Abstract
Target population
Adult patients (older than 18 years of age) with newly diagnosed World Health Organization (WHO) Grade II gliomas (Oligodendroglioma, astrocytoma, mixed oligoastrocytoma).
Question
Is there a role for chemotherapy as adjuvant therapy of choice in treatment of patients with newly diagnosed low-grade gliomas?
Recommendations
Level III
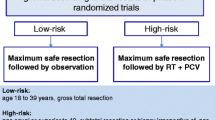
Chemotherapy is recommended as a treatment option to postpone the use of radiotherapy, to slow tumor growth and to improve progression free survival (PFS), overall survival (OS) and clinical symptoms in adult patients with newly diagnosed LGG.
Question
Who are the patients with newly diagnosed LGG that would benefit the most from chemotherapy?
Recommendation
Level III
Chemotherapy is recommended as an optional component alone or in combination with radiation as the initial adjuvant therapy for all patients who cannot undergo gross total resection (GTR) of a newly diagnosed LGG. Patient with residual tumor >1 cm on post-operative MRI, presenting diameter of >4 cm or older than 40 years of age should be considered for adjuvant therapy as well.
Question
Are there tumor markers that can predict which patients can benefit the most from initial treatment with chemotherapy?
Recommendation
Level III
The addition of chemotherapy to standard RT is recommended in LGG patients that carry IDH mutation. In addition, temozolomide (TMZ) is recommended as a treatment option to slow tumor growth in patients who harbor the 1p/19q co-deletion.
Question
How soon should the chemotherapy be started once the diagnosis of LGG is confirmed?
Recommendation
There is insufficient evidence to make a definitive recommendation on the timing of starting chemotherapy after surgical/pathological diagnosis of LGG has been made. However, using the 12 weeks mark as the latest timeframe to start adjuvant chemotherapy is suggested. It is recommended that patients be enrolled in properly designed clinical trials to assess the timing of chemotherapy initiation once diagnosis is confirmed for this target population.
Question
What chemotherapeutic agents should be used for treatment of newly diagnosed LGG?
Recommendation
There is insufficient evidence to make a recommendation of one particular regimen. Enrollment of subjects in properly designed trials comparing the efficacy of these or other agents is recommended so as to determine which of these regimens is superior.
Question
What is the optimal duration and dosing of chemotherapy as initial treatment for LGG?
Recommendation
Insufficient evidence exists regarding the duration of any specific cytotoxic drug regimen for treatment of newly diagnosed LGG. Enrollment of subjects in properly designed clinical investigations assessing the optimal duration of this therapy is recommended.
Question
Should chemotherapy be given alone or in conjunction with RT as initial therapy for LGG?
Recommendation
Insufficient evidence exists to make recommendations in this regard. Hence, enrollment of patients in properly designed clinical trials assessing the difference between chemotherapy alone, RT alone or a combination of them is recommended.
Question
Should chemotherapy be given in addition to other type of adjuvant therapy to patients with newly diagnosed LGG?
Recommendation
Level II: It is recommended that chemotherapy be added to the RT in patients with unfavorable LGG to improve their progression free survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chemotherapy rationale
The main focus of these guidelines is on the supratentorial WHO II gliomas, which include only WHO II astrocytomas, oligodendrogliomas and mixed oligoastrocytomas, in patients older than 18 years of age. [1] Conventional treatment of Grade II gliomas consists of extensive surgical resection, if possible, followed by observation or radiation therapy. Recently, there has been interest in adding chemotherapy as an early treatment. This interest is based on several reports that have demonstrated the high rate of response of anaplastic oligodendrogliomas [2, 3]. Historically, for WHO Grade II gliomas, chemotherapy has been explored mainly in the management of recurrence or progression of the disease and less frequently as the first line of therapy soon after histological diagnosis is made.
Given the increased routine use of molecular classification, it may be possible to identify (1) the group of patients that should be considered for initial chemotherapy treatment, (2) those chemotherapeutic agents that are the most beneficial, (3) the duration of treatment administration, and (4) whether chemotherapy should be given alone, in combination with RT or not at all.
Given this evolving appreciation of the value of chemotherapy for newly diagnosed LGG a comprehensive search and evaluation of the available and relevant literature was carried out in an attempt to formulate guidance for treatment of these lesions and to identify areas that require additional studies.
Chemotherapy methodology
To answer the questions described above, a comprehensive systematic literature review was performed. The search strategy is documented in the methodology paper for these guidelines series written by Olson et al.
Literature review
The following databases were searched from January 1990 to December 2012 using low-grade glioma and surgery relevant search MeSH and non-MeSH search terms: PubMed (National Library of Medicine, http://www.ncbi.nlm.nih.gov) was searched using Endnote® (Thomas Reuters, Inc. http://www.endnote.com). The keywords used during our search in the medical literature search engines cited above are documented in Table 2. Manual searches of the included article’s bibliographies were also conducted.
Article inclusion and exclusion criteria
For literature to be included for consideration, studies published in full as peer review papers had to meet the following criteria:
-
Be published in English.
-
Involve patients with newly diagnosed WHO grade 2 astrocytoma, oligo-astroctyoma, or oligodendroglioma.
-
Involve adult patients (age over 18) or provide isolated results for adult patients in a mixed cohort.
-
Fully published, peer-review articles.
-
The number of study participants with newly diagnosed LGG was at least 5 for each study arm.
-
Use of chemotherapy after diagnosis of LGG has been made.
-
Supratentorial LGG only.
Study selection and quality assessment
After an extensive search, more than 1739 articles were found. The duplicates from the search in different databases were eliminated. By reviewing the titles and/or abstracts, we excluded all articles referring to anaplastic gliomas or glioblastomas, those discussing exclusively chemotherapy in patients younger than 18 years of age, glioma of the spine, optic nerve, brain stem and/or posterior fossa. We excluded as well those publications that discussed exclusively chemotherapy used for treatment of recurrent/progressive LGG and all articles discussing experimental therapy in animal tumor models. The remaining 101 articles underwent full text review. Only 13 articles met all of the inclusion criteria and were used in formulating these evidence-based clinical guidelines (Table 2). The majority of the remaining 88 articles that underwent full review were excluded because they reported the use of chemotherapy at recurrence or progression together with its use for the initial treatment and with results that were not separable, and the remainder because they lacked significance for our topic.
Evidence classification and recommendation levels
Both the quality of the evidence and the eventual strength of the recommendations generated by this evidence were graded according to a three-tiered system for assessing studies addressing diagnostic testing as approved by the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee on criteria.
Conflict of interest
Low Grade Glioma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair may approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs.
Chemotherapy scientific foundation
The use of chemotherapy as the initial treatment for patients with newly diagnosed LGG is controversial. LGG are slow growing tumors and the goal of treatment is to improve symptoms and prolong progression free survival (PFS) and overall survival (OS) [4]. Different groups have reported on the efficacy of chemotherapy for LGG. However, in our review, we found that the literature is complicated because the vast majority of the studies that have reported on the effects of chemotherapy include a mix of Grade I and II gliomas, grade II and III gliomas, pediatric and adult patients, or infra- and supratentorial low grade gliomas, and newly diagnosed and recurrent tumors. These mixed patient populations limit the ability to quantify the impact of chemotherapy in our target population, which are adults with newly diagnosed supratentorial LGG (WHO II glioma). In addition, the majority of the studies that have examined the effect of chemotherapy on LGG have focused on its use at the time of recurrence or progression. We found very few studies that met our criteria regarding the use of chemotherapy alone as frontline treatment for LGG (Table 1). Several other studies were excluded from our review, even though they intended to report on the use of chemotherapy as initial treatment for newly diagnosed LGG because the length of time from the diagnosis was made to the time when the chemotherapy was started. For example, Murphy et al. [5] reported that the chemotherapy was started up to 44 months after initial diagnosis, Hoang-Xuan and colleague, reported starting chemotherapy up to 108 months after the diagnosis was made [6], and in a publication by Peyre et al., the time delay from initial diagnosis and the start of the chemotherapy ranged from 0.1 to 13 years [7]. These studies may be difficult to interpret given the possibilities of selection bias and concerns for changes in tumor biology or malignant transformation.
Another difficulty in evaluating the literature published on adjuvant chemotherapy for LGG consist in the inability to separate the results of patients that are treated after having undergone partial or subtotal resection of the tumor versus those that undergone biopsy alone. It has to be underlined that there has been only one published prospective randomized trial evaluating the role of chemotherapy in newly diagnosed LGG so far [8]. In their publication, Shaw et al., discussed short-term follow up results of the RTOG 9802 trial [8]. As discussed in the appropriate section below, even this trial did not study the efficacy of the chemotherapy as the only adjuvant treatment in LGG versus other treatments, and it was focused in evaluating the role of chemotherapy when added to radiation therapy as compared to RT alone in patients of a certain population diagnosed with LGG.
Given the opportunity for effective salvage treatment with radiation (if not given frontline) or alternative chemotherapy regimens, in defining the role of initial chemotherapy in newly diagnosed LGG, we considered PFS in addition to OS as relevant indicators of treatment effect.
Is there a role for chemotherapy as adjuvant therapy of choice in treatment of patients with newly diagnosed low-grade gliomas?
Ten studies met our inclusion criteria in answering the question of whether there is a role for the use of chemotherapy alone as initial treatment for newly diagnosed LGG, although none were randomized. All of the studies provide class III evidence.
Ricard et al. evaluated the natural progression of low grade gliomas and the impact of temozolomide (TMZ) on tumor diameter changes [9]. They evaluated the mean tumor diameter (MTD) changes of 146 patients with LGG before, during and after TMZ treatment with serial MRIs. They included adult patients with confirmed histological diagnosis of LGG after central review. In the first group, 39 patients were evaluated before, during and after treatment with TMZ. These 39 patients had at least 4 consecutive MRIs with a median follow-up of 3.6 years (range 1 to 9.2 years) before initiation of TMZ. One hundred seven patients, included in the second group, were evaluated only during and after treatment with TMZ. Standard dose of TMZ was used: 200 mg/m2 orally on days 1–5 of 28 days cycle for a mean of 17 cycles (2–30 cycles). Before treatment with TMZ, the MTD increased linearly overtime. The growth was slower in tumors harboring 1p/19q co-deletion (3.4 vs. 5.9 mm/year) and in those that did not express p53 (4.2 vs. 6.3 mm/year; p < 0.05). After starting TMZ, the authors reported that there was a decrease in MTD in 38/39 patients of the first group and in 98/107 of the second group. These changes were calculated on at least 4 consecutive MRIs. In the group demonstrating a decline in the MTD, the decrease was linear and was half of the pretreatment growth rate (9.2 vs. 4.7 mm/year).
The authors defined complete radiologic response as the complete disappearance of all tumors on T2W MRI or FLAIR sequences at 8 weeks. Partial response was defined as more than 50 % reduction in the size (by cross-sectional area), minor response was defined as 25–50 % reduction in size and progressive disease was defined as more than 25 % increase in size of the tumor. Twenty patients achieved a partial radiological response, 45 achieved a minor response, and 35 had tumor stabilization, whereas 7 had tumor progression. Clinical improvement was observed in 68 (63.5 %) patients, whereas 34 (31.8 %) were clinically stable, and 5 deteriorated. Among those with objective response, the decrease of MTD after starting the TMZ was immediate in 77 patients and delayed by a median of 116 days (48–206) in 21 patients. After a median of 367 days (95 % CI 290–403; range 98–756 days), in 36 of 98 patients, tumor regrowth was noticed. The risk of regrowth was significantly greater in patients without 1p/19q co-deletion (60.6 vs. 16.6 %; p < 0.0004). TMZ was discontinued in 25 patients because the clinician believed that the patient had received the optimal course of TMZ. The majority of these tumors resumed their progressive growth within 1 year. The authors concluded that untreated LGG grow continuously on an almost predictable fashion and this is influenced by their genetic alterations. TMZ reverses this pattern at onset, but this effect does not last. Furthermore they conclude that the majority of the tumors will resume their growth when treatment is discontinued.
Lebrun and colleagues retrospectively reviewed their institutional experience in treating 33 patients with low grade oligodendrogliomas that had undergone partial resection and chemotherapy [10]. None of these patients underwent RT. Chemotherapy regimen was as follows: PCV (Lomustine, Procarbazine, Vincristine) in 6 week cycles for 6 cycles. Lomustine 110 mg/m2 on day 1, Procarbazine 60 mg/m2 on days 8–21 and Vincristine 1.4 mg/m2 (maximum 2 mg) on days 8 and 29 in cycles of 6 weeks for a maximum of six cycles. Chemotherapy was started at a mean of 2 months after the surgical procedure. They reported a PFS of more than 30 months (median not reached at the time of the report). The survival rate at 2 and 5 years was 85 and 75 % respectively. The PFS rate at 1 year was 90 %. Clinical response (defined as a reduction of seizure frequency) was observed in 81 % of the patients. They concluded that upfront PCV treatment could be used in symptomatic patients with low-grade oligodendroglioma that cannot undergo GTR to postpone RT and prolong PFS.
Stege et al., reviewed their single institution experience of treating 21 patients with LGG with PCV (16 newly diagnosed and 5 recurrent low grade oligodendroglioma) [11]. Large or multilobar tumors that would have required large radiation volumes were considered for chemotherapy at the time of diagnosis and RT was deferred. Nine of the patients with newly diagnosed tumor had gliomatosis cerebri. Of the 16 patients with newly diagnosed tumors, 3 had a partial radiologic response (defined as 50 % decrease in the size of the lesion) and 10 a minor response (defined as less than 50 % reduction in tumor size). Median time to progression was not reached at 24 months follow up. The authors reported improvement in seizure frequency. They concluded that chemotherapy is effective in patients with large low grade oligodendroglioma or mixed oligoastrocytoma. However, this retrospective review did not report on the timing of starting the chemotherapy after surgical intervention or diagnosis.
Another single institution, prospective, nonrandomized study of 18 adult patients with LGG was reported by Higuchi and colleagues [12]. Five patients who underwent GTR underwent observation only with serial MRIs. Twelve patients that underwent STR or biopsy only received chemotherapy immediately after surgical procedure, and 1 patient refused treatment. The treatment regimen consisted of ACNU 75 mg/m2 for day 1, vincristine 1 mg/m2 on days 8 and 29 and procarbazine 100 mg/day for days 8–21 for 4 cycles a year for 2 years. They estimated the tumor response to chemotherapy using MRI classifying patients as responders when there was more than a 50 % reduction in tumor volume, and non-responders if there was more than a 25 % increase in tumor volume and no change in all other situations. There were seven responders and the remaining 5 were categorized as having no change in tumor size. The authors reported that 94 % of the LGG tumors could be controlled without RT during a median follow up of 4.7 years. The authors concluded that chemotherapy can be used safely to control PFS and OS in patients undergoing partial/subtotal surgical resection or biopsy alone. Furthermore, they stated that surgical resection and chemotherapy for residual tumor is an adequate initial treatment of low-grade oligodendroglioma, permitting delay of RT until tumor progression or recurrence.
In a phase II trial of PCV for LGG, Buckner and colleagues evaluated the efficacy of chemotherapy in treatment of 28 patients with low-grade oligodendroglioma and oligoastrocytoma that had undergone biopsy or subtotal resection only [13]. Patients that had undergone GTR were not eligible. This was a prospective non-randomized phase II trial. Chemotherapy was started 3–12 weeks after surgery and repeated every 8 weeks for 6 cycles. Ten weeks after completion of chemotherapy, or earlier if there was evidence of tumor progression, RT was started. All patients had histologic confirmation by central review. Twenty-five of the 28 patients had both baseline and pre-irradiation MRI scans available for central review. Radiologic assessment after chemotherapy showed objective response in 8 patients, disease stability in 17 and tumor progression in 3. The authors stated that the response rate to initial chemotherapy observed by the clinicians was 29 % (95 % CI 13–49 %). However, in this study patients received the “intensive” PCV regimen (Procarbazine 75 mg/m2 PO on day 8–21, Lomustine 130 mg/m2 PO on day 1, Vincristine 1.4 mg/m2 IV on day 8 & 29. This regimen was repeated every 8 weeks for total of 6 cycles.) The conclusion of this phase II study was that PCV produces tumor regression in a meaningful proportion of patients with LGG, but toxicity consisting of grade 3 and 4 thrombocytopenia and leucocytopenia, pulmonary histiocytosis and neurotoxicity (lethargy and peripheral neuropathy), with the intensive PCV regimen was significant.
Two studies have exclusively evaluated the benefits of chemotherapy in treating the symptoms caused by the LGG. Sherman and colleagues evaluated the impact of TMZ on seizure control in patients with LGG [14]. This was a retrospective review of patients with LGG that presented with new onset of seizures. There were 39 patients in the treatment group and 30 in the control group. In the treatment group, patients underwent surgical resection or biopsy and then started chemotherapy, whereas patients in the control group did not receive chemotherapy after the surgical procedure. The authors did not report the extent of tumor resection, including the number of patients that underwent GTR. Twelve (28 %) of patients in the treatment group and 14 (47 %) in the control group experienced complete seizure control following treatment with AED. Five (13 %) patients in the treatment group had no improvement in seizure control as compared to 12 (40 %) in the control group despite manipulation of anti-epileptic drugs (AED). There was a greater than 50 % improvement in seizure control with and without AEDs in 23 (59 %) of patients in the treatment group and only in 5 (13 %) of patients in the control group. Seven (18 %) of these patients in the treatment group experienced greater than 50 % seizure control compared to none in the control group. The authors concluded that TMZ treatment appears to lower the seizure frequency in a subset of patients with LGG. However, the interpretation of these results is limited because the timing when the TMZ was started after surgical intervention was not reported. Furthermore the reasons why the patients in the control group did not receive chemotherapy were not provided. In another study Frenay et al. reported on the efficacy of chemotherapy in controlling the symptoms in patients with LGG [1]. This was a single institution, retrospective review of 10 patients with unresectable fibrillary astrocytoma. All 10 patients underwent biopsy prior to starting chemotherapy and none were treated with RT. Chemotherapy was started 2–4 months after the biopsy. They reported that there was no tumor progression with a mean follow up of 6.5 years. All 10 patients experienced improvement of aphasia (if present) and seizure control (reduction of seizure frequency in 3 patients, resolution of seizures in 4 patients). They reported that there was evidence of radiologic response in 6 patients. The authors concluded that in patients with refractory epilepsy, caused by unresectable grade II astrocytoma, adjuvant PCV can improve the neurological status and first line chemotherapy may allow postponement of RT. The study did not have a control arm, limiting the conclusions regarding efficacy.
Olson and colleagues performed a retrospective review to evaluate the benefit of adjuvant therapy for LGG. One hundred and six patients fulfilled inclusion criteria [15]. Nineteen underwent GTR, 41—subtotal resection (STR), 28 underwent only a biopsy and 18 were diagnosed based on radiologic criteria only. Patients underwent different treatment modalities: 20 underwent RT, 12 received chemotherapy (PCV 14, carmustine 1 and cisplatin 1), 6 received chemoradiation and 68 did not undergo any adjuvant treatment. Recurrence was diagnosed in 72 patients after a median of 6 years. Median time to progression (MTTP) was 3.9 years for observation patients, 5.7 years for RT patients, 5.5 years for chemotherapy patients and 8.6 years for patient treated with RT + chemotherapy. The authors concluded that the timing and treatment modality (Observation, RT, chemotherapy or chemoradiation) did not appear to affect progression or survival. As such a judicious approach was suggested for the management of these patients and withholding treatment until necessary was advised. Furthermore, the authors recommended that since the toxicities due to chemotherapy were acute, but overall reversible, unlike the RT induced toxicities that are delayed and irreversible, chemotherapy may be preferable as initial therapy.
Iwadate et al. prospectively followed 36 patients with oligodendroglioma and mixed glioma [16]. Patients that underwent GTR (n = 15) did not undergo adjuvant treatment until the time of recurrence or progression. Patients that had a STR underwent PVA treatment (ACNU 75 mg/m2 day 1, Vincristine 1 mg/m2 on days 8 and 29, procarbazine 10 mg/day on days 8–21, 4 times/year) for 2 years. Chemotherapy was started within 12 weeks of surgery. PFS at 5 and 10 years was 75 and 46.9 %. No significant difference was found in PFS between the 2 groups. The authors concluded that the best way to treat the LGG is to attempt the greatest possible surgical resection without neurological deterioration followed by simple observation. Furthermore they concluded that those patients who do not undergo GTR may benefit from addition of chemotherapy within 12 weeks of surgery.
Nakamura et al., reported a retrospective experience from a single institution study that did not find clear evidence that addition of chemotherapy to RT had any benefit in treatment of LGG [17]. Eighty-eight patients were included in this study. The chemotherapy regimen was a combination of ACNU and vincristine that was administered intravenously during RT and for 2 cycles thereafter at 5 weeks interval. Forty-three patients underwent radical resection and 45 underwent subtotal resection. After surgery 52 patients received RT alone, 14—received chemotherapy combined with RT and 22 did not receive either RT or chemotherapy. Median PFS for all patients was 5.9 years with the PFS rate at 5 and 10 years of 45 and 7 %, respectively. Median overall survival was 8.5 years for patients that received postoperative RT and 7.6 years for the 14 patients that received chemotherapy with RT. The patients who underwent radical resection without adjuvant therapy had a median overall survival of 5.1 years. Chemotherapy was used in combination with RT and not alone. The report does raise questions about the selection criteria for treatment of individual patients and this impacts the interpretation of these results. For example, could the decision to give 14 patients chemotherapy with radiation have been based on worse molecular, histological and imaging characteristics?
In summary, with the acknowledged limitations of the cited studies reporting control of tumor growth, prolonging PFS, OS and improving symptoms, there is level III support for the use of chemotherapy as a treatment option for the newly diagnosed LGG. In addition, several studies have suggested using chemotherapy as initial treatment for LGG alone in order to postpone RT treatment [1, 9, 10, 11, 12, 15, 16].
Who are the patients with newly diagnosed LGG that would benefit the most from chemotherapy?
Studies to definitively answer this question do not exist. In the 13 studies that met our inclusion criteria, initial adjuvant chemotherapy either alone or in combination with RT was limited to patients who did not undergo radiologic confirmed GTR (Table 2). Therefore, the role on chemotherapy in all patients with LGG cannot be ascertained. Shaw and colleagues published data from the RTOG 9802 trial, a prospective randomized study, where they reported on the recurrence rate following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma [18]. Patients were divided in 2 different risk groups based on age and neurosurgeon-determined extent of resection. The favorable group included all patients with age <40 year-old that underwent GTR, and the unfavorable group included patients of >40 years of age that had undergone any amount of resection of the tumor and the patients <40 years of age that had undergone less than GTR. One hundred eleven patients in the favorable group were followed with serial MRIs after surgical resection and did not undergo any additional therapy. We would like to underline that even though the assignment of patients in each of the two groups was based on neurosurgeon-determined post-operative amount of resection, the results and the conclusions were reported after considering the radiologic-determined residual disease on post-operative MRI. Median follow up duration was 4.4 years. There were 8 deaths due to disease progression and 49 patients had tumor progression. Overall survival at 2 and 5 years was 99 and 93 %, respectively and PFS at 2 and 5 years were 82 and 48 % respectively. For the remaining 253 patients deemed to have unfavorable LGG, OS at 2 and 5 years was 87 and 66 % (p < 0.0001) and PFS was 73 and 50 % (p < 0.13). The latter patients were randomized to receive either RT alone or RT with PCV (RTOG 9802 trial). Median PFS was 4.9 years for the favorable (observation) group and 5.5 years for the unfavorable group. Ninety-eight of 111 patients of favorable group underwent post-operative imaging evaluation of sufficient quality to determine the amount of residual disease. Although all these patient were declared to have undergone GTR by neurosurgical report, post-operative imaging showed that 58 of 98 patients (59 %) had a residual disease of <1 cm in all directions as measured on T2 and FLAIR MRI sequences, 31 (32 %) had a residual of 1–2 cm and 9 (9 %) had >2 cm residual disease. The authors identified 3 factors that were predictive of poorer OS: preoperative tumor diameter larger than 4 cm, histologic diagnosis of astrocytoma or mixed oligoastrocytoma and radiologic-determined post-operative residual of >1 cm. Extent of radiologic-determined surgical resection correlated with progression, as the recurrence rate was 26 % in patients with <1 cm postoperative residual, 68 % if 1–2 cm postoperative residual and 89 % if residual tumor was >2 cm. The authors of this study concluded that patients in the favorable risk group with radiologic-determined post-operative tumor residual of <1 cm, presenting diameter of <4 cm and histologic diagnosis of oligodendroglioma can be observed after initial surgery, but all other subsets of patients in the study-defined “favorable LGG risk group” should still be considered for additional treatment as are the patients in the unfavorable risk group. This study did not specifically address the role of chemotherapy alone in LGG, but it provides some insights for considering which patients should undergo adjuvant therapy after post hoc reassessment and recombination of the randomized groups. Furthermore, this article underlined that neurosurgeon-determined amount of resection status should be validated by post-operative imaging prior to decide on post-operative treatment plans.
Are there tumor markers that can predict those patients that benefit the most from initial treatment with chemotherapy?
Stege and colleagues studied 21 patients with oligodendroglioma, 16 of which were newly diagnosed [11]. All 16 patients only underwent a biopsy followed by PCV treatment. The tumor 1p/19q status of these patients was determined and they reported that even patients without 1p/19q deletions responded to chemotherapy. The authors asserted that although the response duration seemed shorter in the tumors with intact 1p and/or 19q, and relatively more “true” partial responses were observed in patients with loss of 1p/19q that in the other patients, the small number of patients studied prevents further conclusions.
As mentioned earlier, Ricard et al. evaluated the impact of TMZ on natural growth of LGG diameter [9]. They found that TMZ alters the growth pattern of LGG. Furthermore they reported that TMZ effects were brief in tumors that carried p53 mutation and those that do not harbor 1p/19q co-deletion.
Buckner and his group in evaluating the efficacy of PCV as initial therapy in 28 adult patients with low grade oligodendroglioma and mixed oligoastrocytoma found that the loss of 1p and 19q was not associated with better response to treatment [13].
Iwadate et al., studied 36 adult patients with diagnosis of LGG in a prospective, but not randomized study [16]. Twenty-one patients who underwent subtotal resection or partial resection received initial adjuvant chemotherapy and 15 patients that underwent GTR were observed and underwent chemotherapy treatment only at the time of progression (5 patients). Twenty-three patients from the entire cohort had tumors with 1p/19q co-deletion. PFS of these patients was 121 months, but was not significantly different from those with tumors without 1p/19q co-deletion (101 months). The authors concluded that the outcome of all the patients with LGG was generally favorable irrespective of 1p/19q status. This report, however, did not relate patient outcomes to both 1p19q status and the use of chemotherapy.
In conclusion, one article suggested that patients with 1p/19q co-deletion showed better response to temozolomide [9]. The majority of the other retrospective studies that used PCV or PAV regimen suggested that LGG response to chemotherapy is irrespective of 1p/19q status [11, 13].
In a single institution, retrospective study, Okita and colleagues, evaluated the predictive value of IDH1 and IDH2 mutations in patients with LGG treated with adjuvant therapy [19]. Seventy-two adult patients underwent surgical resection and biopsy for diagnosis and then treated with RT (58 patients), of which 46 received chemotherapy as well. None of these patients received chemotherapy alone. They found that patients with tumors with IDH1/2 mutations that were treated with adjuvant chemoradiation had longer PFS (9.3 years) that those treated with RT alone (3.1 years) (p < 0.01). In contrast, there was no difference in PFS in patients with wild type IDH whether they received adjuvant chemoradiation treatment or not.
In summary, the data regarding the use of 1p19q co-deletion to determine treatment is inconclusive. Okita’s study [19], however, provides class III data regarding the predictive value of IDH mutations that is intriguing but requires additional investigation and validation.
How soon should the chemotherapy be started once the diagnosis of LGG is confirmed?
The timing of initiating chemotherapy after surgery has been quite varied. Shaw and colleagues, in the RTOG 8902 trial, reported starting the chemotherapy within 12 weeks after surgical resection in patients who also were undergoing radiation therapy [8]. The same timeframe was reported by Iwadate et al. [16]. and Buckner et al., (range 3–12 weeks) [13]. Frenay and colleagues started adjuvant therapy 8–16 weeks after the tumor biopsy [1]. Lebrun et al. reported that the adjuvant therapy was started at a mean of 2 months after surgical procedure (range 0.5–9 months) [10]. Because none of these studies have addressed directly the timing of starting adjuvant chemotherapy in LGG, definitive recommendations cannot be given. However since the majority of the studies reported starting chemotherapy within 12 weeks from the surgical procedure, it appears reasonable to suggest this as the maximal timeframe for initiation of adjuvant chemotherapy in newly diagnosed LGG.
What chemotherapeutic agents should be used for treatment of newly diagnosed LGG?
There are no studies that have directly compared different regimens or agents as the initial treatment of newly diagnosed LGG. As outlined in Table 2, the majority of the studies have used PCV (Procarbazine, Lomustine and Vincristine) [1, 8, 10, 11, 13, 15], and a few have used TMZ [9, 14, 19, 20]. Few other studies have used ACNU, vincristine and procarbazine regimen [12, 16, 17, 19].
What is the optimal duration and dosing of chemotherapy as initial treatment for LGG?
We did not find any reports that had evaluated the difference in efficacy and toxicity of different duration of specific chemotherapeutical agents’ regimens in newly diagnosed LGG that fulfilled our inclusion criteria. Hence, insufficient evidence exist regarding the duration of any specific cytotoxic drug regimen for treatment of newly diagnosed LGG.
Pouratian and colleagues retrospectively studied the toxicity of the protracted, low dose of TMZ in the dose dense schedule of 75 mg/m2/d for 21 days in a 28 days in distinction to the standard dose and schedule of TMZ (200 mg/m2/d for 5 days of a 28 days cycle) [20]. Twenty-five patients (15 after initial diagnosis and 10 at time of recurrence) with histologically confirmed diagnosis of LGG (11 underwent biopsy and 14 underwent STR) were included. Three patients were changed to standard dose of TMZ due to intolerable side effects and 3 other patients stopped chemotherapy earlier due to tumor progression. Objective response was seen in 52 % of all patients and the disease control rate was 84 %. The PFS rate at 6 and 12 months was 92 and 74 % respectively. In the newly diagnosed patient group, none achieved a complete response; partial response was seen in 3, minimal response in 6, stable disease in 3 and progression in 3. Toxicity consisted of fatigue, lymphopenia, constipation, nausea, electrolyte value alteration, vomiting, arthralgia, herpes zoster infection, secondary malignancy (diffuse large B cell lymphoma), cognitive disturbance and leucopenia. The authors concluded that protracted low dose schedule of TMZ is advantageous because provides increased cumulative drug exposure, but it changed the toxicity profile. They suggested that patients can be started on protracted low dose of TMZ and then changed to the standard dose when the former regimen is not tolerated. As this was a non-comparative study, more randomized trials are needed to confirm the efficacy of the protracted regimen before it is a treatment recommendation. Furthermore as the authors point out this regimen would increase the cost of treatment.
There has been another study that has evaluated the difference in efficacy and toxicity of 2 different dosing regimens of PCV in patients with LGG, but this study did not fulfill our inclusion criteria because did not report separately the outcome of patient with newly diagnosed LGG from other patients’ groups [21].
Should chemotherapy be given in conjunction with RT or alone as initial therapy for LGG?
We did not find any study that has compared initial chemotherapy treatment of LGG with RT alone or combined chemoradiation. Hence no recommendations can be given whether chemotherapy alone is better than RT alone or combined chemoradiation in initial treatment of LGG, although as mentioned earlier, several studies have suggested using chemotherapy as initial treatment for LGG alone and postpone RT treatment [1, 9, 10, 11, 12, 15].
Should chemotherapy be given in addition to other types of adjuvant therapies to patients with newly diagnosed LGG?
Shaw et al. reporting the initial results of RTOG 9802 trial studied RT alone versus chemotherapy combined with RT, in patients with newly diagnosed LGG [8]. In this trial, patients were divided in 2 prognostic groups based on neurosurgeon-determined amount of resection; favorable LGG group that included only patients less than 40 year-old that had undergone neurosurgeon-determined gross total resection (GTR) and unfavorable LGG group that included all the other patients, those patients less than 40 years of age that had undergone only subtotal or partial resection, or biopsy only of the tumor, and all patients older than 40 years of age with any extent of resection. The focus of the trial was to evaluate the potential benefits of adding PCV to conventional radiation therapy (RT) as compared to RT alone in the unfavorable group of LGG patients. Two hundred fifty-one patients were randomized in 2 groups. At the time of the initial publication, median survival in RT + PCV group was not reached (>8.5 years) as compared to 7.5 years in patients that received RT alone, but this did not reached statistical significance. Progression free survival (PFS) at 2 and 5 years was 74 and 63 % respectively for patients in the RT + PCV group and 75 and 46 % respectively for patients in the RT alone. Although the initial analysis did not demonstrate a survival benefit, a post hoc analysis demonstrated that among patients surviving beyond the 2-year mark, the combined treatment had an improvement in progression free survival over RT alone. Interestingly, overall, patients surviving to 2 years were more likely to have had a more complete resection as defined by radiology studies, and oligodendroglial histology. The authors speculated that perhaps this is the subset of the patients that may benefit from chemoradiation therapy. The primary analysis, although a prospective randomized study, does not provide support for early use of chemoradiation. However, the post hoc analysis with recombining of groups from the original study does provide class II evidence supporting early use of chemotherapy with RT.
Olson and colleagues, showed that median time to progression (MTTP) was 3.9 years for observation patients, 5.7 years for RT, 5.5 years for chemotherapy and 8.6 years for chemoradiation patients [15]. None of the treatment regimens was statistically better than the others. They concluded that the type of the adjuvant treatment did not appear to affect MTTP or overall survival.
The same observation came from the study by Nakamura and his group, that in a retrospective study of 88 adult patients with low grade astrocytoma stated that patients that received chemotherapy combined with RT did not have better overall survival when compared to patients that received RT alone [17].
Instead, Okita and colleagues found that adjuvant chemotherapy added to RT had a positive effect on PFS and OS in patient with LGG and IDH mutation when compared to patients that received RT alone [19]. However in patients with wild type IDH, the addition of chemotherapy to RT did not impact PFS and/or OS.
In the single arm phase II study by Buckner et al., 28 adult patients received chemotherapy alone within 12 weeks of surgery followed by RT 10 weeks after chemotherapy. Eight patients had a radiologic response, disease stability in 17 and immediate progression in 3 [13].
In summary, addition of chemotherapy to RT may be of benefit to patients with newly diagnosed LGG. However it appears that the use of chemotherapy combined with RT may benefit more those patients with IDH mutation, but this finding requires additional studies for confirmation.
Conclusions
Despite the large number of publications that have examined the role of chemotherapy in treatment of newly diagnosed LGG, class I evidence providing definitive treatment guidelines is lacking.
Key points for future investigation
Currently, while recognizing their limitations, the existing publications can be used to consider treatment options, but more importantly, to frame the important questions for future clinical trials (Table 2). There is a need for well-designed prospective randomized clinical trials to evaluate the role and efficacy of chemotherapy alone in newly diagnosed LGG as compared to observation, RT alone or combination of RT and chemotherapy. Furthermore, these studies need to define the optimal treatment regimen and timing of starting the chemotherapy, based on patient clinical parameters, tumor histologic and molecular characteristics and extent of tumor resection. Given the overall good prognosis for patients with LGG, treatment evaluations need to provide long-term determination of treatment-related effects so that risk to benefit analyses can be utilized.
Abbreviations
- LGG:
-
Low grade glioma
- RT:
-
Radiation therapy
- PFS:
-
Progression free survival
- OS:
-
Overall survival
- TMZ:
-
Temozolomide
- PCV:
-
Procarbazine, lomustine or CCNU, vincristine
- ACNU:
-
Nimustine
- MTD:
-
Mean tumor diameter
- AEDs:
-
Anti-epileptic drugs
- GTR:
-
Gross total resection
- STR:
-
Subtotal resection
- PR:
-
Partial resection
- RTOG:
-
Radiation therapy oncology group
References
Frenay MP, Fontaine D, Vandenbos F, Lebrun C (2005) First-line nitrosourea-based chemotherapy in symptomatic non-resectable supratentorial pure low-grade astrocytomas. Eur J Neurol 12:685–690. doi:10.1111/j.1468-1331.2005.01028.x
Cairncross JG (1998) Cognition in survivors of high-grade glioma. J Clin Oncol 16:3210–3211
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343. doi:10.1200/jco.2012.43.2674
Schomas DA, Laack NN, Rao RD, Meyer FB, Shaw EG, O’Neill BP, Giannini C, Brown PD (2009) Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol 11:437–445. doi:10.1215/15228517-2008-102
Murphy PS, Viviers L, Abson C, Rowland IJ, Brada M, Leach MO, Dzik-Jurasz AS (2004) Monitoring temozolomide treatment of low-grade glioma with proton magnetic resonance spectroscopy. Br J Cancer 90:781–786. doi:10.1038/sj.bjc.6601593
Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Criniere E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broet P, Sanson M, Delattre JY (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Peyre M, Cartalat-Carel S, Meyronet D, Ricard D, Jouvet A, Pallud J, Mokhtari K, Guyotat J, Jouanneau E, Sunyach MP, Frappaz D, Honnorat J, Ducray F (2010) Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro-oncology 12:1078–1082. doi:10.1093/neuonc/noq055
Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta MP (2012) Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 30:3065–3070. doi:10.1200/jco.2011.35.8598
Ricard D, Kaloshi G, Amiel-Benouaich A, Lejeune J, Marie Y, Mandonnet E, Kujas M, Mokhtari K, Taillibert S, Laigle-Donadey F, Carpentier AF, Omuro A, Capelle L, Duffau H, Cornu P, Guillevin R, Sanson M, Hoang-Xuan K, Delattre JY (2007) Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol 61:484–490. doi:10.1002/ana.21125
Lebrun C, Fontaine D, Bourg V, Ramaioli A, Chanalet S, Vandenbos F, Lonjon M, Fauchon F, Paquis P, Frenay M (2007) Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol 14:391–398. doi:10.1111/j.1468-1331.2007.01675.x
Stege EM, Kros JM, de Bruin HG, Enting RH, van Heuvel I, Looijenga LH, van der Rijt CD, Smitt PA, van den Bent MJ (2005) Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer 103:802–809. doi:10.1002/cncr.20828
Higuchi Y, Iwadate Y, Yamaura A (2004) Treatment of low-grade oligodendroglial tumors without radiotherapy. Neurology 63:2384–2386
Buckner JC, Gesme D Jr, O’Fallon JR, Hammack JE, Stafford S, Brown PD, Hawkins R, Scheithauer BW, Erickson BJ, Levitt R, Shaw EG, Jenkins R (2003) Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol 21:251–255
Sherman JH, Moldovan K, Yeoh HK, Starke RM, Pouratian N, Shaffrey ME, Schiff D (2011) Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg 114:1617–1621. doi:10.3171/2010.12.jns101602
Olson JD, Riedel E, DeAngelis LM (2000) Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 54:1442–1448
Iwadate Y, Matsutani T, Hasegawa Y, Shinozaki N, Higuchi Y, Saeki N (2011) Favorable long-term outcome of low-grade oligodendrogliomas irrespective of 1p/19q status when treated without radiotherapy. J Neurooncol 102:443–449. doi:10.1007/s11060-010-0340-4
Nakamura M, Konishi N, Tsunoda S, Nakase H, Tsuzuki T, Aoki H, Sakitani H, Inui T, Sakaki T (2000) Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology 58:108–116
Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta M (2008) Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg 109:835–841. doi:10.3171/JNS/2008/109/11/0835
Okita Y, Narita Y, Miyakita Y, Ohno M, Matsushita Y, Fukushima S, Sumi M, Ichimura K, Kayama T, Shibui S (2012) IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol. doi:10.3892/ijo.2012.1564
Pouratian N, Gasco J, Sherman JH, Shaffrey ME, Schiff D (2007) Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol 82:281–288. doi:10.1007/s11060-006-9280-4
Mason WP, Krol GS, DeAngelis LM (1996) Low-grade oligodendroglioma responds to chemotherapy. Neurology 46:203–207
Acknowledgments
We acknowledge the significant contributions of Laura Mitchell, Senior Manager of Guidelines for the CNS, the AANS/CNS Joint Guidelines Committee (JGC) for their review, comments and suggestions, and Anne Woznica and Mary Bodach for their assistance with the literature searches. We also acknowledge the following individual JGC members for their contributions throughout the review process: Kevin Cockroft, MD, Sepideh Amin-Hanjani, MD, Kimon Bekelis, MD, Isabelle Germano, MD, Daniel Hoh, MD, Steven Hwang, MD, Cheerag Dipakkumar Upadhyaya, MD, Christopher Winfree, MD, and Brad Zacharia, MD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Kalkanis is a consultant for Arbor and Varian. Dr. Olson is a consultant for the American Cancer Society; has received research funding from the National Cancer Institute, Genentech, and Millennium; and has received investigational drug provision from Merck.
Rights and permissions
About this article
Cite this article
Ziu, M., Kalkanis, S.N., Gilbert, M. et al. The role of initial chemotherapy for the treatment of adults with diffuse low grade glioma. J Neurooncol 125, 585–607 (2015). https://doi.org/10.1007/s11060-015-1931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1931-x