Abstract
Patients with gliomas are at risk of cerebrovascular accidents (CVA) with potential consequences on survival, function, and local tumor control. Our objective was to provide information about CVA in patients with gliomas and to estimate survival in this group. We reviewed all adult glioma patients with ischemic CVA at the University of Texas-M.D. Anderson Cancer Center from 2003 through 2014. We extracted demographic, clinical, imaging, treatment and outcome data. We used descriptive summary data and estimated or compared survival rates where appropriate. 60 of 6500 patients (0.1 %) with high-grade (HGG, n = 47) or low-grade glioma (LGG, n = 13) had ischemic CVA Thirty-two (53 %) patients had postoperative strokes, and 20 (33 %) had CVA after 2 weeks of surgery. Forty-one patients (68 %) had gross total resection. For HGG and CVA, the poststroke median overall survival was 17 months versus 61 months in LGG and CVA (P = 0.03; hazard ratio (HR): 2.8; 95 % CI 1.07–4.60). Survival stratified by modified Rankin Scale grade was significant (X 2 = 9.8, P = 0.007). Five patients received bevacizumab before stroke onset; none responded to antiangiogenic therapy. There was no stroke-related death. At our institution for 10 years, ischemic CVA in glioma patients was a rare complication, clearly associated in half of cases to surgery, and with a variable negative impact on performance status and neurologic function. In this group, patients with more neurological deficits lived less. The survival difference between and within subgroups was most likely due to tumor grade. More research is necessary to improve prevention of postoperative stroke in glioma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk for ischemic stroke or ischemic cerebrovascular accidents (we use both terms interchangeably) is higher in patients with gliomas, reaching up to 9 % [1, 2], compared to the general population (2.7 %) [3]. This complication may have a detrimental effect on the outcome of this group [2]. The diagnosis of an ischemic CVA is rare in the initial presentation of glioma [1, 4] and the clinical diagnosis and imaging may be challenging. A close clinical and radiological follow-up is often necessary to correctly diagnose a stroke [4].
The onset of neurological deficits in patients with glioma could be secondary to tumor progression, brain edema, seizures and stroke [5]. Stroke has been described more frequently as a postoperative complication [2] or as a late complication of radiotherapy [6], but also associated with tumor-induced hypercoagulability or nonbacterial thrombotic endocarditis [2, 6]. Moreover, infectious vasculopathy, certain chemotherapy and antiangiogenic agents are associated to a higher risk for stroke [6, 7]. All these factors are crucial to suspect a CVA in patients with gliomas to make a correct diagnosis and to start the appropriate treatment as early as possible.
The aim of this study was to collect information about risk factors and clinical presentation of ischemic strokes in patients with gliomas, to explore any relation between CVA and glioma, and to estimate survival in this group. As a secondary objective, we derived information on performance status and neurological disability after the stroke.
Methods
We reviewed all adult glioma patients registered in the University of Texas-M.D. Anderson Cancer Center (UT-MDACC) institutional database from 2003 through 2014 under a protocol with waiver of consent approved by the Institutional Review Board. We identified a total database population of 6500 patients. All of these patients had a biopsy or surgical resection with a tissue diagnosis of glioma (grade II-IV astrocytomas; grade II and III oligodendrogliomas, and grade II and III ependymomas) [8]. 60 of them developed an ischemic stroke during the course of disease. All patients had a preoperative MRI 24 h within surgery, and postoperatively 24 h after surgery. The treating neurologist or neurosurgeon made the diagnosis of CVA in all patients with confirmation by brain magnetic resonance imaging (MRI) using hyperintense diffusion-weighted imaging (DWI); and hypointense attenuated diffusion coefficient (ADC) within 1 and 24 h of symptom onset. The scanners were 1.5-Tesla or 3-Tesla units using field of view of 22 cm, 5-mm slice thickness with a 1.5-mm gap. We also examined brain computed tomography (CT) scans for early ischemic findings. All the patients underwent brain MRI after surgery for newly diagnosed glioma or tumor recurrence and they were performed within the first 48 h, 3 weeks after radiation therapy and every 2-month in case of HGG or every 3 months for LGG.
We defined postoperative CVA as a stroke within 2 weeks of surgery. We excluded cases of transient ischemic attacks (TIA) if there was no radiological evidence of infarction, or if there was clear, unequivocal evidence of seizure, migraine, tumor edema or progression of disease. We also excluded cases of hemorrhagic stroke, or intracranial hemorrhage (ICH). Based on the data review, we assigned Modified Rankin Scale (mRS) grades at onset of stroke, and the Karnofsky performance status (KPS) before and after the stroke.
We extracted demographic, clinical, radiographic, therapeutic, and survival data from the database. We first summarized the data using standard descriptive statistics and frequency tabulation. Time-to-event endpoints including overall survival (OS) and progression-free survival (PFS) after the stroke were estimated using the Kaplan–Meier method. If appropriate, we used the log-rank test to compare OS and PFS between subgroups, and within HGG, between grade III gliomas and glioblastomas (GB). We confirmed death by review of medical records, death certificates, or querying the Social Security Index. We defined death associated to stroke as any death occurring within 4 weeks of stroke onset [9]. We gathered additional clinical, pathology, and neuroimaging data from the institutional electronic medical records.
Results
Demographics, clinical presentation, and initial effect of CVA on function
From July 1, 2003, through June 1, 2014, we identified 60 patients with low-grade glioma (LGG; n = 13, grade II gliomas) or high-grade glioma (HGG; n = 47, grade III gliomas and glioblastomas) with acute ischemic stroke from a total database population of 6500 patients (0.1 %; 0.017 % occurred presurgically and 0.083 % postsurgically). This corresponds to 7500 patient-years of observation. Thirty-seven of them were men (60 %) with a mean age of onset for glioma and stroke of 50 and 52 years, respectively, compared to 51 and 54 years in women.
Most patients developed symptoms with a time course that suggested an ischemic stroke (n = 57, 95 %); three patients (5 %) presented clinically as a transient ischemic attack (TIA). Of these 60 patients, 32 (53 %) had the event postoperatively; in 20 patients (30 %) the ischemic stroke occurred after the postoperative period and in eight patients (17 %) the ischemic stroke occurred preoperatively, within 6 months of surgery. The Karnofsky performance status (KPS) ranged between 40 and 100, whereas 47 (78 %) of them had a prestroke KPS between 80 and 100. The poststroke KPS scores declined in 28 (47 %) patients: by 10 (n = 13); 20 (n = 5); 30 (n = 5); 40 (n = 1); 50 (n = 3), and 60 (n = 1) units. Thirty-two (53 %) patients maintained a poststroke KPS between 80 and 100. The glioma type, its location and stroke risk factors are detailed in Table 1.
Most of CVAs localized adjacent to the resection cavity in patients with a gross total resection (n = 41, 68 %), subtotal resection (n = 16, 27 %) and biopsy (n = 3, 5 %). Twenty-five (42 %) patients underwent one surgery before the stroke, 21 (35 %) had two surgeries and seven (12 %) patients had three or more brain surgeries. Seven other patients (12 %) did not have surgery before the onset of stroke. Thirty-one (52 %) patients had received radiation therapy before the stroke. Half of them received a total radiation dose of 60 Gy. Thirty-six (60 %) patients received chemotherapy before the stroke. Twenty-eight (47 %) patients received radiation and chemotherapy before the stroke.
The clinical presentation of stroke included hemiparesis (52 %); hemihypoesthesia (19 %); language deficits (18 %); visual field deficits (10 %); headaches (8 %); gait imbalance (8 %), and seizures (5 %). Thirteen (22 %) patients were asymptomatic, and the diagnosis of stroke was incidental in the postoperative MRI. Twenty-eight (47 %) patients had mild residual deficits (mRS 1–2), 25 (42 %) had moderate residual deficits (mRS 3–4) and five (8 %) had severe deficits (mRS 5) within the first 72 h of stroke onset. In all, half of patients experienced moderate to severe neurologic disability ranging from requiring help but able to walk without assistance to bedridden, incontinent, and dependent on constant nursing care and attention.
Characteristics of the CVA and associated factors
Only 20 (33 %) patients had a brain CT scan. Acute CT scan findings included hypodensity (70 %); sulcal effacement (15 %); loss of white–gray matter differentiation (5 %), and a hyperdense middle cerebral artery (5 %). 5 % of patients had a normal or unremarkable CT scan. All the patients had a brain MRI. All 60 cases had DWI restriction. We found correlation between ADC and DWI sequences in 46 patients (77 %), and contrast enhancement in seven cases (12 %) (Fig. 1a–f). The most common vascular territory was the middle cerebral artery (MCA; n = 46, 77 %); the posterior cerebral artery (PCA; n = 9, 15 %); the anterior cerebral artery (ACA; n = 7, 12 %), and the posterior inferior cerebellar artery (PICA; n = 1, 2 %).
Brain MRI findings of cerebrovascular accidents. Case 1 a Brain MRI 24 h after a brain biopsy (arrow) shows DWI hyperintensity with corresponding ADC hypointensity b involving the territory irrigated by the lateral lenticulostriate arteries, branches of the right middle cerebral artery. Case 2 c Brain MRI 24 h after a right temporal craniotomy with gross total resection of a glioblastoma shows DWI restriction in the posterior aspect of the resection cavity, without corresponding ADC findings (d). Case 3 Four months after a right parietal craniotomy with gross total resection of a glioblastoma, brain MRI shows T1-contrast enhancement (e) (arrow) surrounding the region of DWI restriction (f). Brain biopsy showed no residual/recurrent tumor but acute inflammation, gliosis and foamy macrophages. Case 4 4 days before surgery, brain MRI shows DWI restriction (g) and ADC hypointensity (h) in both occipital lobes and left thalamus (arrow) far from tumor location
The ischemic areas were adjacent to the surgical cavity in 50 (83 %) patients; the rest occurred away from the resection cavity (Fig. 1g, h). 21 patients had transthoracic echocardiogram; five had heart failure, two had an intracardiac shunt, and two had a valvulopathy. Electrocardiograms revealed atrial fibrillation (n = 3), tachyarrhythmia (n = 1) and right bundle branch block (n = 1). None of the patients had Holter monitoring. 37 patients had a lipid panel within 12 months before the stroke. The mean LDL was 133 mg/dL, HDL 54 mg/dL, and triglycerides 207 mg/dL. Seven patients had carotid Doppler, of which one had significant ipsilateral carotid stenosis. The hypercoagulation panel in two patients was unremarkable. The mean HbA1c in 13 patients was 5.7 %. The mean platelet count was 231 × 109/L.
Regarding the tumor status at the time of the stroke, 42 (70 %) patients were newly diagnosed with glioma and 18 (30 %) had recurrence or progression. The use of steroids was common (n = 36, 60 %), and the average daily dose was 12 mg. Postoperative CVA was the most common occurrence (n = 32, 53 %), compared with non-postoperative causes of CVA such as radiation-related (n = 10), lacunar (n = 5), cardioembolic (n = 4), and large artery disease due to radiation (n = 1). In eight patients the CVA occurred within 6 months before surgery (Fig. 2). Five patients had received bevacizumab by the time they had a stroke. The time between the start of bevacizumab to stroke was 12 weeks (range: 2–31 weeks). None of these patients responded to antiangiogenic therapy after the stroke.
Treatment
Thirteen (22 %) patients could safely receive some treatment for the acute stroke. The most common treatment was antiplatelet therapy; aspirin alone or with dipyridamole (n = 9, 14 %) or clopidogrel (n = 3, 5 %). One patient (2 %) was treated with tissue plasminogen activator (tPA). This patient tolerated tPA well and did not have treatment-related complications. The rest of patients did not or could not receive any treatment.
Survival
There were no stroke-related deaths (mRS grade 6). The median OS for all 60 glioma patients after the acute ischemic stroke was 15 months; the median PFS was 7 months. Patients with a postoperative stroke had a median OS of 21 months. Twenty six (43 %) patients had recurrent tumor after the stroke, of which 11 (41 %) developed recurrence within the first 6 months after the CVA.
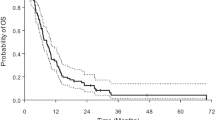
The median poststroke OS in the HGG and LGG subgroups was 17 and 61 months (P = 0.03; HR: 2.8; 95 % CI 1.07–4.60, log-rank test). The median PFS was 6 and 10 months (P = 0.15; HR: 2.1; 95 % CI 0.83–5.38, log-rank test) (Fig. 3). Patients with GB and postoperative stroke had a median OS of 11 months and a PFS of 5 months. Progression after the stroke occurred in 24 (40 %) patients, half of them within 6 months of the CVA.
The relationship between the degree of disability and shorter survival based on mRS was significant (X 2 = 9.8, P = 0.007); the OS for the three groups (mRS 1–2), (mRS 3–4) and (mRS 5) was 18, 7, and 4 months, respectively. The median OS of patients with a poststroke KPS between 80 and 100 was 17 months; the median OS of patients with a poststroke KPS <80 was 4 months (X 2 = 2.8, P = 0.09).
Discussion
In addition to risk factors for stroke such as cardiovascular disease, cancer predisposes patients to thrombosis through tissue-specific pathways, and brain tumors are not an exception [10]. Mechanical compression and vascular infiltration by malignant cells might contribute as well. Strokes in patients with gliomas may occur as early surgical, chemotherapeutic or late radiation complications [2]. Previous radiotherapy could also increase the risk for postoperative ischemia [2].
In our series, ischemic strokes occurred in 0.1 % from a total database population of 6500 patients (7500 patient-years). These episodes were frequent in the postoperative period, especially in patients with prior chemotherapy and radiation. Most of the postoperative strokes localized adjacent to the resection cavity suggesting iatrogenic stroke due to manipulation during surgery as the main mechanism, general prothrombotic state due to the underlying glioma may also contribute to develop stroke. The low incidence of CVA found in our population may be related to several reasons including the neurosurgeon’s skills, the restricted availability of postoperative DWI and ADC sequences in the early 2000s at The UT-MDACC, difficulty to detect early CVA findings on CTs and low clinical suspicion of CVA in primary brain tumor (PBT) patients.
Given the influence of astrocytes in controlling vascular tone, cerebral blood flow, nutrient diffusion, and circuit reorganization [11], gliomas may affect vasodilation and neuronal plasticity after injury. Furthermore, because astrocytes endocytose and metabolize glutamate, their malfunction can lead to seizures [12], not frequent in our series (5 %) when compared with hemiparesis (52 %). These factors make more difficult to distinguish between clinical tumor progression, hemorrhage, TIA, ischemic stroke, and partial motor or sensory seizures that may be followed by postictal deficits. In the pre-MRI era, some authors suggested that unilateral focal motor seizures, pure sensory deficits, loss of consciousness and speech arrest should prompt for brain imaging for evaluation of a brain tumor regardless of risk factors for stroke such as carotid stenosis or other vascular disease [13]. Furthermore, ischemic CVA is an important comorbidity in cancer patients and possibly acts as inducer of tumor progression. Mohyeldin and colleagues stipulated that stem cell niches in the subventricular zone maintain an undifferentiated state by keeping one of the lowest partial pressures of oxygen in brain tissue [14]. Interestingly, progression of disease occurred in 40 % of the patients after the stroke, 42 % of which did so within the first 6 months of the CVA. On the other hand, and making the recognition of stroke more complicated, many asymptomatic and nonclassic presentations of stroke may go unnoticed if DWI sequences in MRI studies are missing. Yet, there is debate whether DWI truly reflects ischemia as opposed to atypical necrosis or VEGF inhibitor-induced chronic hypoxia [15].
Most studies agree that the majority of ischemic strokes in glioma patients are postoperative. A large study on glioma complications reported 61 % of ischemic infarcts as postoperative [16], similarly to our data, with 53 % suffering a decline in KPS. The degree of disability after the CVA affected the OS. It was statistically significant when comparing three groups (mRS 1–2), (mRS 3–4) and (mRS 5) with an OS of 18, 7, and 4 months, respectively. A study of patients with PBT had a similar trend [2]. CVAs provide additional risk to HGG patients, already with dismal prognosis; we found a poststroke OS of 15 and 11 months in grade III glioma and GB patients, respectively. Another study reported an OS of 1.7 years (20.4 months) after the stroke in patients with primary brain tumors [2].
Neurosurgical studies have also addressed the prevention of postoperative stroke. An extent of resection (EOR) of <80 % was associated with better early postoperative outcomes (within 30 days of surgery) than larger EOR [17]. However, other studies have associated the extent of resection (gross total or near total) in grade III gliomas and glioblastomas with longer survival, recommending to attempt the greatest EOR when motor-evoked potentials and intra-operative mapping are available [18]. Most of our patients underwent gross total resection; unfortunately, it is difficult to measure how postoperative ischemia may induce tumor progression. Current studies on CVA with focus on PBT evaluate CVA incidence during specific treatments for glioblastoma patients or as complications of long-term glioblastoma survivors [19].
An European study that compared ischemic stroke in patients receiving anti-VEGF therapy with patients who received temozolomide and radiotherapy, found that the stroke rate significantly increased with bevacizumab (6.2 vs. 0.6 %) [16]. Another study found no difference between the groups, with a stroke frequency of 1.9 % [7]. In our series, only five patients were receiving bevacizumab at the onset of stroke, which occurred within the first 12 weeks of starting bevacizumab. Furthermore, bevacizumab did not show any clinical or radiological benefit once the CVA occurred.
We acknowledge this study has limitations. The main shortcoming was its retrospective design with a lack of a control group to which we could estimate the stroke risk. Also, survival comparisons between groups stratified by diagnosis, mRS, or KPS are post hoc; therefore, these results should be interpreted and extrapolated with caution. With our low incidence of ischemic strokes, we are not sure that strict preoperative screening to identify high-risk patients can lower this rate even more.
References
Cestari DM, Weine DM, Panageas KS, Segal AZ, DeAngelis LM (2004) Stroke in patients with cancer: incidence and etiology. Neurology 62(11):2025–2030
Kreisl TN, Toothaker T, Karimi S, DeAngelis LM (2008) Ischemic stroke in patients with primary brain tumors. Neurology 70(24):2314–2320
Fang J, Shaw KM, George MG (2012) Prevalence of stroke–United States, 2006-2010. MMWR Morb Mortal Wkly Rep 61(20):379–382
Obeid M, Ulane C, Rosenfeld S (2010) Pearls & Oysters: large vessel ischemic stroke secondary to glioblastoma multiforme. Neurology 74(13):e50–e51
Aoki N, Sakai T, Oikawa A, Takizawa T, Koike M (1999) Dissection of the middle cerebral artery caused by invasion of malignant glioma presenting as acute onset of hemiplegia. Acta Neurochir (Wien) 141(9):1005–1008
Bitzer M, Topka H (1995) Progressive cerebral occlusive disease after radiation therapy. Stroke 26(1):131–136
Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM (2011) Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neurooncol 105(2):281–289
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) WHO Classification of Tumours of the Central Nervous System, 4th edn. International Agency for Research on Cancer, Lyon
Brønnum-Hansen H, Davidsen M, Thorvaldsen P, Danish MONICA Study Group (2001) Long-term survival and causes of death after stroke. Stroke 32(9):2131–2136
Pabinger I, Thaler J, Ay C (2013) Biomarkers for prediction of venous thromboembolism in cancer. Blood 122(12):2011–2018
Filosa JA, Iddings JA (2013) Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol 305(5):H609–H619
Mungenast AE (2011) Diacylglycerol signaling underlies astrocytic ATP release. Neural Plast 2011:537659
The UK TIA Study Group (1993) Intracranial tumours that mimic transient cerebral ischaemia: lessons from a large multicentre trial. J Neurol Neurosurg Psychiat 56(5):563–566
Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7(2):150–161
Rieger J, Bähr O, Müller K, Franz K, Steinbach J, Hattingen E (2010) Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol 99(1):49–56
Seidel C, Hentschel B, Simon M et al (2013) A comprehensive analysis of vascular complications in 3,889 glioma patients from the German Glioma Network. J Neurol 260(3):847–855
Oppenlander ME, Wolf AB, Snyder LA et al (2014) An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 120(4):846–853
McGirt MJ, Chaichana KL, Gathinji M et al (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110(1):156–162
Hottinger AF, Yoon H, DeAngelis LM, Abrey LE (2009) Neurological outcome of long-term glioblastoma survivors. J Neurooncol 95(3):301–305
Funding
This study does not involve use of any grant funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors report no disclosure.
Additional information
Carlos Kamiya-Matsuoka and David Cachia contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Kamiya-Matsuoka, C., Cachia, D., Yust-Katz, S. et al. Ischemic stroke in patients with gliomas at The University of Texas-M.D. Anderson Cancer Center. J Neurooncol 125, 143–148 (2015). https://doi.org/10.1007/s11060-015-1880-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1880-4