Abstract
Background
Diffuse intrinsic pontine glioma (DIPG) is a devastating cancer of childhood and adolescence.
Methods
The study included patients between 3 and 20 years with clinically and radiologically confirmed DIPG. Primary endpoint was 6-month progression-free survival (PFS) following administration of nimotuzumab in combination with external beam radiotherapy (RT). Nimotuzumab was administered intravenously at 150 mg/m2 weekly for 12 weeks. Radiotherapy at total dose of 54 Gy was delivered between week 3 and week 9. Response was evaluated based on clinical features and MRI findings according to RECIST criteria at week 12. Thereafter, patients continued to receive nimotuzumab every alternate week until disease progression/unmanageable toxicity. Adverse events (AE) were evaluated according to Common Terminology Criteria for Adverse Events (CTC-AE) Version 3.0 (CTC-AE3).
Results
All 42 patients received at least one dose of nimotuzumab in outpatient settings. Two patients had partial response (4.8%), 27 had stable disease (64.3%), 10 had progressive disease (23.8%) and 3 patients (7.1%) could not be evaluated. The objective response rate (ORR) was 4.8%. Median PFS was 5.8 months and median overall survival (OS) was 9.4 months. Most common drug-related AEs were alopecia (14.3%), vomiting, headache and radiation skin injury (7.1% each). Therapy-related serious adverse events (SAEs) were intra-tumoral bleeding and acute respiratory failure, which were difficult to distinguish from effects of tumor progression.
Conclusions
Concomitant treatment with RT and nimotuzumab was feasible in an outpatient setting. The PFS and OS were comparable to results achieved with RT and intensive chemotherapy in hospitalized setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brainstem tumors constitute approximately 10–15% of all pediatric central nervous system (CNS) tumors [1]. Diffuse intrinsic pontine glioma (DIPG) is the most common brainstem tumor of childhood, accounting for more than 80% of brainstem gliomas in this age group. It has recently been re-classified as H3 K27M-mutant diffuse midline glioma according to World Health Organization Classification of CNS Tumors (2016) [1, 2]. Approximately 300 children in the U.S. and in Europe are diagnosed with DIPG every year [3]. There is no effective treatment against the condition till date, with less than 10% patients surviving for 2 years following diagnosis, and less than 1% surviving for 5 years. The median survival time is less than 1 year following diagnosis [4, 5].
Studies on pediatric high-grade glioma (HGG) have demonstrated the over-expression of epidermal growth factor receptor (EGFR) protein in about 80–85% of tumors tested [6]. However, amplification of the EGFR protein is relatively rare in childhood HGG. Only 7% of the cases with EGFR over-expression were found to have EGFR amplification indicating that unlike adult HGG, a mechanism other than gene amplification is responsible for EGFR overexpression in childhood HGG [6].
Nimotuzumab is a recombinant humanized monoclonal antibody against the human receptor for epidermal growth factor (EGF). In vivo experiments using human brain tumor cell line xenografted into nude mice have shown that nimotuzumab is effective in reducing the number of CD133+ cancer stem cells when used as monotherapy or in combination with radiotherapy (RT), compared to RT alone [7]. A study by Diaz-Miqueli et al. [7] concluded that co-administration of nimotuzumab with RT increased the radiosensitivity of human glioblastoma cell line (U87MG), resulting in a significant delay in subcutaneous tumor growth, significant reduction in size of tumor blood vessels and proliferating cells in subcutaneous tumors. These data suggest that anti-EGFR monoclonal antibodies play the role of radiosensitizers in human glioblastoma cells.
It was postulated that nimotuzumab in combination with radiation would have potential use in the treatment of pediatric brain tumors, and be effective in children with recurrent or relapsed high grade gliomas [8].
The main goal of this study was to assess the median PFS in children and adolescents with newly diagnosed DIPG treated with the anti-EGFR humanized monoclonal antibody nimotuzumab and local fractionated RT.
Materials and methods
Study design
An open-label, single arm, phase III multinational clinical study was conducted with patients enrolled from Germany, Italy and Russia. The study included patients with newly diagnosed clinically and radiologically confirmed DIPG, in age group of 3–20 years and with Lansky or Karnofsky index > 40%. In addition, the patients had to have adequate renal, liver and haematological functions. All patients had a short disease history with symptoms (cranial nerve paresis, ataxia, lower or upper extremity paresis) duration of less than 3 months, and characteristic features of DIPG on MRI (tumor primarily located in the pons, infiltrating diffusely the pons and with a tumor diameter more than half of the pons) [9].
Major exclusion criteria were: (i) patients having pontine glioma as secondary malignancy or a low grade brain stem glioma, (ii) prior anti-neoplastic therapy, including chemotherapy, immunotherapy, radiotherapy and (iii) prior administration of a recombinant human or murine antibody or known hypersensitivity to antibodies. Biopsy of the tumour was neither among inclusion nor exclusion criteria and therefore these data were not reported.
Interventions
The dose of nimotuzumab was calculated based on studies in adults and the phase II study conducted in children and adolescents [8]. It was assumed that an absolute dose of 200–400 mg in adults corresponds to a dose of 115–230 mg/m2 body surface area (BSA), and the dose for this study was adjusted to 150 mg/m2 BSA [8]. Nimotuzumab (150 mg/m2) was diluted in 0.9% sodium chloride to a total volume of 250 mL and was administered once weekly by intravenous infusion over 30 min for 12 weeks. Concomitantly, external beam RT with a total dose of 54 Gy was administered, divided in 1.8 Gy fractions/day for 5 days/week for 6 weeks (“induction” therapy). Thereafter, nimotuzumab treatment was continued every alternate week in patients with stable disease or positive response until disease progression, appearance of unacceptable toxicity or withdrawal of consent (“maintenance” therapy).
Efficacy assessment
The primary endpoint of the study was estimation of progression-free survival (PFS) following the administration of nimotuzumab in combination with external beam RT. Progression-free survival was defined as the time from patient enrollment till the day of documented progression of disease or death, whichever happened earlier. Time was censored at the latest follow-up for patients still alive and relapse-free.
The secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety and tolerability. Disease control rate (DCR) was defined as the number of participants experiencing complete response (CR), partial response (PR), or stable disease (SD) at week 12, divided by the number of enrolled participants. Overall response rate (ORR) was defined as all patients with partial or complete response divided by number of enrolled participants. Overall survival (OS) was defined as the time from either diagnosis or recurrence to death due to any cause. Time was censored at the latest follow-up for patients still alive. Tumor assessment using MRI was performed at baseline, not more than 2 weeks before the start of treatment, and then repeated after every 12 weeks. Evaluation of tumors was performed by investigator and Independent Response Review Committee (IRRC). Continuation of treatment was based on IRRC evaluation. Tumor response criteria were determined according to RECIST by changes in size using width (W), transverse (T), and length (L) measurements on either T2 or FLAIR-weighted MRIs [10]. Complete response (CR) was defined as disappearance of all measurable disease. Partial response (PR) was defined as reduction in greatest diameter (GD) of target lesion by at least 30% from baseline value. Progressive disease (PD) was defined as increase in GD of target lesion by at least 20% from baseline value.
In case length of lesion could not be determined, the product of the longest diameter and its longest perpendicular diameter (TxW) was used for comparison. Treatment was discontinued in case of global deterioration in physical or neurological conditions, regardless of radiological assessment. Hence, clinical deterioration was considered as tumor progression even in the absence of confirmatory MRI findings [8, 11].
Safety assessment
All patients who had received at least one dose of antibody were evaluated for drug safety. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTC-AE) Version 3.0 of the National Cancer Institute.
Statistical methods
The study population was defined as intention-to-treat (ITT) population and included all study patients who had received at least one dose of nimotuzumab. Primary efficacy endpoint was progression-free survival (PFS). Intention-to-treat population was also evaluated for overall survival (OS), tumor response (ORR and DCR), safety and tolerability.
A non-parametric one-sided sign-test was performed to assess if the median PFS in ITT population was significantly over 6 months. Setting-up H0: p ≤ 0.5 and H1: p > 0.5, assuming H1: p = 0.75, with a type I error rate α = 0.025 and type II error rate β = 0.20, a sample size of n = 40 patients was needed, including a 20% drop out rate. The median PFS was calculated with 95% confidence interval (CI). Primary statistical analysis of the efficacy endpoint was performed 6 months after registration of the last patient for the study.
The OS curves and cumulative incidence for PFS were estimated using the Kaplan–Meier method. A Drug Monitoring and Safety Committee (DMSC) was established and conducted regular safety reviews.
Results
Patient characteristics
From April 2006 to July 2009, 42 children and adolescents (16 male and 26 female) with newly diagnosed brain stem gliomas suspected to be DIPG were included in this trial. Median age of participants was 7.4 years with a range of 3 to 15 years. In the intention-to-treat (ITT) population Lansky or Karnofsky Performance Status (KPS) was evaluated at baseline visit for all patients. There were 9 patients (21.4%) with Lansky or KPS of 90, 11 patients (26.2%) with 80, 12 patients (28.6%) with 70, 6 (14.3%) with 60 and 4 (9.5%) with 40. At baseline 41 patients (97.6%) had a typical DIPG, while one patient (2.4%) suffered from a medulla oblongata tumor (Table 1).
Clinical efficacy
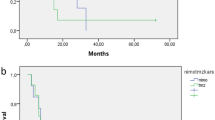
The primary endpoint of the study was not met according to the statistical design proposed because the median PFS (95% CI) was 5.8 months (4.8 months, 6.1 months) (Fig. 1). The PFS rate at 6 months was 36.9% (21.2%, 52.8%), one-sided sign test: p = 0.0541 and the PFS rate at 1 year was 5.3%, respectively.
Thirty-three patients died due to tumor progression during the study period. During follow up, another 8 patients died due to disease progression. Two children were alive without progression for 26 and 34 months and one patient was still alive with stable disease at last follow up (30th June 2010, OS 47.4 months). The median OS (95% CI) was 9.4 months (7.9, 11.6) (Fig. 2). The 1- and 2-year overall survival probabilities were 33.3% and 4.8%, respectively.
After 12 weeks of treatment with radiotherapy in combination with nimotuzumab, 2 patients had PR (4.8%), 27 patients had SD (64.3%), 10 patients had PD (23.8%) and 3 patients (7.1%) could not be evaluated for response (1 patient had an early death, 1 could not be radiologically evaluated and 1 did not have typical DIPG). The ORR at week 12 was 4.8% and the DCR was 69.1%.
Safety and tolerability
Patients received a median of 18 infusions of nimotuzumab (range 1–24 infusions) for a median duration of 5.4 months (range 0.1–9.6 months). Table 2 summarizes the incidence of AEs in subjects related to nimotuzumab and RT classified by Primary System Organ Class and Preferred Terms (MedDRA).
A total of 368 AEs were reported in 42 patients (100%). Sixty-six AEs (in 35 patients, 83.3%) were classified as SAEs and 302 as non-serious AEs. Eight of the SAEs (reported in 2 patients, 4.8%) and 64 of the non-serious AEs (reported in 21 patients, 50%) were assessed to be possibly, probably or definitely related to administration of nimotuzumab and RT; 238 AEs and 58 SAEs were assessed as having no association with the treatment. The most frequently reported treatment-related AEs were alopecia (14.3%), vomiting (7.1%), headache (7.1%), and radiation skin injury (7.1%). Headaches, fatigue, vomiting, nausea and temporary neurological deterioration occurred, particularly during the first weeks of the RT, possibly as a consequence of radiation induced oedema and required therapy with dexamethasone. The most severe therapy-related SAEs reported were recurrent intra-tumoral bleeding and acute respiratory failure occurring in two different patients.
Discussion
Diffuse intrinsic pontine glioma is a devastating cancer that is fatal in most cases and is the leading cause of brain tumor-related death in children. DIPG is not amenable to surgical resection due to its critical location and infiltrative nature [12]. Till date, RT is the only treatment that offers a transient benefit. Long-term survivors with DIPG are extremely rare, with very few patients surviving beyond 2 years [3, 12, 13]. Unfortunately, despite four decades of research aimed at expanding treatment options, the disease remains incurable [3, 4, 12, 13].
Radiotherapy delays the inevitable progression of disease by a few months, and can be repeated to prolong survival [14,15,16]. Despite improvement in the clinical outcomes with temozolomide observed in adult patients with glioblastoma, combination of radiation and temozolomide has not improved outcomes in DIPG over the past 15 years [17,18,19]. Chemotherapeutic strategies including multi-agent neoadjuvant chemotherapy, concurrent chemotherapy with RT, and adjuvant chemotherapy have not provided any consistent survival benefit when compared with standard RT [12, 15, 18].
In an effort to alter the dismal prognosis in most children, numerous studies have been undertaken with hopes of improving outcomes. Nimotuzumab is an anti-EGFR humanized monoclonal antibody that blocks EGF and TGF-α from binding to EGFR and inhibits its intrinsic tyrosine kinase activity. Nimotuzumab requires bivalent interaction for binding to EGF receptors and therefore accumulates predominantly on tumor tissue with high of EGFR density. Tumor cell surface studies have shown that nimotuzumab has a binding affinity of the order of 10−9 M to EGFR and specifically recognizes EGFR/HER-1 but not HER-2, HER-3, or HER-4. It also demonstrates positive recognition to fetal and adult tissues rich in EGFR and tumors of epithelial origin [20, 21].
The antibody mediates anti-neoplastic effects via inhibition of signal transduction, thus inhibiting proliferation of EGFR-bearing tumor cells, increasing apoptosis, and reducing angiogenesis. Nimotuzumab inhibits cell proliferation, induces cell cycle arrest in G1 phase of cell division and induces down-regulation of Vascular Endothelial Growth Factor (VEGF) production [22]. Furthermore, in vitro studies have demonstrated that nimotuzumab has immunological activity, specifically in activation of CD8+ T cells [23, 24]. Results reported by the study show favorable outcomes when nimotuzumab is used in combination with RT, especially with regards to the low toxicity of the combination [8, 11]. Other studies have shown that concomitant treatment with EGFR inhibitors and radiation was feasible in DIPG, even though the effect on clinical outcomes was minimal [25,26,27,28].
During the study, drug-related AEs of all grades were most frequently reported during concomitant treatment with radiation or suspected to be correlated with tumor progression [29].
Clinical experience with nimotuzumab has demonstrated that its combination with radiotherapy does not produce toxicities commonly associated with other EGFR-targeting agents, such as severe acneiform rash, skin rash, hypokalemia and hypomagnesaemia. Better drug tolerability has allowed researchers to perform pilot studies with other drug combinations as well with interesting results [30, 31].
This study was classified as a phase III study (i) after a phase II study in children suffering from a recurrent high grade glioma or DIPG has shown lack of severe toxicity besides modest efficacy using nimotuzumab as a single drug in a BSA adjusted dose [8], and (ii) as the main goal of this study was defined as the evaluation of efficacy of combined therapy of nimotuzumab in the previously evaluated drug dose with a standard treatment of local radiotherapy.
In summary, nimotuzumab administered concomitantly and continued after RT was well tolerated and had comparable efficacy to the combination of intensive chemotherapy and RT. While other chemotherapeutic regimens are often associated with prolonged hospitalization, this treatment regimen could be administered within a short period of time in outpatient settings, with longer intervening intervals at home or attending school. Further translational research on molecular alterations in DIPG is needed to identify patients who may benefit from therapy with nimotuzumab and other EGFR inhibitors and to provide molecular rationale for combination with other targeted therapies, cytotoxic agents, immunotherapy and radiotherapy [11]. Possibility of adding other drugs without harming patients gives a hope of improving clinical outcomes in DIPG.
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Introduction to DIPG. DIPG registry. http://dipgregistry.org/medical-professionals/introduction-to-dipg/. Accessed 23 Oct 2018
Vanan MI, Eisenstat DD (2015) DIPG in children—what can we learn from the past? Front Oncol 5:237. https://doi.org/10.3389/fonc.2015.00237
Warren KE (2012) Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2:205. https://doi.org/10.3389/fonc.2012.00205
Bredel M, Pollack IF, Hamilton RL, James CD (1999) Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res 5:1786–1792
Diaz Miqueli A, Rolff J, Lemm M, Fichtner I, Perez R, Montero E (2009) Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer 100:950–958. https://doi.org/10.1038/sj.bjc.6604943
Bode U, Massimino M, Bach F, Zimmermann M, Khuhlaeva E, Westphal M, Fleischhack G (2012) Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther 12:1649–1659. https://doi.org/10.1517/14712598.2012.733367
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248. https://doi.org/10.1016/S1470-2045(06)70615-5
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Bartels U, Wolff J, Gore L, Dunkel I, Gilheeney S, Allen J, Goldman S, Yalon M, Packer RJ, Korones DN, Smith A, Cohen K, Kuttesch J, Strother D, Baruchel S, Gammon J, Kowalski M, Bouffet E (2014) Phase 2 study of safety and efficacy of nimotuzumab in pediatric patients with progressive diffuse intrinsic pontine glioma. Neuro Oncol 16:1554–1559. https://doi.org/10.1093/neuonc/nou091
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38:27–35. https://doi.org/10.1016/j.ctrv.2011.06.007
Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C, Bartels U, Bouffet E, Goldman S, Leary S, Foreman NK, Packer R, Warren KE, Broniscer A, Kieran MW, Minturn J, Comito M, Broxson E, Shih CS, Khatua S, Chintagumpala M, Carret AS, Escorza NY, Hassall T, Ziegler DS, Gottardo N, Dholaria H, Doughman R, Benesch M, Drissi R, Nazarian J, Jabado N, Boddaert N, Varlet P, Giraud G, Castel D, Puget S, Jones C, Hulleman E, Modena P, Giagnacovo M, Antonelli M, Pietsch T, Gielen GH, Jones DTW, Sturm D, Pfister SM, Gerber NU, Grotzer MA, Pfaff E, von Bueren AO, Hargrave D, Solanki GA, Jadrijevic Cvrlje F, Kaspers GJL, Vandertop WP, Grill J, Bailey S, Biassoni V, Massimino M, Calmon R, Sanchez E, Bison B, Warmuth-Metz M, Leach J, Jones B, van Vuurden DG, Kramm CM, Fouladi M (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma [DIPG]: a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36:1963–1972. https://doi.org/10.1200/JCO.2017.75.9308
Langmoen IA, Lundar T, Storm-Mathisen I, Lie SO, Hovind KH (1991) Management of pediatric pontine gliomas. Childs Nerv Syst 7:13–15
Massimino M, Spreafico F, Biassoni V, Simonetti F, Riva D, Trecate G, Giombini S, Poggi G, Pecori E, Pignoli E, Casanova M, Ferrari A, Meazza C, Luksch R, Terenziani M, Cefalo G, Podda M, Polastri D, Clerici CA, Fossati-Bellani F, Gandola L (2008) Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neurooncol 87:355–361. https://doi.org/10.1007/s11060-008-9525-5
Janssens GO, Gandola L, Bolle S, Mandeville H, Ramos-Albiac M, van Beek K, Benghiat H, Hoeben B, Morales La Madrid A, Kortmann RD, Hargrave D, Menten J, Pecori E, Biassoni V, von Bueren AO, van Vuurden DG, Massimino M, Sturm D, Peters M, Kramm CM (2017) Survival benefit for patients with diffuse intrinsic pontine glioma [DIPG] undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73:38–47. https://doi.org/10.1016/j.ejca.2016.12.007
Lashford LS, Thiesse P, Jouvet A, Jaspan T, Couanet D, Griffiths PD, Doz F, Ironside J, Robson K, Hobson R, Dugan M, Pearson AD, Vassal G, Frappaz D, United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study (2002) Temozolomide in malignant gliomas of childhood: a United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol 20:4684–4691. https://doi.org/10.1200/JCO.2002.08.141
Verschuur AC, Grill J, Lelouch-Tubiana A, Couanet D, Kalifa C, Vassal G (2004) Temozolomide in paediatric high-grade glioma: a key for combination therapy? Br J Cancer 91:425–429. https://doi.org/10.1038/sj.bjc.6601997
Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, Pollack IF (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 13:410–416. https://doi.org/10.1093/neuonc/noq205
Ledon N, Casaco A, Casanova E, Beausoleil I (2011) Comparative analysis of binding affinities to epidermal growth factor receptor of monoclonal antibodies nimotuzumab and cetuximab using different experimental animal models. Placenta 32:531–534. https://doi.org/10.1016/j.placenta.2011.04.008
Perez R, Moreno E, Garrido G, Crombet T (2011) EGFR-targeting as a biological therapy: understanding nimotuzumab’s clinical effects. Cancers 3:2014–2031. https://doi.org/10.3390/cancers3022014
Diaz Miqueli A, Blanco R, Garcia B, Badia T, Batista AE, Alonso R, Montero E (2007) Biological activity in vitro of anti-epidermal growth factor receptor monoclonal antibodies with different affinities. Hybridoma 26:423–431. https://doi.org/10.1089/hyb.2007.0516
Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A (2002) Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: a humanized anti-EGFR antibody. Int J Cancer 101:567–575. https://doi.org/10.1002/ijc.10647
Garrido G, Sanchez B, Rodriguez HM, Lorenzano P, Alonso D, Fernandez LE (2004) 7A7 MAb: a new tool for the pre-clinical evaluation of EGFR-based therapies. Hybrid Hybrid 23:168–175. https://doi.org/10.1089/1536859041224280
Geoerger B, Hargrave D, Thomas F, Ndiaye A, Frappaz D, Andreiuolo F, Varlet P, Aerts I, Riccardi R, Jaspan T, Chatelut E, Le Deley MC, Paoletti X, Saint-Rose C, Leblond P, Morland B, Gentet JC, Méresse V, Vassal G, ITCC (Innovative Therapies for Children with Cancer) European Consortium (2011) Innovative therapies for children with cancer: pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol 13:109–118. https://doi.org/10.1093/neuonc/noq141
Pollack IF, Stewart CF, Kocak M, Poussaint TY, Broniscer A, Banerjee A, Douglas JG, Kun LE, Boyett JM, Geyer JR (2011) A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol 13:290–297. https://doi.org/10.1093/neuonc/noq199
Broniscer A, Baker JN, Tagen M, Onar-Thomas A, Gilbertson RJ, Davidoff AM, Pai Panandiker AS, Leung W, Chin TK, Stewart CF, Kocak M, Rowland C, Merchant TE, Kaste SC, Gajjar A (2010) Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol 28:4762–4768. https://doi.org/10.1200/JCO.2010.30.3545
Broniscer A, Baker SD, Wetmore C, Pai Panandiker AS, Huang J, Davidoff AM, Onar-Thomas A, Panetta JC, Chin TK, Merchant TE, Baker JN, Kaste SC, Gajjar A, Stewart CF (2013) Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 19:3050–3058. https://doi.org/10.1158/1078-0432.CCR-13-0306
Broniscer A, Laningham FH, Kocak M, Krasin MJ, Fouladi M, Merchant TE, Kun LE, Boyett JM, Gajjar A (2006) Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer 106:1364–1371. https://doi.org/10.1002/cncr.21749
Massimino M, Biassoni V, Miceli R, Schiavello E, Warmuth-Metz M, Modena P, Casanova M, Pecori E, Giangaspero F, Antonelli M, Buttarelli FR, Potepan P, Pollo B, Nunziata R, Spreafico F, Podda M, Anichini A, Clerici CA, Sardi I, De Cecco L, Bode U, Bach F, Gandola L (2014) Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 118:305–312. https://doi.org/10.1007/s11060-014-1428-z
Massimino M, Biassoni V, Miceli R, Schiavello E, Warmuth-Metz M, Modena P, Casanova M, Pecori E, Giangaspero F, Antonelli M, Buttarelli FR, Potepan P, Pollo B, Nunziata R, Spreafico F, Podda M, Anichini A, Clerici CA, Sardi I, De Cecco L, Bode U, Bach F, Gandola L (2018) Correction to: Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. https://doi.org/10.1007/s11060-018-2893-6
Acknowledgements
Authors are thankful to all patients and their families who were a part of the study. We would also like to thank all pediatricians, medical doctors, neurologists, medical oncologists and nurses who participated and without whose contribution this study would not have been possible.
Funding
This study was funded by Oncoscience GmbH, Schenefeld, Germany (Study No.: OSAG 101-BSC-05). Oncoscience GmbH has also provided the investigational drug nimotuzumab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
GF, MM, MWM, EK, GJ, NG, SR, AB, IS, VB, SKG, CK, HR, PGS, RDK and UB have received research funding and expense allowance for study conduct from Oncoscience GmbH. GF and UB have received support from Oncoscience GmbH for meeting travel to present study results. NEIE is an employee of the Center of Molecular Immunology, the research institution that patented and manufactures nimotuzumab. DR is an employee of Oncoscience GmbH. FB was an employee and has had stock options of Oncoscience GmbH that funded this study and has provided the investigational drug.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national committees. The trial was conducted in accordance with 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The institutional review boards or ethics committees of all participating centers reviewed and approved the protocol. All parents/guardians and patients, where appropriate, gave their written informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F. Bach—deceased.
Rights and permissions
About this article
Cite this article
Fleischhack, G., Massimino, M., Warmuth-Metz, M. et al. Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): a phase III clinical study. J Neurooncol 143, 107–113 (2019). https://doi.org/10.1007/s11060-019-03140-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03140-z