Abstract
Malignant giant cell tumor (MGCT) in the spine is extremely rare and there is little published information regarding this subject in the literature. We attempted to correlate different treatment options and outcomes over time. A retrospective study of patients with spinal MGCT who were surgically treated in our center between 2006 and 2012 was performed. Overall, three surgical management strategies, including subtotal resection, piecemeal total resection, and total en bloc spondylectomy were applied. Postoperative radiotherapy was carried out in 4 cases. Clinical data and efficacy of surgical treatment strategy were analyzed via chart review. A total of 14 patients with spinal MGCT were included in the study. Three cases were diagnosed as primary MGCT (PMGCT), while the other 11 patients were secondary MGCT (SMGCT). The mean follow-up period was 41 (range 3–75) months. Recurrence was found in 7 patients after surgery in our center, while distant metastasis and death occurred in 4 and 6 cases, respectively. MGCT of bone is always a high-grade sarcoma with a poor prognosis and complete excision, while also preserving neural function, is recommended. In our study, patients who underwent total en bloc spondylectomy had significantly lower local recurrence rate for MGCT in the spine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Giant cell tumor of bone (GCT) is an aggressive skeletal tumor that consists of three major cell types: osteoclast-like multinucleated giant cells, spindle-like stromal cells, and monocytic round cells [1, 2]. Although GCT is predominantly regarded as benign lesion, it has malignant potential and could completely transform into malignant one [11]. Only 1.4–9.4 % of GCTs appear in the spine and occur most commonly between the ages of 20–40 years, with a male-to-female ratio of 1:2.5 [1, 3–5].
Malignant giant cell tumor of bone (MGCT) accounts for 2–9 % of all GCT cases [6–8]. WHO used the term “malignancy in GCT” to describe MGCT and subdivided it into either primary or secondary [9]. Primary MGCT (PMGCT), which is often diagnosed at the time of first treatment, has a juxtaposition of conventional giant cell areas and pleomorphic spindle cell areas that are clearly malignant [10]. Secondary malignant giant cell tumor (SMGCT) is a high-grade sarcoma occurring as a recurrent lesion at the site of a benign GCT either after surgery, radiotherapy, or both [9, 11]. SMGCT is more common than PMGCT and mainly originates after irradiation treatment for the primary lesion [8, 12–16].
Due to the rarity of MGCT, there is only little published information in the literature, and there have been no reports of MGCT of the spine. The low incidence of spinal MGCT makes it difficult to define appropriate therapy and prognosis. There is no consensus regarding treatment recommendations of MGCT. Treatment protocols include surgery alone or surgery combined with chemotherapy or radiotherapy, but radical excision is considered to be associated with reduced recurrence rates of MGCT of bone [10]. However complete resection is difficult to achieve in the spine. In this series, a retrospective review of 14 cases with spinal MGCT that were treated with surgery at our center was performed, and to our best knowledge, represents the largest cohort reported to date.
Patients and methods
A total of 14 patients with spinal MGCT who were surgically treated and documented in our center were identified from April 2006 to December 2012. The diagnosis of MGCT was confirmed by an independent pathologist in all patients. The clinical and pathological data of all patients were retrieved from the previously maintained database of our center. This study was approved by Ethics Committee of our hospital and informed consent was obtained from the surviving patients or family members of those who had died.
Preoperative neurologic status was classified according to the Frankel score [17]. Tumor extension was described according to the Weinstein–Boriani–Biagini (WBB) system (except for one case with tumor in the sacrum evaluated by Enneking grading system) and Campanacci grading systems based on CT and MRI [18, 19]. All the patients accepted surgery in our center, and surgical strategy was decided for each patient according to WBB system and Enneking stage. A screw-rod system in combination with autologous or artificial bone grafts was used to reconstruct the stability of the spine for all the 14 cases, and an anterior titanium plate was also used for some patients with cervical lesion who were surgically treated in a combination of both posterior and anterior approach.
Postoperative radiotherapy (RT) which was used as adjuvant therapy was undertaken 4–6 weeks after surgery with the total dose ranging from 30 to 50 Gy [20, 21]. RT was forbidden for those with adequate radiation exposure before. Except one patient who was treated before 2007, 13 patients received one dose of intravenous bisphosphonate before surgery and one dose every month after surgery for 2 years [1].
All cases were advised to accept radiographic assessment by radiograph and CT/MR of the surgical segment as well as the adjacent vertebrae. Regular assessment were done at 0, 3, 6, and 12 months after surgery, every 6 months for the next 2 years, and then annually for life [22]. Follow-up data were obtained from office visits and telephone interviews. In the follow-up visit in 3 months after surgery, neural function was re-evaluated based on the Frankel score system. The follow-up period was defined as the interval from the date of surgery to death, or until June 2014 for patients alive.
Results
Patient features
The series was comprised of 4 men and 10 women, with a mean age of 35 (median 32, range 15–63) years old. Six cases (42.9 %) were between 20 and 40 years, while five patients (35.7 %) were more than 40 years. Three patients were admitted for PMGCT and the other 11 cases were SMGCT. Lesions were detected in the cervical spine (n = 4), thoracic spine (n = 8), lumbar spine (n = 1), and sacrum (n = 1) (Table 1).
Localized pain in the spine was the most consistent complaint. The duration of preoperative symptom was 1–25 months, with an average of about 8.2 months. Additional patient characteristics included 1 patients presented with a palpable mass, 2 patients had secondary aneurysmal bone cyst, 6 patients presented with radicular pain, and 8 cases had different degrees of cord compression at diagnosis (Supplementary Table 1). For the eight patients with spinal cord compression, three of them suffered incomplete paralysis and the other five patients presented with myelopathy.
Radiologic studies
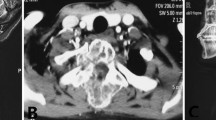
The radiologic features of MGCT were similar to those of conventional GCT [11]. A radiographic appearance of osteolytic lesion by X-ray, Computed tomography (CT), and Magnetic resonance imaging (MRI) was found in the 14 cases. Cortical breakthrough and absence of well-circumscribed borders in CT images presented in all patients with MGCT. MRI revealed a common phenomena of soft tissue mass formation in the 14 patients, which was reflected by WBB system as extraosseous involvement (layer A) and epidural space involvement (layer D) (Fig. 1). A higher Campanacci stage was also found in MGCT in the spine: all the 14 patients were classified as Campanacci grade III [44].
Radiologic images of a patients with SMGCT (case, #5). a MRI image in June 2008 revealed a osteolytic lesion in vertebral body and the accessories of C2 (benign GCT). b Postoperative lateral radiograph in June 2008 showed the reconstruction performed from C1 to C4. c MRI performed in January 2009 exhibited soft tissue mass in C2 (malignant GCT). d Lateral radiograph after second surgery showed the reconstruction by occipitocervical fixation
Treatment history
Three patients, who did not receive surgical intervention and any other treatment before admission into our institution, were regarded as PMGCT. The other 11 patients had been diagnosed as benign GCT before and were admitted into our center for SMGCT in the spine: 6 of them received surgical resection; 1 patient (case. #13, Supplementary Table 2) was treated by radiotherapy (3 months before admission, total dose of 35 Gy in 15 fractions); 4 patients accepted both surgery and radiotherapy (case. #2, case. #3, case. #8, case. #14, Supplementary Table 2). Six patients with only surgical resection were regarded as postoperative SMGCT. The patient who was just treated by radiotherapy before admission was considered to be with radiation-associated SMGCT and radiotherapy was considered to be the main cause for the transition of benign GCT to MGCT. For the 4 patients with both surgical treatment and radiotherapy, the cause of the malignant transformation could not be easily confirmed (surgery, radiotherapy, or both) (Table 1).
Treatment and outcome
Needle biopsy was carried out on 3 cases with PMGCT, and intraoperative fast pathological examination was performed in all 14 cases. The results of intraoperative fast pathological examination were confirmed to be correct in 12 cases. However the result of needle biopsy consistent with the final pathological diagnosis was found in only one case (33 %, case, # 12), and the other two patients were misdiagnosed as benign GCT. Sampling error, small needle samples, or over-conservative judgment of pathologist might lead to the misdiagnosis.
Three different surgical strategies were pursued: subtotal resection, piecemeal total resection, and total en bloc spondylectomy. Subtotal resection was performed in 3 cases (case. #2, nearly 80 % resected; case. #3, approximately 90 % resected; case. #8, more than 95 % resected), piecemeal total resection was carried out in 7 cases, and total en bloc spondylectomy was undertaken in 4 cases (Table 1). Intraoperative blood loss ranged from 800 to 5000 (mean ~2686) ml. Bisphosphonate treatment by either zoledronic acid or incadronate disodium which was used in our center since 2007 was applied in 13 patients. Postoperative RT was delivered with megavoltage beams, and the total dose ranged from 30 to 50 Gy, with the dose limits of 50 Gy for the spinal cord. It was performed in 4 cases (case. #1, case. #7, case. #11, case. #12, Supplementary Table 2).
For 8 patients diagnosed with spinal cord compression before surgery, their pain significantly alleviated or disappeared, and neurological status showed a decrease in Frankel scores of 1–2 grades by their 3-month follow-up visit. Local recurrence occurred in 7 patients, lung metastasis was found in 4 cases, and finally 6 patients died in the follow-up. Surprisingly, all those bad prognoses occurred in patients with SMGCT, and 3 cases with PMGCT were alive with no evidence of disease (NED). The treatment options and outcomes were listed in Table 2. Three patients with subtotal resection suffered progression of residual disease, and two of them died. Of the 7 patients with piecemeal total resection, 4 patients had local recurrence and finally died including 2 cases receiving postoperative RT. Howerver all the 4 patients who accepted total en bloc spondylectomy were alive with NED.
Pathology
Histologic diagnosis was obtained in all cases, and margins were submitted for pathological examination at the same time to decide further treatment. Reported sarcoma types in MGCT of bone include fibrosarcoma, osteosarcoma, malignant fibrous histiocytoma, undifferentiated high-grade pleomorphic sarcoma (UPS), and undifferentiated sarcoma [23–27]. In our series, chondrosarcoma was found in one case, malignant fibrous histiotoma was confirmed in 2 cases, osteosarcoma was found in 6 cases, and the rest 5 cases were considered to be with undifferentiated sarcoma (Fig. 2).
Discussion
Spinal MGCT is extremely rare with limited information in the literature. In this study, we analyzed the clinical and histological data of 14 cases with spinal MGCT, and reported our experience in the treatment of it. To our knowledge, this is the largest cohort about spinal MGCT by far.
Jaffe et al. firstly described malignant GCT (Jaffe Grade III), but the grading system is unable to predict the clinical behavior and prognosis of GCT [28]. Unni used the term “malignancy in giant cell tumor” to describe MGCT and subdivided it into primary or secondary, which was recorded as WHO recommendations [9, 15]. SMGCT can be further subdivided into two types: postsurgical and radiotherapy-associated, which are believed to have different etiologies but cannot be distinguished from each other on the basis of radiographic and histological presentation [7]. PMGCT is considered to be less common than SMGCT, and the similar finding (3 cases vs 11 cses) was achieved in our series. Further classification of SMGCT might be very difficult to be applied for patients previously treated with both surgery and radiotherapy.
Our cohort had a broad age demographic structure (range 15–63 years; mean 35 years). GCT in the spine occurs most commonly between the ages of 20–40 years, while patients with MGCT were thought to be older than patients with benign GCT [1, 15, 29]. The current study shows the same tendency with more than one third of patients more than 40 years old. Female gender predominance was also found in spinal MGCT in our series, which was similar with benign GCT in the spine.
Clinical and radiographic information are of limited value for the diagnosis of MGCT in the spine. The most frequent clinical feature of spinal MGCT was localized pain and neurologic deficits, which was common in spine tumors. In our series, MGCT was most likely to infringe upon the thoracic spine. The radiologic features of MGCT were similar to those of conventional GCT: osteolytic lesions, however MGCT showed more aggressive features, with a less distinct margin and more cortical breakthrough.
Histologically, the morphologic features of a classic GCT exists in PMGCT, while residual GCT elements in SMGCT might not be obvious and patient’s hospital history needs to be investigated to make the diagnosis [11]. Fibrosarcoma, osteosarcoma, malignant fibrous histiotoma, UPS, and undifferentiated sarcoma were the reported sarcoma types in MGCT with osteosarcoma as the main sarcoma type [23–27]. In our studied, the malignant components included chondrosarcoma, malignant fibrous histiocytoma, osteosarcoma and undifferentiated sarcoma, with osteosarcoma and undifferentiated sarcoma as the main sarcoma types.
Surgical treatment is the foundational treatment strategy for spinal MGCT with the aim of preserving functionality, relieving pain, controlling local recurrence, and promising prolonged survival [20, 30]. Surgical procedures applicable to spine vary from the simplest subtotal resection (curettage) to the most complex total en bloc spondylectomy [19]. Although only a limited number of cases were analyzed, it was evident that patients who underwent total en bloc spondylectomy had better prognosis when compared to patients with the other two surgical options.
Subtotal resection is a common surgical option in the spine due to its anatomical complexity, but it is confirmed to be insufficient for MGCT in the spine [1, 31, 32]. Piecemeal total resection is considered to be superior to subtotal resection, but it is also associated with a possibility of tumor cell contamination in the surgical field which might cause serious consequences for spinal MGCT. Total en bloc spondylectomy is a procedure aimed at surgically removing a tumor in a single, intact piece, fully encased by a continuous shell of healthy tissue (margin) [19]. Anatomical complexity of the spine makes it technically demanding, and careful surgical planning according to the Enneking stage, and WBB system is of great importance [20]. Total en bloc spondylectomy is also considered to cause more complications than the other two surgical procedures in the spine, which have been widely discussed in the literature [33–35].
Radiotherapy and chemotherapy were used as adjuvant treatment for MGCT of bone, but their positive effect on recurrence and overall survival remains controversial [7, 32, 36, 37]. With the development of focal irradiation treatment, the recent studies reported the safety and efficacy of radiotherapy in the management of MGCT, but its value is still debated because MGCT is initially thought to be radioresistant and malignant transformation following radiation treatment has occurred [38–40, 43]. Although chemotherapy was considered to be effective in controlling local disease in surgically inaccessible and radioresistant tumors by several reports, a chemotherapeutic protocol for MGCT has not yet been standardized [37, 41]. In our series, postoperative radiotherapy was used in 4 cases, but no significantly positive effect was found. Bisphosphonate treatment which was confirmed to reduce recurrence rate of spinal GCT might provide another adjuvant treatment choice for spinal MGCT.
Distant metastasis is not uncommon in MGCT of bone and the lung serves as the most frequent site [7, 32]. Distant metastasis makes disease control difficult and further threats the survival of patients. Lung metastasis occurred in 4 patients in our series and was thought to be the leading cause of death in these patients.
Preoperative biopsy is needed for surgery protocol formulation, although there is risk of possible nerve damage. However, the diagnosis of PMGCT might initially be missed when a biopsy shows only areas of benign GCT. Intraoperative fast pathological examination which also has a guiding significance for surgery seemed to be more credible in our study.
The prognosis of MGCT is still indefinite due to the rarity of the disease, but the existing cases indicated poor prognosis and short life expectation [36, 42]. Six of the fourteen patients (42.9 %) died in our series with mean survival time of 31 (range 3–64) months. Nascimento et al. and Lihua Gong et al. reported that PMGCT had a better prognosis than SMGCT [11, 29]. Similar outcome was found in our series, all 3 patients with PMGCT were alive with NED, but more than half of patients with SMGCT died.
References
Xu W, Li X, Huang W et al (2013) Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol 20(3):804–810
Wu Z, Yin H, Liu T et al (2014) MiR-126-5p regulates osteoclast differentiation and bone resorption in giant cell tumor through inhibition of MMP-13. Biochem Biophys Res Commun 443(3):944–949
Werner M (2006) Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop 30(6):484–489
Junming M, Cheng Y, Dong C et al (2008) Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine (Phila Pa 1976) 33(3):280–288
Wu Z, Yang X, Xiao J et al (2011) Aneurysmal bone cyst secondary to giant cell tumor of the mobile spine: a report of 11 cases. Spine (Phila Pa 1976) 36(21):E1385–E1390
Rock MG, Sim FH, Unni KK et al (1986) Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Jt Surg Am 68(7):1073–1079
Bertoni F, Bacchini P, Staals EL (2003) Malignancy in giant cell tumor of bone. Cancer 97(10):2520–2529
Dahlin DC, Cupps RE, Johnson EW Jr (1970) Giant-cell tumor: a study of 195 cases. Cancer 25(5):1061–1070
Reid R, Banerjee SS, Sciot R (2002) Giant cell tumour. In: Fletcher CDM, Unni KK, Mertens F (eds) World health organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon, pp 309–314
Domovitov SV, Healey JH (2010) Primary malignant giant-cell tumor of bone has high survival rate. Ann Surg Oncol 17(3):694–701
Gong L, Liu W, Sun X et al (2012) Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch 460(3):327–334
Sakkers RJ, van der Heul RO, Kroon HM et al (1997) Late malignant transformation of a benign giant-cell tumor of bone. A case report. J Bone Jt Surg Am 79(2):259–262
Mori Y, Tsuchiya H, Karita M et al (2000) Malignant transformation of a giant cell tumor 25 years after initial treatment. Clin Orthop Relat Res 381:185–191
Marui T, Yamamoto T, Yoshihara H et al (2001) De novo malignant transformation of giant cell tumor of bone. Skelet Radiol 30(2):104–108
Unni KK (1996) Dahlin’s bone tumors-general aspects and data on 11,087 cases, 5th edn. Lippincott-Raven Publishers, Philadelphia, pp 263–287
Hutter RV, Worcester JN Jr, Francis KC et al (1962) Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer 15:653–690
Frankel HL, Hancock DO, Hyslop G et al (1969) The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia, I. Paraplegia 7(3):179–192
Enneking WF (1986) A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 204:9–24
Boriani S, Weinstein JN, Biagini R (1997) Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976) 22(9):1036–1044
Yin H, Zhou W, Meng J et al (2014) Prognostic factors of patients with spinal chondrosarcoma: a retrospective analysis of 98 consecutive patients in a single center. Ann Surg Oncol 21:3572–3578
Yin H, Zhou W, Yu H et al (2014) Clinical characteristics and treatment options for two types of osteoblastoma in the mobile spine: a retrospective study of 32 cases and outcomes. Eur Spine J 23(2):411–416
Yin H, Zhang D, Wu Z et al (2013) Desmoplastic fibroma of the spine: a series of 12 cases and outcomes. Spine J. doi:10.1016/j.spinee.2013.09.042
Boriani S, Sudanese A, Baldini N et al (1986) Sarcomatous degeneration of giant cell tumours. Ital J Orthop Traumatol 12(2):191–199
Gitelis S, Wang JW, Quast M et al (1989) Recurrence of a giant-cell tumor with malignant transformation to a fibrosarcoma twenty-five years after primary treatment. A case report. J Bone Jt Surg Am 71(5):757–761
Kenan S, Abdelwahab IF, Klein MJ et al (1995) Case report 863. Osteosarcoma associated with giant cell tumor. Skelet Radiol 24(1):55–58
Ortiz-Cruz EJ, Quinn RH, Fanburg JC et al (1995) Late development of a malignant fibrous histiocytoma at the site of a giant cell tumor. Clin Orthop Relat Res 318:199–204
Zhu XZ, Steiner GC (1990) Malignant giant cell tumor of bone: malignant transformation of a benign giant cell tumor treated by surgery. Bull Hosp Jt Dis Orthop Inst 50(2):169–176
Sanerkin NG (1980) Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer 46(7):1641–1649
Nascimento AG, Huvos AG, Marcove RC (1979) Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer 44(4):1393–1402
Rao G, Suki D, Chakrabarti I et al (2008) Surgical management of primary and metastatic sarcoma of the mobile spine. J Neurosurg Spine 9(2):120–128
Mendenhall WM, Zlotecki RA, Scarborough MT et al (2006) Giant cell tumor of bone. Am J Clin Oncol 29(1):96–99
Beebe-Dimmer JL, Cetin K, Fryzek JP et al (2009) The epidemiology of malignant giant cell tumors of bone: an analysis of data from the Surveillance, Epidemiology and End Results Program (1975–2004). Rare Tumors 1(2):e52
Boriani S, Bandiera S, Donthineni R et al (2010) Morbidity of en bloc resections in the spine. Eur Spine J 19(2):231–241
Bandiera S, Boriani S, Donthineni R et al (2009) Complications of en bloc resections in the spine. Orthop Clin North Am 40(1):125–131
McDonnell MF, Glassman SD, Dimar JR 2nd et al (1996) Perioperative complications of anterior procedures on the spine. J Bone Jt Surg Am 78(6):839–847
Karamanakos PN, Jaaskelainen JE, Alafuzoff I et al (2010) Malignant giant cell tumor in the posterior fossa of a neonate. J Neurosurg Pediatr 5(3):277–282
Sasagawa Y, Tachibana O, Shiraga S et al (2012) Secondary malignant giant cell tumor of the clivus: case report. Clin Neurol Neurosurg 114(6):786–788
Kishima H, Miyao Y, Shimizu K (2001) Radiosensitive giant cell tumour of the sphenoid bone. Br J Neurosurg 15(2):171–174
Martins AN, Dean DF (1974) Giant cell tumor of sphenoid bone: malignant transformation following radiotherapy. Surg Neurol 2(2):105–107
Watkins LD, Uttley D, Archer DJ et al (1992) Giant cell tumors of the sphenoid bone. Neurosurgery 30(4):576–581
Yamamoto M, Fukushima T, Sakamoto S et al (1998) Giant cell tumor of the sphenoid bone: long-term follow-up of two cases after chemotherapy. Surg Neurol 49(5):547–552
Anract P, De Pinieux G, Cottias P et al (1998) Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop 22(1):19–26
Mahadevan A, Miksad R, Goldstein M et al (2011) Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 81(4):e615–e622
Campanacci M, Baldini N, Boriani S, Sudanese A (1987) Giant-cell tumor of bone. J Bone Jt Surg Am 69(1):106–114
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81402222, 81402223, 81401355).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Huabin Yin, Mo Cheng, and Bo Li contributed equally to this work, and all should be considered first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, H., Cheng, M., Li, B. et al. Treatment and outcome of malignant giant cell tumor in the spine. J Neurooncol 124, 275–281 (2015). https://doi.org/10.1007/s11060-015-1835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1835-9