our practice, surgery for LGG is directed at maximum resection of the area of T2 hyperintensity with preservation of the functionally important cortical and subcortical neuronal and vascular structures. EOR was determined based on the volumetric comparison of the hyperintensity area on T2-weighted MRI performed before surgery and within 72 h thereafter.
Histopathological diagnosis
Histopathological tumor typing and grading was based on the standard criteria of the 4th edition of the World Health Organization (WHO) classification of central nervous system (CNS) tumors (2007) [19] with additional consideration of changes reflected in its updated version (2016) [20]. In particular, combined complete loss of the chromosomal arms 1p and 19q (1p/19q co-deletion) was considered as prerequisite for diagnosis of oligodendroglioma (OD) unless cytogenetic testing was not done. Immunohistochemical staining for Ki-67 with MIB-1 antibodies was performed in all cases, and for encoded protein products of mutant IDH1R132H in 80 tumors (99%). Assessment of 1p/19q co-deletion was done in 74 tissue samples (91%).
Adjuvant therapy
Strategy of postoperative treatment for LGG was generally based on the previously reported protocol adopted in our practice [21]. Briefly, in cases with EOR ≥ 90% adjuvant therapy was usually omitted and patients underwent observational follow-up with regular MRI examinations. In cases with EOR < 90%, postoperative local FRT alone (total dose, 50 Gy; 25 fractions; 2 Gy per fraction) or combined with chemotherapy (nimustine hydrochloride [ACNU], 80 mg/m2 i.v. once in 8 weeks; 6–8 cycles in total) was recommended routinely in cases of 1p/19q non-codeleted and co-deleted tumors, respectively [21]. Nevertheless, due to different reasons even after EOR ≥ 90% some patients, considered as “high-risk,” received adjuvant therapy, whereas in few cases with EOR < 90% it was delayed until tumor recurrence.
Follow-up
Patients were followed with regular clinical and radiological evaluations scheduled each 3 months during first year after surgery and each 4–6 months thereafter. In case of clinical deterioration examination was done urgently. The median length of follow-up was 6.7 years (range 1.0–14.8 years).
Diagnosis of recurrence
Radiological diagnosis of tumor recurrence was generally based on criteria of Response Assessment in Neuro-Oncology (RANO) working group [22] and included ≥ 25% increase of the product of perpendicular diameters of the lesion or appearance of new hyperintense area(s) on T2-weigthed and/or FLAIR images not attributable to effects of adjuvant therapy, or appearance or expansion of the contrast-enhanced areas on T1-weighted images, in association with “tumor pattern” on proton magnetic resonance spectroscopy (1H-MRS) and definite radioisotope uptake on positron emission tomography with 11C-methionine (MET-PET). Appearance of a new area of contrast enhancement on T1-weighted MRI was considered as a sign of malignant transformation [22]. Differentiation of tumor recurrence and radiation-induced necrosis (if required) was mainly based on MET-PET. Histopathological diagnosis of tumor recurrence and malignant transformation (if presented) was established upon examination of the pathological tissue after re-resection of the lesion, which was done in 62 cases (77%).
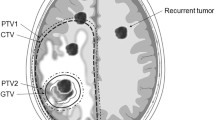
The time of recurrence onset was defined according to date of the first MRI examination at which it was suspected. Early and non-early recurrences were considered if it were diagnosed, respectively, within 2 years or > 2 years after primary surgery. With regard to speed of progression, recurrences were categorized as fast and slow, if time interval from initial imaging changes to definitive radiological diagnosis was, respectively, ≤ 6 months and > 6 months. With regard to location, the following recurrence patterns were considered [5]: regional (in the wall of resection cavity), marginal (within 20 mm from the margin of resection cavity), distant (> 20 mm from the margin of resection cavity), multiple (several discontiguous recurrences in various brain areas), and CSF dissemination (Fig. 1). Regional and marginal recurrences were defined as local, and distant, multiple, and CSF dissemination as non-local.
Statistical analysis
Group comparisons were done with chi-square test. Survival analysis was performed with construction of Kaplan–Meier curves and their comparison by log-rank test. PFS and OS were calculated from the time of primary surgery. Statistically significant difference was defined if P-value of two-tailed test was < 0.05. All calculations were done with commercially available software JMP® Pro 13, version 13.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
The study group comprised 51 DA and 30 OD. Median MIB-1 index was 4.8% (range 1–21%). Immunopositivity for mutant IDH1R132H was identified in 64 cases (79%) and 1p/19q co-deletion was revealed in 28 cases (35%). The group of DA included 32 cases of IDH1R132H immunopositive and 1p/19q non-codeleted tumors, 13 cases of IDH1R132H immunonegative and 1p/19q non-codeleted tumors, 1 case of IDH1R132H non-tested and 1p/19q non-codeleted tumor, as well as 2 cases of IDH1R132H immunopositive and 3 cases of IDH1R132H immunonegative tumors which were not tested for 1p/19q status, but histologically were diagnosed as DA. The group of OD comprised 28 cases of IDH1R132H immunopositive and 1p/19q co-deleted tumors, and 2 cases of IDH1R132H immunopositive tumors which were not tested for 1p/19q status, but histologically were diagnosed as OD.
The median EOR at primary surgery was 90% (range 20–100%); in 56 patients (69%) EOR was ≥ 90%. There was no statistically significant difference of EOR between molecularly defined groups of tumors. Overall, 23 patients received postoperative FRT and 15 were treated with chemotherapy.
During follow-up malignant transformation of the tumor was noted in 38 patients (47%), and was diagnosed histopathologically in 29 cases and radiologically in 9. It was encountered significantly more often in cases with EOR < 90% than with EOR ≥ 90% (64% vs. 39%; P = 0.0395). Median PFS of patients in the study group was 3.3 years (range 0.4–12.0 years), median OS was 12.6 years (range 1.0–14.8 years), and actuarial 10-year OS rate was 56%.
Characteristics of tumor recurrences and associated factors
Tumor recurrence characteristics and associated factors in the entire study group are presented in Table 2.
Early tumor recurrence was noted in 23 cases (28%) and was diagnosed at a median interval of 1.0 year after primary surgery (range 0.4–1.9 years). Non-early tumor recurrence was noted in 58 cases (72%) cases and was diagnosed at a median interval of 4.1 years after primary surgery (range 2.1–11.9 years). Early tumor recurrence was identified more frequently in men (P = 0.0140), in cases of DA (P = 0.0049), without IDH1R132H mutation (P = 0.0066), absent 1p/19q co-deletion (P = 0.0085), and in presence of malignant transformation of the neoplasm during follow-up (P = 0.0022). OS of patients with early recurrence of LGG (median, 5.1 years; range 1.0–13.5 years) was shorter than in cases with non-early recurrence (median not reached; range 3.4–14.8 years) and this difference was statistically significant (P = 0.0005; Fig. 2a).
In 2 cases (2%) speed of radiological progression of tumor recurrence could not be assessed because of lack of corresponding images. Fast recurrence progression was noted in 31 cases (38%) and lasted at median 0.2 month (range 0.0–5.5 months) from the initial imaging changes to definitive radiological diagnosis of relapse. Slow recurrence progression was noted in 48 cases (59%) and lasted at median 16.1 months (range 6.2–95.5 months) from the initial imaging changes to definitive radiological diagnosis of relapse. Fast recurrence progression was noted more frequently in cases without IDH1R132H mutation (P = 0.0266), EOR < 90% (P < 0.0001), if postoperative FRT was administered (P = 0.0004), and in presence of malignant transformation of the neoplasm during follow-up (P = 0.0011). OS of patients with fast recurrence progression (median, 6.7 years; range 1.0–14.1 years) was shorter than in cases with slow recurrence progression (median not reached; range 1.3–14.8 years) and this difference was statistically significant (P = 0.0021; Fig. 2b).
With regard to location, tumor recurrence was defined as regional in 67 cases (83%), marginal in 4 (5%), distant in 4 (5%), and multiple in 5 (6%); CSF dissemination was noted in 1 case (1%). Local tumor recurrence (71 cases) was identified more frequently in cases of OD (P = 0.0096), with MIB-1 index < 5% (P = 0.0313), presence of 1p/19q co-deletion (P = 0.0080), EOR ≥ 90% (P = 0.0331), if postoperative FRT was not administered (P < 0.0001), and without malignant transformation of the neoplasm during follow-up (P = 0.0035). OS of patients with non-local recurrence of LGG (median, 5.5 years; range 1.1–7.0 years) was shorter than in cases with local recurrence (median not reached; range 1.0–14.8 years) and this difference was statistically significant (P = 0.0003; Fig. 2c).
Characteristics of tumor recurrences in different types of tumors
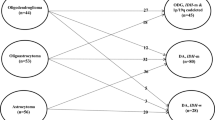
Since molecular features of LGG were one of the most important determinants of the recurrence characteristics, comparison was done between 3 definitively determined groups of tumors, namely IDH1R132H immunopositive, 1p/19 co-deleted OD (Group I; N = 28), IDH1R132H immunopositive, 1p/19 non-codeleted DA (Group II; N = 32), and IDH1R132H immunonegative, 1p/19 non-codeleted DA (Group III; N = 13).
Risk of early recurrence was significantly lower in Group I (11%) in comparison with Groups II (34%; OR 4.37; 95% CI 1.07–17.74; P = 0.0197) and III (54%; OR 9.72; 95% CI 1.93–49.11; P = 0.0030); the difference between Groups II and III was not statistically significant (OR 2.23; 95% CI 0.6–8.27). Risk of fast recurrence progression was 32% in Group I, 32% in Group II (OR 1.01; 95% CI 0.34–3.00), and 62% in Group III (OR 3.38; 95% CI 0.86–13.30), but differences did not reach statistical significance. There was no non-local recurrences in Group I, whereas their risks in Groups II (22%) and III (15%; OR 0.65; 95% CI 0.12–3.64) did not demonstrate statistically significant difference.
Characteristics of tumor recurrences after aggressive surgery
In cases with EOR ≥ 90% at primary surgery early and non-early tumor relapses were noted in 13 (23%) and 43 (77%) cases, respectively. Early tumor recurrence was identified more frequently in men (P = 0.0104), in cases of DA (P = 0.0440), and if postoperative FRT was administered (P = 0.0121). Fast and slow recurrence progression was noted in 12 (22%) and 42 (78%) cases, respectively. Fast recurrence progression was noted more frequently if postoperative FRT was administered (P = 0.0084) and in presence of malignant transformation of the neoplasm during follow-up (P = 0.0382). Local and non-local tumor recurrences were identified in 52 (93%) and 4 (7%) cases, respectively. Local tumor recurrence was noted more frequently in younger patients (P = 0.0439), in cases with MIB-1 index < 5% (P = 0.0379), if postoperative FRT was not administered (P < 0.0001), and without malignant transformation of the neoplasm during follow-up (P = 0.0099).
After aggressive surgery no statistically significant difference in evaluated recurrence characteristics was found between 3 molecularly defined groups of tumors. Risk of early recurrence comprised 10% in Group I, 32% in Group II (OR 4.24; 95% CI 0.79–22.85), and 29% in Group III (OR 3.60; 95% CI 0.40–32.37). Risk of fast recurrence progression was 25% in Group I, 16% in Group II (OR 0.60; 95% CI 0.14–2.62), and 43% in Group III (OR 2.25; 95% CI 0.37–13.71). There was no non-local recurrences in Group I, and their risks in Groups II (12%) and III (14%; OR 1.22; 95% CI 0.11–13.97) did not demonstrate statistically significant difference. In particular, aggressive resection has resulted in significant decrease of proportion of early recurrences in Group III, and proportions of fast progressing and non-local recurrences in Group II (Table 3).
Discussion
Median OS of patients with LGG varies from 2.7 to 16.7 years [7, 14, 23,24,25,26]. The mostly accepted prognostic factors are age at diagnosis, Karnofsky performance scale (KPS) score and neurological status before primary surgery, maximum diameter, eloquent location, and bi-hemispheric extension of the tumor, EOR and residual lesion volume, histological type of the neoplasm, and presence of IDH1/IDH2 mutations and 1p/19q co-deletion [7, 13, 27,28,29]. As was demonstrated in the present study, recurrence of LGG by itself also negatively influences the outcome. Among patients in the study group, median PFS and actuarial 10-year OS rate were 3.3 years and 56%, respectively, which is much worse than in the entire cohort of 227 consecutive cases of LGG operated on in our clinic within the same time span (median PFS, 12.0 years; actuarial 10-year OS rate, 80.1%; data not shown). Moreover, individual recurrence characteristics have statistically significant impact on prognosis. Early onset, fast radiological progression, and non-local site of relapsing tumor, each has negatively influenced OS of our patients. In addition, these unfavorable characteristics were significantly associated with malignant transformation of the neoplasm during follow-up.
Current WHO classification of CNS tumors [20] presumes assessment of IDH1/IDH2 mutational status and 1p/19q co-deletion for definitive diagnosis of DA and OD. Both IDH1/IDH2 mutations and 1p/19q co-deletion in gliomas carry favorable prognostic and predictive values and are associated with prolonged survival of patients [14, 15, 21, 29, 30]. According to our data, these molecular alterations may be also considered as one of the main determinants of recurrence characteristics after resection of LGG. As has been shown herein, IDH1-mutant tumors significantly more often demonstrate non-early onset and slow progressing recurrences, whereas 1p/19q co-deletion has been associated with non-early onset of relapse and its regional or marginal location. Thus, it may be hypothesized that realization of the positive prognostic impact of IDH1/IDH2 mutations and 1p/19q co-deletion may be in part related to more favorable characteristics of relapse at the time of its development.
Presented series includes 16 cases (20%) of IDH wild-type DA (IDH1R132H immunonegative), which, according to the updated WHO classification of CNS tumors (2016) [20], are considered as separate provisional pathological entity. These neoplasms comprise 7–30% of LGG, and usually do not display TP53 mutation and 1p/19q co-deletion as well, thus designated as “triple-negative” gliomas [15]. Such molecular fingerprint is frequently considered to be associated with unfavorable clinical course and dismal prognosis. Nevertheless, presence of wild-type IDH in DA may not be invariably linked to poor outcome, unless these tumors carry other glioblastoma-like genetic alterations, e.g., activating mutation or high-level amplification of EGFR, gain of chromosome 7, loss of chromosome 10, activating mutation of TERT promoter, etc.[15, 31]. In fact, based on DNA methylation profiling, IDH wild-type astrocytomas of WHO grade II and III additionally carrying aforementioned genetic features have been shown to cluster tightly with IDH wild-type glioblastomas [31]. Therefore, as suggested by the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW), identification of such molecular abnormalities in IDH wild-type LGG should be considered as a possible sign of highly aggressive biological behavior and allows for their designation as WHO grade IV tumors [31]. If additional clinicopathological studies validate this expert opinion based recommendation, it may be considered for inclusion into standard diagnostic criteria and histopathological grading scheme of the next edition of the WHO classification of CNS tumors. Optimal treatment strategies of IDH wild-type DA should be determined as well. An important finding of our study is that their aggressive resection may carry positive impact on prognosis and leads to similar recurrence characteristics as observed in IDH1-mutant DA and OD.
In cases of LGG, EOR ≥ 90% is associated with prolongation of both PFS and OS, reduced rates of malignant transformation, and better seizure control [9, 21, 26, 32]. Our data have shown that aggressive surgery may also result in more favorable characteristics of tumor relapse at the time of its development. It was demonstrated both in the entire cohort of patients, and in individual molecularly defined tumor types: EOR ≥ 90% led to reduced proportion of early recurrences in IDH wild-type DA, and was associated with more frequent appearance of slow progressing and local relapses of IDH1-mutant DA. In contrast, postoperative chemotherapy, which is currently considered as an important adjunct for treatment of LGG [33, 34], did not show any impact on the investigated characteristics of recurrence. Moreover, FRT was associated with more frequent fast progressing and distantly located relapses. These findings may have several explanations. First, in our practice adjuvant therapy is usually administered if EOR of LGG is < 90% or there are some other “high-risk” factors, thus focal FRT might simply carry limited efficacy on tumor control in such cases. Second, irradiation might indeed improve local tumor control, but was unable to provide it in distant areas of the brain. Third, FRT might result in malignant transformation of LGG, which in turn led to faster tumor growth and dissemination. Further studies are definitely needed to clarify this issue.
The RTOG 9802 study included 111 adult patients (aged from 18 to 39 years) with supratentorial LGG after neurosurgeon-determined aggressive resection (EOR > 90%) into the observation arm [16, 34]. In this cohort, in 82% of cases the recurrence was located within 2 cm of the resection cavity [16]. Such proportion of local relapses seems comparable with results of EORTC 22845 trial; in its early FRT arm in-field recurrences were noted in 90.4% of cases [7]. In the present series local recurrences were noted in 88% of patients in the entire study group, and in 93% of cases with EOR ≥ 90%. In the latter cohort postoperative FRT was significantly associated with early onset, fast radiological progression, and non-local site of relapse. Such finding justifies our current strategy to omit early adjuvant therapy for LGG in cases with EOR ≥ 90% in favor of observational follow-up with regular MRI examinations [21].
Conclusions
Tumor recurrence patterns after surgical resection of LGG are characterized by significant variability and differ in distinct molecularly defined types of tumors. OD typically demonstrate late and local relapses, whereas DA usually recur earlier and relatively more often in distant locations. In particular, IDH wild-type DA are prone for early recurrence with fast radiological progression. Early onset, fast progression, and non-local site of relapsing tumor negatively influence OS of patients and are often associated with malignant transformation of LGG. Nevertheless, aggressive resection (EOR ≥ 90%) at the time of primary surgery may alleviate effects of molecularly defined tumor type on recurrence characteristics and result in their more favorable patterns, whereas administration of postoperative FRT may have opposite effects.
References
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911
Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, Wilson CB (1986) Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 65:654–658
Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409
Liang BC, Thornton AF Jr, Sandler HM, Greenberg HS (1991) Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg 75:559–563
Konishi Y, Muragaki Y, Iseki H, Mitsuhashi N, Okada Y (2012) Patterns of intracranial glioblastoma recurrence after aggressive surgical resection and adjuvant management: retrospective analysis of 43 cases. Neurol Med Chir (Tokyo) 52:577–586
Ferguson SD, Momin EN, Weinberg JS (2018) Surgical management of recurrent intracranial gliomas. Prog Neurol Surg 30:218–231
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990
Lebrun C, Fontaine D, Bourg V, Ramaioli A, Chanalet S, Vandenbos F, Lonjon M, Fauchon F, Paquis P, Frenay M (2007) Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol 14:391–398
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Ahmadi R, Dictus C, Hartmann C, Zürn O, Edler L, Hartmann M, Combs S, Herold-Mende C, Wirtz CR, Unterberg A (2009) Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 151:1359–1365
Martino J, Taillandier L, Moritz-Gasser S, Gatignol P, Duffau H (2009) Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 151:427–436
Chaichana KL, McGirt MJ, Laterra J, Olivi A, Quiñones-Hinojosa A (2010) Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg 112:10–17
Daniels TB, Brown PD, Felten SJ, Wu W, Buckner JC, Arusell RM, Curran WJ, Abrams RA, Schiff D, Shaw EG (2011) Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys 81:218–224
Okita Y, Narita Y, Miyakita Y, Ohno M, Matsushita Y, Fukushima S, Sumi M, Ichimura K, Kayama T, Shibui S (2012) IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol 41:1325–1336
Komori T, Muragaki Y, Chernov MF (2018) Pathology and genetics of gliomas. Prog Neurol Surg 31:1–37
Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta M (2008) Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg 109:835–841
Muragaki Y, Iseki H, Maruyama T, Kawamata T, Yamane F, Nakamura R, Kubo O, Takakura K, Hori T (2006) Usefulness of intraoperative magnetic resonance imaging for glioma surgery. Acta Neurochir Suppl 98:67–75
Muragaki Y, Iseki H, Maruyama T, Tanaka M, Shinohara C, Suzuki T, Yoshimitsu K, Ikuta S, Hayashi M, Chernov M, Hori T, Okada Y, Takakura K (2011) Information-guided surgical management of gliomas using low-field-strength intraoperative MRI. Acta Neurochir Suppl 109:67–72
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Nitta M, Muragaki Y, Maruyama T, Ikuta S, Komori T, Maebayashi K, Iseki H, Tamura M, Saito T, Okamoto S, Chernov M, Hayashi M, Okada Y (2015) Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus 38(1):E7
van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, Armstrong T, Choucair A, Waldman AD, Gorlia T, Chamberlain M, Baumert BG, Vogelbaum MA, Macdonald DR, Reardon DA, Wen PY, Chang SM, Jacobs AH (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593
Olson JD, Riedel E, DeAngelis LM (2000) Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 54:1442–1448
Schomas DA, Laack NN, Brown PD (2009) Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer 115:3969–3978
Iwadate Y, Matsutani T, Hasegawa Y, Shinozaki N, Higuchi Y, Saeki N (2011) Favorable long-term outcome of low-grade oligodendrogliomas irrespective of 1p/19q status when treated without radiotherapy. J Neurooncol 102:443–449
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, Solheim O (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308:1881–1888
Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084
Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, Barbaro NM, Parsa AT, Berger MS, McDermott MM (2008) Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg 109:817–824
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O'Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508
Gozé C, Bezzina C, Gozé E, Rigau V, Maudelonde T, Bauchet L, Duffau H (2012) 1p/19q loss but not IDH1 mutations influences WHO grade II gliomas spontaneous growth. J Neurooncol 108:69–75
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol 136:805–810
Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, Skrap M (2012) Low-grade glioma surgery in eloquent areas volumetric analysis of extent of resection and its impact on overall survival: a single-institution experience in 190 patients. J Neurosurg 117:1039–1052
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary JP, Fisher BJ, Kim H, Murtha AD, Bell EH, Won M, Mehta MP, Curran WJ Jr (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374:1344–1355
Narita Y (2018) Chemotherapy of diffuse astrocytoma (WHO grade II) in adults. Prog Neurol Surg 31:145–215
Funding
None. The authors do not have any personal or institutional financial interests in drugs, materials, or devices described in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study contains no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fukuya, Y., Ikuta, S., Maruyama, T. et al. Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J Neurooncol 144, 519–528 (2019). https://doi.org/10.1007/s11060-019-03250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03250-8