Abstract
Protracted low dose temozolomide (75 mg/m2/day on days 1–21 of 28 days) offers potential advantages over standard temozolomide schedules (200 mg/m2/day on days 1–5 of 28 days) including greater cumulative drug exposure and depletion of O6-alkylguanine DNA alkyltransferase levels, theoretically overcoming intrinsic chemoresistance. We retrospectively review our experience in 25 patients with pathologically proven low grade gliomas (LGG) treated with protracted low dose temozolomide to primarily quantify its toxicity and secondarily to assess efficacy. None had previously received radiation. Tumor response was graded based on changes in tumor size, steroid requirements, and clinical exam. About 243 cycles of protracted low dose temozolomide were administered. Three patients (12%) were changed to standard temozolomide dosing due to side effects, including intractable nausea (n = 2) and multiple cytopenias (n = 1). The most frequent toxicities were fatigue (76%), lymphopenia (72% [48% high grade]), constipation (56%), and nausea (52%). High grade toxicities (other than lymphopenia) included secondary malignancy, pruritis, hyponatremia, neutropenia, leukopenia, and cognitive decline (n = 1 for each). Tumor response rate was 52% and and disease control rate was 84%. Six month PFS was 92% and 12 month PFS was 72%. Response rates and PFS were independent of pathological subtype, deletion status, and indication for chemotherapy. Protracted low dose temozolomide has a distinct spectrum of toxicities compared to standard dosing but is well tolerated in most patients and may provide improved response rates compared to standard dosing. The results of larger randomized trials are needed to assess its potential advantages over other management schemes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temozolomide (TMZ) is an oral alkylating with excellent bioavailability and a favorable toxicity profile that induces DNA damage by methylating the O6 position of guanine. It has been shown to be efficacious for the treatment of anaplastic gliomas and glioblastomas [1–3]. Encouraging results have also been obtained in low grade gliomas (LGG) using a standard dosing regimen (200 mg/m2 for 5 days every 28 days) [4–10]. Despite the emphasis on the standard regimen (based on early phase I studies [11]), experimental and in vivo studies suggest that the antitumor activity of TMZ is schedule-dependent and that continuous or protracted dosing may be more effective than the standard schedule commonly used [12].
Protracted low dose TMZ is theorized to have improved efficacy as a result of a combination of increased cumulative drug exposure and depletion of O6-alkylguanine DNA alkyltransferase (AGAT) [13–16]. AGAT (which is synonymous with O6-methylguanine-DNA methyltransferase [MGMT]) is a DNA repair enzyme that is irreversibly inactivated when it removes alkyl groups from the O6 position of guanine. Because it is irreversibly inactivated and de novo AGAT synthesis is required to replenish AGAT function, protracted low dose TMZ results in greater AGAT depletion than less frequent administrations [17]. The depletion of AGAT may be critical since AGAT expression has been implicated in chemoresistance; tumoral AGAT expression is inversely related to chemosensitivity [18–22]. Based on these considerations, a joint study of the European Organization for Research and Treatment of Cancer (EORTC) Brain Tumor Group, EORTC Radiotherapy Group, and the National Cancer Institute of Canada’s Clinical Trials Group (NCIC-CTG) is underway to compare continuous low dose TMZ (75 mg/m2 daily for 21 days every 28 days) with radiotherapy (50.4 Gy) in patients with LGG who require treatment due to either tumor progression, uncontrolled symptoms, or unfavorable prognostic factors [23].
Despite the potential advantages, reports of clinical investigations using protracted low dose TMZ have been limited [15, 16, 24–26]. The toxicity and chemotherapeutic efficacy of protracted low dose TMZ still need further elucidation before widespread use can be advocated. The objective of this study was primarily to evaluate the toxicity and secondarily to assess the efficacy of protracted, low dose TMZ (75 mg/m2 on days 1–21 of 28 days) in a series of patients with LGG treated at the University of Virginia using this dosing regimen.
Methods
Patient selection and evaluation
After receiving Human Investigation Committee (HIC) approval, we retrospectively reviewed the University of Virginia NeuroOncology database to identify all patients with pathologically proven LGG treated with protracted low dose TMZ between October 2003 and June 2006. Twenty-five patients were identified. Diagnoses included oligodendroglioma (n = 15), oligoastrocytoma (n = 6), astrocytoma (n = 1), and unspecified LGG (n = 3). Patient charts were retrospectively reviewed to identify relevant clinical history, past medical history, details of pathology, and details of treatment.
Treatment
Patients received TMZ 75 mg/m2/day orally for 21 days every 28 days. The original plan in each patient was to administer between 12 and 15 cycles of TMZ. Patients were evaluated every two to three cycles. Dosing was reduced in the setting of some high-grade toxicities, as described in the results. The treatment was discontinued in the presence of disease progression or due to unacceptable toxicity (as described in the results). Patients were maintained on prophylaxis against Pneumocystis carinii with trimethorpim–sulfamethoxazole. Antiemetics and stool softeners were prescribed as needed.
Toxicity evaluation
Toxicity was assessed at each clinical follow-up by patient history and laboratory examination. Laboratory examination included complete blood counts with differentials and comprehensive metabolic panels. Toxicities were graded according to the NCI Common Toxicity Criteria v3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf).
Tumor response criteria
Tumor responses were evaluated according to established response criteria that incorporate changes in tumor size, steroid requirements, and patient neurological status [27]. Complete MRI (including T1 without and with contrast, T2, and FLAIR sequences) were obtained every 2–3 months in all patients as part of routine neuro-oncological follow-up. For this study, we retrospectively measured the maximum perpendicular cross-sectional diameters of each patient’s tumor as it appeared on FLAIR sequences before and after treatment. A complete response (CR) was defined as complete disappearance of all enhancing and nonenhancing tumor, with the patient not receiving steroid and being neurologically stable or improved. A partial response (PR) was defined as ≥50% reduction in FLAIR tumor volume from baseline and maintained for at least 8 weeks with the patient using stable or reduced steroids and being clinically stable or improved. A minor response (MR) was defined as ≥25% but less than 50% reduction in tumor volume or no change in volume but disappearance of tumor enhancement and maintained for at least 8 weeks, with the patient using stable or reduced steroids and being clinically stable or improved. Progressive disease (PD) was defined as ≥25% increase in tumor volume, any new distant tumor, or tumor-related neurological progression while on stable or increased steroids. Stable disease (SD) was defined as any other clinical status that does not meet any of the above criteria.
Statistical analysis
Survival was measured from day 1 of treatment. Progression free survival (PFS) was measured until the first sign of radiological or clinical progression (whichever came first) or last follow-up visit if no progression was noted. Survival was estimated by the Kaplan–Meier method and differences were evaluated by the log-rank test. The overall response rate and its 95% confidence intervals (95% CI) were calculated by pooling CR, PR, and MR rates.
Results
From October 2003 to June 2006, 25 patients were treated with protracted low dose TMZ (75 mg/m2/day on days 1–21 of a 28 day cycle) for a pathologically proven diagnosis of low grade glioma, including 15 oligodendrogliomas (WHO grade II), 6 oligoastrocytomas (WHO grade II), 1 astrocytoma (WHO grade II), and 3 low grade gliomas, not otherwise specified. Of these 25 patients, 10 were treated for progressive disease and 15 were treated at the time of initial diagnosis. Further pretreatment patient characteristics are provided in Table 1. On MRI FLAIR images, the median and range of maximum tumor diameters were 47 mm and between 19 and 98 mm. Gadolinium enhancement was noted in 24% of patients’ pretreatment MRIs.
About 243 cycles of chemotherapy were administered to the 25 patients. The number of cycles of protracted low dose TMZ administered per patient ranged from one to 16 (median = 12 cycles). Three patients did not complete their originally prescribed course of protracted low dose TMZ due to adverse effects of chemotherapy, including grade 2 nausea (two patients) and multiple combined high grade cytopenias (one patient). In these patients, chemotherapy was changed to standard dose TMZ (150–200 mg/m2/day on days 1–5 of a 28 day cycle). In addition, four patients did not complete their originally prescribed course of chemotherapy due to disease progression on therapy. These patients were referred for radiation therapy. The median follow-up for all patients was 19.8 months (range 5.3–26.2 months).
Toxicity
The primary purpose of this study was to evaluate the toxicity and tolerability of protracted low dose TMZ for the treatment of LGG. Adverse events are summarized in Table 2. There were no deaths (grade 5 toxicity) associated with treatment. Lymphopenia was the most common high grade toxicity (grade 3), occurring in 48% of patients. No grade 4 lymphopenias were noted. Grade 3 lymphopenias occurred between cycles 4 and 16 (median was during cycle 6). In two patients, localized herpes zoster infections requiring antiviral therapy were noted associated with grade 3 lymphopenias. No other negative sequelae accompanied lymphopenias. In one patient, however, after experiencing combined grade 3 lymphopenia, grade 2 leukopenia, and grade 2 neutropenia during the 4th cycle of protracted low dose temozolomide, a patient was changed to standard dose TMZ due to the combination of multiple cytopenias. High grade cytopenias resolved with the change in regimen. Other high grade toxicities were uncommon; six events were identified in 5 patients including neutropenia, leucopenia, hyponatremia, cognitive disturbance, pruritis, and secondary malignancy (diffuse large B-cell lymphoma). The secondary malignancy and pruritis were identified in the same patient during the 12th cycle of therapy.
The most frequent toxicities were fatigue (76%), lymphopenia (72%, as described above), constipation (56%), and nausea (52%). Although fatigue was common, it was generally limited and did not interfere significantly with patients’ daily activities. With regards to constipation, in all affected patients, symptoms were effectively treated with the use of stool softeners. While nausea was effectively treated in most patients with antiemetic medications, intolerable nausea (grade 2) required changes in TMZ dosing in two patients. In one patient, the daily dose of TMZ was reduced to 50 mg/m2/day for 3 cycles before changing to a standard TMZ regimen after 3 additional cycles due to persistent nausea. In the other case, the patient, who had a history of nausea, was immediately changed to a standard TMZ regimen after experiencing intolerable nausea during the first cycle of chemotherapy. In both cases, the patients tolerated standard dose TMZ without significant or disabling nausea and vomiting.
Delay in chemotherapy was not required in this series of patients for adverse events. Dose reductions and regimen changes however were implemented in the setting of some adverse events as described above.
Outcomes
Although the primary purpose of this study was to report the toxicity and tolerability of protracted low dose TMZ for the treatment of LGG, we evaluated tumor responses as a secondary measure. The overall response rate (ORR, including CR, PR, and MR) was 52% (95% CI, 33–70%) and disease control rate (DCR, including CR, PR, MR, and SD) was 84% (95% CI, 65–94%). Tumor responses were detected between the completion of 2nd and 10th cycle of chemotherapy, with a median time to radiographic response of 4 cycles. There were no significant differences in ORR and DCR when patients were stratified according to indication for chemotherapy (i.e., initial therapy versus progressive disease), histology, 1p/19q deletion status, or the presence or absence or pretreatment imaging enhancement (P > 0.05 for each comparison, Kruskal–Wallis nonparametric test, Table 3). This study was not however designed or powered to detect such differences. Steroid use during chemotherapy was also assessed independently of other response rates. Out of eight patients that were on steroids at the initiation of treatment, four patients (50%) discontinued and 2 patients (25%) significantly decreased use of steroids after starting treatment with protracted low dose TMZ. The two remaining patients (both of whom were considered to have progressive disease) required increasing steroids during treatment.
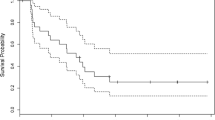
Progression free survival (PFS) was also evaluated. For the entire series, six month PFS was 92% and 12 month PFS was 74% (Table 4). Median PFS was not reached for the entire series despite a median follow-up of 19.8 months (range 5.3–26.2 months). There was no difference in PFS when patients were stratified according to indication, histology, deletion status, the presence or absence of imaging enhancement, or by age (P > 0.05 for each comparison). As explained before, this study was not designed or powered to necessarily detect such differences.
Four patients did not complete their originally prescribed course of protracted low dose TMZ due to disease progression while on therapy (during cycles 4 [n = 2], 7, and 10). Patients noted to have progression were all referred for radiation therapy since none had had such therapy prior to initiation of chemotherapy.
Discussion
TMZ induces a substantial rate of clinical and radiographic response and can be used as a first-line, single-agent treatment in patients with recurrent LGG when administered in a standard dosing regimen (Table 5) [4, 5, 7–10]. Several factors may alter the success of treatment. Specifically, the expression of AGATis inversely related to tumoral chemosensitivity [8, 20, 22, 28]. Because of the apparent importance of AGAT levels in regulating chemosensitivity, means of depleting intra-tumoral AGAT levels have been sought, including co-administering O6-benzylguanine (an AGAT substrate that irreversibly inactivates AGAT) with TMZ and using extended, or protracted, TMZ dose schedules [13, 14, 29, 30]. Protracted low dose TMZ is also potentially advantageous because it provides increased cumulative drug exposure [13–16]. While the modified dosing schedule may result in greater chemotherapeutic efficacy, it also changes the toxicity profile of treatment [15, 16, 24–26]. This study represents the first report of the toxicity and efficacy of protracted low dose TMZ for the treatment of LGG.
Toxicity of protracted low dose TMZ
A limited number of studies have reported on the toxicity of protracted low dose TMZ for the treatment of various tumors including gliomas, soft tissue sarcomas, melanomas, and renal cell carcinomas [15, 16, 24–26, 31]. Similar to prior studies, we found the most common and severe toxicities to be hematologic, especially lymphopenia (Table 6). Two opportunistic infections were identified in our series (both Herpes Zoster) and, like the report of Tosoni and colleagues, were associated with lymphopenia rather than neutropenia [25]. Despite the high rate of high grade lymphopenia, there were few clinically significant consequences. Lymphopenia seems to be a unique toxicity of protracted low dose TMZ administration, never having been reported with standard TMZ schedules [4, 5, 7]. Although corticosteroid use (in eight patients) may have contributed in part to the observed lymphopenias, high grade lymphopenias (total incidence = 12) occurred in patients both receiving (n = 5) and not receiving corticosteroids (n = 7). Other high grade hematological toxicities that are commonly reported with standard TMZ regimens, including neutropenias and thrombocytopenias (at a combined rate of between 8 and 25%) are rare with protracted low dose TMZ (Table 2) [25].
In addition to the hematological toxicities, fatigue, nausea, and constipation are noted at high rates, although never greater than grade 2. Garcia del Muro and colleagues reported fatigue in 58% of patients and nausea and vomiting in 56% of patients [26]. Fatigue was well managed with appropriate rest and occasional stimulant medication (methylphenidate, n = 1) and never interfered with daily activities. Likewise, although nausea and constipation were common, these were almost always well managed with antiemetics (as needed or scheduled) and stool softeners, respectively. As in previous reports, in two patients, protracted low dose TMZ could not be tolerated due to intractable nausea, necessitating conversion to standard dose TMZ (which relieved the nausea) [26]. Like Tosoni and colleagues who found a low grade transaminitis in 35% of patients and rise in creatinine in 6% of patients, we found similar toxicities in our patient population, at a rate of 28% and 8%, respectively [25]. The similarity across studies suggests these observed toxicities are treatment related and may be due to increased cumulative drug exposure to end organs. Like them, however, all our patients received Bactrim prophylaxis, which may in part have contributed to renal dysfunction.
Overall, treatment was well tolerated with few clinical sequelae resulting from the reported adverse effects. No delays in treatment were required due to chemotherapeutic toxicities in this series of patients. Three patients (12%) who started protracted low dose TMZ for the treatment of LGG did not complete therapy due to adverse effects of treatment, similar to the report of Tosoni and colleagues, which is greater than that reported for standard TMZ dosing [25]. Nonetheless, there were no permanent sequelae of treatment in those patients who had to convert to standard dosing due to the toxicities of protracted low dose TMZ. This suggests that if protracted low dose TMZ is in fact more efficacious than standard dose TMZ, it is safe to initiate protracted low dose treatment in all patients and convert to standard dosing if the protracted dosing is not tolerated. A secondary malignancy (large B-cell lymphoma) was identified in one patient after 12 cycles of protracted low dose TMZ, presumably due to treatment. This has not been previously reported in any series of patients treated with protracted low dose TMZ. We must be cognizant of the potential complications of prolonged use of an alkylating agent, such as secondary malignancies and aplastic anemia, especially in young patients who have a relatively good prognosis [32]. While concerning, the true rate of secondary malignancy induction will have to be verified in larger Phase 2/3 trials, such as that planned by the EORTC and NCIC-CTG [23].
Efficacy of protracted low dose TMZ
Reports of the clinical efficacy of protracted low dose TMZ are limited. Garcia del Muro and colleagues found that protracted low dose TMZ was effective in a significant proportion of patients (15.5%) with advanced soft tissue sarcomas in whom second-line chemotherapy and standard TMZ was not effective [26]. Conversely, its efficacy in patients with malignant gliomas and metastatic renal cell carcinoma has been questioned [15, 24]. Establishing efficacy is particularly important in light of the pharmacoeconomic impact of this schedule change: low dose protracted TMZ roughly doubles the cost of chemotherapy compared to standard regimens, corresponding to approximately $6,000 greater cost per cycle.
Because this study was primarily intended to assess chemotherapy-related toxicity, follow-up is relatively short (median 19.8 months). Nevertheless, we provide a preliminary assessment of efficacy based on short term follow-up. The overall 6 and 12 month PFS reported in this study suggest improved or equivalent tumor control rates relative to those reported in studies of LGG using standard TMZ dosing (Table 5). Moreover, we found that tumor response rate to chemotherapy (CR+PR+MR, 52%) was similar to that reported in other studies (between 31 and 61%) (Table 5). While prior studies identified differences in treatment efficacy based on chromosome 1p deletion status, no significant effect was seen in this series of patients [7, 8]. Moreover, no differences were seen between groups of patients stratified by indication for chemotherapy, histology, age, or imaging enhancement pattern. While outcomes with protracted low dose TMZ are at least equivalent to other studies of TMZ therapy for LGG, the 12 month PFS appears inferior to that reported for XRT (>90%) and not significantly different than that reported for patients with LGG who have only undergone surgery (biopsy or resection) and never received XRT or chemotherapy (between 70 and 80%) [33]. This study however was not designed to make such comparisons or to compare the relative risks and benefits of various therapies or observation. Larger scale, prospectively designed studies such as the ongoing EORTC trial are needed to adequately answer such questions [23]. Nonetheless, relative to other studies of TMZ for LGG, these preliminary outcomes are encouraging and suggest further characterization of the efficacy of protracted low dose TMZ for the treatment of LGG is warranted.
References
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12:259–266
van den Bent MJ, Taphoorn MJ, Brandes AA, Menten J, Stupp R, Frenay M, Chinot O, Kros JM, van der Rijt CC, Vecht Ch J, Allgeier A, Gorlia T (2003) Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol 21:2525–2528
Brada M, Viviers L, Abson C, Hines F, Britton J, Ashley S, Sardell S, Traish D, Gonsalves A, Wilkins P, Westbury C (2003) Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol 14:1715–1721
Byrne TN (2004) Response of low-grade oligodendroglial tumors to temozolomide. J Neurooncol 70:279–280
Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Criniere E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broet P, Sanson M, Delattre JY (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Levin N, Lavon I, Zelikovitsh B, Fuchs D, Bokstein F, Fellig Y, Siegal T (2006) Progressive low-grade oligodendrogliomas: response to temozolomide and correlation between genetic profile and O6-methylguanine DNA methyltransferase protein expression. Cancer 106:1759–1765
Pace A, Vidiri A, Galie E, Carosi M, Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B, Carapella CM (2003) Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol 14:1722–1726
Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Provenzale JM, McLendon RE, Gururangan S, Bigner DD, Herndon JE 2nd, Avgeropoulos N, Finlay J, Tourt-Uhlig S, Affronti ML, Evans B, Stafford-Fox V, Zaknoen S, Friedman HS (2003) Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol 21:646–651
Newlands ES, Blackledge GR, Slack JA, Rustin GJ, Smith DB, Stuart NS, Quarterman CP, Hoffman R, Stevens MF, Brampton MH et al. (1992) Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer 65:287–291
Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA et al. (1987) Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47:5846–5852
Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011
Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL (2001) Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res 7:2309–2317
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE (2002) A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-oncol 4:39–43
Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH, Rustin GJ (1998) Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res 58:4363–4367
Pegg AE (1990) Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res 50:6119–6129
Gerson SL (2002) Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20:2388–2399
Silber JR, Blank A, Bobola MS, Ghatan S, Kolstoe DD, Berger MS (1999) O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin Cancer Res 5:807–814
Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M (2006) O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem 96:766–776
Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, Margison GP (1993) Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer 67:1299–1302
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
van den Bent MJ, Stupp R, Brandes AA, Lacombe D (2004) Current and future trials of the EORTC brain tumor group. Onkologie 27:246–250
Bex A, Kerst J, Mallo H, Van Tinteren H, Horenblas S, De Gast GC (2005) Extended continuous oral temozolomide in patients with progressive metastatic renal cell carcinoma not responding to interferon alpha 2b. J Chemother 17:674–678
Tosoni A, Cavallo G, Ermani M, Scopece L, Franceschi E, Ghimenton C, Gardiman M, Pasetto L, Blatt V, Brandes AA (2006) Is protracted low-dose temozolomide feasible in glioma patients? Neurology 66:427–429
Garcia del Muro X, Lopez-Pousa A, Martin J, Buesa JM, Martinez-Trufero J, Casado A, Poveda A, Cruz J, Bover I, Maurel J (2005) A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: a study by the Spanish Group for Research on Sarcomas. Cancer 104:1706–1712
Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Bobola MS, Silber JR, Ellenbogen RG, Geyer JR, Blank A, Goff RD (2005) O6-methylguanine-DNA methyltransferase, O6-benzylguanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res 11:2747–2755
Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE 2nd, Bigner DD, Friedman HS (2005) Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol 23:7178–7187
Middleton MR, Lee SM, Arance A, Wood M, Thatcher N, Margison GP (2000) O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 hr schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study. Int J Cancer 88:469–473
Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB (2004) Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol 22:610–616
Villano JL, Collins CA, Manasanch EE, Ramaprasad C, van Besien K (2006) Aplastic anaemia in patient with glioblastoma multiforme treated with temozolomide. Lancet Oncol 7:436–438
Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, Darcel F, Stenning S, Pierart M, Van Glabbeke M (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52:316–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouratian, N., Gasco, J., Sherman, J.H. et al. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol 82, 281–288 (2007). https://doi.org/10.1007/s11060-006-9280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9280-4