Abstract
Malignant giant cell tumors of bone (MGCTB) are rare, and the diagnosis can be difficult due to the occurrence of a variety of malignant tumors containing giant cells. To better understand its clinicopathological features, we have reviewed our experience with 17 cases of MGCTB. Five cases were primary malignant giant cell tumor of bone (PMGCTB), and 12 cases were giant cell tumors of bone initially diagnosed as benign but malignant in a recurrent lesion (secondary MGCTB, SMGCTB). The patients included six women and 11 men (age ranged from 17 to 52 years; mean, 30.5 years). The tumor arose in the femur (six cases), the tibia (seven cases), the humerus (three cases), and the fibula (one case). Microscopically, PMGCTB showed both conventional giant cell tumor and malignant sarcoma features. SMGCTB were initially diagnosed as conventional giant cell tumor of bone, the recurrent lesion showing malignant features. Histologically, the malignant components included osteosarcoma (11 cases), undifferentiated high-grade pleomorphic sarcoma (two cases), and fibrosarcoma (four cases). SMGCTB cases showed strong expression of p53. Follow-up information revealed that four patients died of lung metastasis, two patients are alive with lung metastases, and 11 patients are alive without tumor. MGCTB should be considered as a high-grade sarcoma. It must be distinguished from GCTB and other malignant tumors containing giant cells. p53 might play a role in the malignant transformation of GCTB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant cell tumor of bone (GCTB) is a benign and locally aggressive neoplasm that is composed of sheets of mononuclear cells interspersed with uniformly distributed large, osteoclast-like giant cells [1]. Malignant giant cell tumor of bone (MGCTB) is a high-grade sarcoma diagnosed either at the onset in a giant cell tumor of bone (primary MGCTB or PMGCTB) or at the site of a recurrent lesion, previously diagnosed as a conventional giant cell tumor of bone (secondary MGCTB or SMGCTB) [1, 2]. The high-grade sarcoma component of PMGCTB often exists side by side with histologically benign GCTB components. SMGCTB occurs after surgery, radiotherapy, or both. It occurs more frequently than PMGCTB [2, 3]. MGCTB is uncommon and accounts for 1.8–7% of all GCTBs [4, 5]. It can be confused with other malignant lesions containing giant cells, such as osteosarcoma, fibrosarcoma, and undifferentiated high-grade pleomorphic sarcoma (UPS). To better understand the characteristics of MGCTB and the features that distinguish it from other malignant bone tumors, we report the clinicopathological features of 17 such cases.
Materials and methods
Seventeen cases were retrieved from the surgical pathology files between 1990 and 2010 in the Department of Pathology in Beijing Jishuitan Hospital, Beijing. In all cases, the tissues derived from surgical operation in the Department of Orthopedic Oncology were fixed in neutral buffered formalin and processed routinely with paraffin embedding. The sections were prepared and stained with hematoxylin and eosin. The histopathological features were reviewed by three pathologists. Clinical and radiological information was obtained from the electronic medical records and the surgeons. Immunohistochemistry was performed by an automated immunostainer (Autostainer 720, Labvision) using standard heat-induced epitope retrieval and an avidin–biotin–peroxidase complex method. Antibodies against the following antigens were used: p53, p16, vimentin, desmin, CD68, SMA, and myogenin. Appropriate positive and negative controls were included with each batch of staining (Table 3). This study was approved by the Ethics Committee of the Beijing Jishuitan Hospital.
Results
Clinical features
The patient series included six women and 11 men, with an age range from 17 to 52 years (mean age, 30.5 years). In the PMGCTB group (five cases), one case was from a female patient, and four cases were from male patients, whose ages ranged from 18 to 47 years (mean age, 29.2 years). In the SMGCTB group (12 cases), five cases were from female patients and seven cases were from male patients, whose ages ranged from 17 to 52 years (mean age, 30 years). The tumors involved the proximal tibia (two cases), distal tibia (one case), and proximal femur (two cases) in the PMGCTB, and distal femur (four cases), proximal tibia (four cases), proximal humerus (one case), distal humerus (one case), graft humerus (one case), and fibula (one case) in SMGCTB (Tables 1 and 2). None of the patients reported a history of prior radiation treatment.
Radiologic features
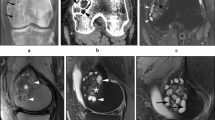
The radiologic features of the PMGCTB were similar to those of conventional GCTB: osteolytic lesions with well-circumscribed margins in the epiphyses of long bones (Fig. 1). In a few cases, cortical breakthrough, with or without soft tissue mass, was seen on plain films. In SMGCTB, the initial radiographic appearance was similar to that of conventional GCTB (Fig. 4a). The radiographic appearance of SMGCTB showed more aggressive features, with a less distinct margin of the lesion and more prominent sclerotic components. Cortical breakthrough and soft tissue mass formation were more common in these cases (Fig. 4b).
Gross features
The gross features of PMGCTB did not differ from those of GCTB. The tissue was soft and brown, and some areas were gray red to gray white because of hemorrhage and fibrosis; cyst formation and a thin bone shell were also often found. The tumor tissue of SMGCTB was white and soft; in some areas, it appeared randomly granular, suggesting the production of osteoid. The tumor transgressed the cortex and extended into the soft tissue in some cases.
Histologic features
In PMGCTB, the conventional GCTB features were prominent (Figs. 2a and 3a). The multinuclear giant cells were sparsely spread in the ovoid/round or spindle mononuclear stromal cells. The number of nuclei of giant cells ranged from 50 to 100, and the nuclei of the osteoclasts were similar to those of the stromal cells. The cytoplasm of stromal cells was ill defined, and mitotic figures were present, but atypical mitosis was not found. In addition to the GCTB characteristics, malignant features were also present. In our study, three cases showed fibrosarcoma characteristics and two cases features of osteosarcoma. In the fibrosarcoma component, tumor cells were densely arranged in long intersecting fascicles, and storiform areas were found (Fig. 2b). The cells had hyperchromatic nuclei with variably prominent nucleoli. Mitotic activity was almost always present, and atypical mitosis could also be found. Malignant spindle cells could be found infiltrating the normal bone trabecula (Fig. 2c). In the osteosarcoma component, the cells were highly anaplastic and pleomorphic with large hyperchromatic nuclei. Necessary characteristic for a diagnosis of osteosarcoma was the production of osteoid by malignant tumor cells: dense, pink, and amorphous intercellular material to be distinguished from non-osseous collagen (Fig. 3b). Collagen was linear and fibrillar, appearing around tumor cells without atypia or other malignant features found in osteosarcoma cells (Fig. 2d). In neighboring osteosarcoma areas, residual giant cell tumor areas were found (Fig. 3c).
In the cases of PMGCTB, between conventional giant cell tumor areas and high-grade malignant sarcoma, a distinct line of demarcation as described in the WHO classification [6] was not observed. The tumor gradually transited from the giant cell tumor area to the malignant area. In the transitional zone, the number of giant cells decreased, and the number of mononuclear cells increased. The mononuclear cells were arranged more densely and their shape changed to spindle, nuclear atypia appeared, and finally the characteristics of a high-grade malignant tumor (Fig. 4).
For SMGCTB, the initial lesions were conventional GCTB without evidence of malignancy. After one or more recurrences, the histological characteristics became malignant. We found one case with fibrosarcoma similar to conventional fibrosarcoma. Histological features of two cases were similar to UPS, with striking cytological atypia and nuclear pleomorphism, frequent and occasionally atypical mitotic figures. Some tumor cells showed prominent eosinophilic cytoplasm resembling rhabdomyosarcoma (Fig. 5b). Immunohistochemical stains showed reactivity for CD68 and vimentin, while stains for other antigens were negative, confirming a diagnosis of UPS and excluding rhabdomyosarcoma and leiomyosarcoma. Staining for p16 was negative in both UPS and original GCTB lesions (Fig. 8). p53 was diffusely positive in the nuclei of tumor cells in UPS, but expressed only focally and weakly in original GCTB lesions (Fig. 9; Table 3).
Nine cases of SMGCTB were osteosarcoma. In one case, uniform undifferentiated small round cells constituted the major malignant component (Fig. 6c); other areas showed a chondroid matrix (Fig. 6b) as in mesenchymal chondrosarcoma, but osteoid formation by markedly atypical tumor cells confirmed the diagnosis (Fig. 6a). Another case of osteosarcoma showed areas of aneurysmal bone cyst-like structure (Fig. 7), and one case invaded into the soft tissue and nerve fibers.
A remnant of conventional GCTB was found in four of 11 cases (Fig. 5a), suggesting the diagnosis of SMGCT. In cases without GCTB areas, the combination of malignant features in the recurrent lesion with the histopathology of the original conventional CGTB justified the diagnosis of SMGCTB. SMGCTB can occur after surgery or radiation therapy. In our hospital, radiation therapy was not used, so all of our 12 SMGCTB cases occurred without irradiation (Fig. 8).
Prognosis
The patients had been followed up for 8 to 221 months. One patient with PMGCTB had developed lung metastasis but is alive after 8 months follow-up. The remaining four patients with PMGCTB showed no evidence of recurrence. One patient with SMGCTB died of UPS. Four patients with SMGCTB developed lung metastasis, of whom three patients died. The remaining seven patients with SMGCTB showed no evidence of recurrence (Fig. 9).
Discussion
GCTB was originally described by Jaffe et al. and Lichtenstein et al. They classified GCTB into three categories: grade I (benign) without appreciable atypia of stromal cells, few mitoses, none abnormal; grade II (intermediate) with stromal cells showing only slight or more marked atypia, but not enough to justify a diagnosis of malignancy; and grade III (malignant) with obvious features of malignancy [7, 8]. However, this grading system is difficult to apply in practice and is unable to predict the clinical behavior and prognosis of this tumor [9]. Thus, this grade system is not used any more.
In Jaffe’s grade system, grade III represents malignant GCTB. The nomenclature and diagnostic criteria of malignant GCTB were further elaborated by Hutter et al. and Dalin et al. [10, 11]: Malignant GCTB is a sarcoma either in a pre-existing GCTB or in conjunction with it. Their term malignant GCTB included several types of giant-cell-rich tumor, such as osteosarcoma, UPS, metastatic or recurrent GCTB, and de novo malignant transformation of formerly conventional GCTB [3, 12]. To better describe malignant GCTB, Unni [2] introduced the name “malignancy in giant cell tumor” and subdivided it into primary and secondary malignancy in GCTB. The PMGCTB are those lesions in which a high-grade sarcoma component is present de novo in conjunction with GCTB, while SMGCTB is a high-grade sarcoma occurring as a recurrent lesion at the site of previously treated GCTB. According to this definition, PMGCTB and SMGCTB must include a high-grade sarcoma component. Some lesions need to be differentiated from MGCTB. Some features of GCTB do not justify a diagnosis of MGCTB. This can be pseudoanaplasia in the GCTB [22]. In addition, hemorrhage and necrosis are not uncommon in GCTB. The mononuclear stromal cells adjacent to the hemorrhage and necrosis show mild nuclear atypia, with enlarged and hyperchromatic or condensed hyperchromatic nuclei because of pyknosis. In some areas, only densely arranged mildly atypical spindle cells occur without giant cells, features insufficient to classify the lesion as malignant (similar to grade II as Jaffe described). Mitotic activity may be increased but without pathological mitosis. None of these features justify classification of the lesion as malignant. Reactive bone formation is often found at the border of the tumor. It is more regular and mature than malignant osteoid, and it is surrounded by mature osteoblasts, features that help to distinguish them from osteosarcoma cells. Furthermore, MGCTB needs to be differentiated from other malignant tumors containing giant cells such as osteosarcoma and UPS. Sufficient sampling to explore the GCTB features is helpful to distinguish it from the PMGCTB and other malignant tumors. The key points to differentiate between SMGCTB and other malignant tumors are the GCTB history and the recurrence information.
Histologically, in MGCTB, the reported sarcoma types include fibrosarcoma [15, 16], osteosarcoma [17, 18], UPS [19], and undifferentiated sarcoma [20]. In our cases, the malignant components included fibrosarcoma, osteosarcoma (one case with undifferentiated component), and UPS.
In SMGCTB, the original diagnosis is GCTB, but the recurrent secondary tumor is malignant. In some cases, residual giant cell tumor elements can also be found, justifying a diagnosis of SMGCTB. In other cases, the patient’s hospital history needs to be investigated to make the diagnosis of SMGCTB. In our cases, osteosarcoma was the major malignant tumor type. Sakkers et al. [21] proposed a theory about the malignant transformation of GCTB treated by curettage and bone grafting, stating that in this situation, the reparative proliferation occurring at the border of the dead bone might serve as a nidus for the formation of a malignant tumor. Currently, curettage and bone grafting constitute the routine surgical treatment of GCTB, and this may also be the element that favors malignant transformation.
Regarding the immunophenotype, the giant cells express RANK, CD51, and CD33 but are negative for CD14. The mononuclear cells express RANK, CD51, CD33, and CD14 [26, 27]. These cells are believed to derive from blood monocyte-macrophage lineage cells and infiltrate the tumor by chemotactic factors secreted by spindle-shaped stromal cells [28, 29]. GCTB is frequently regarded as a polyclonal tumor. Cytogenetically, telomeric associations are the most common chromosomal aberrations [30], but other genetic alterations have been reported. Moskovszky et al. found an elevated number of individual-cell aneusomies in recurrent GCTB compared with nonrecurrent cases. Eusomic polysomy has been seen in several tetraploid nonrecurrent cases, and balanced aneusomy has been found more frequently in diploid recurrent than in diploid nonrecurrent cases. These findings suggest that chromosomal abnormalities superimposed on telomeric associations might be responsible for the aggressive behavior of GCTB [31]. The expression of proteins involved in cell cycle regulation/oncogenesis, such as p53, p63, ki-67, cyclinD1, or Bcl-2, are not predictive for the clinical behaviour of GCTB [32]. We found the expression of p53 in UPS stronger than that in original GCTB, which suggests that it may play a role in the malignant transformation of GCTB.
Clinical and radiographic information are of limited value for the diagnosis of MGCTB, especially for PMGCTB. According to Compannacci et al. [13] radiographic features allow grading of the GCTB as three types: quiescent (type I), active (type II), and aggressive (type III). Clinically aggressive behavior of GCTB has suggested a surgical staging system with stages 1–3, respectively, representing the clinically latent, active, and aggressive forms of GCTB [14]. However, these stages do not correspond to histological benign or malignant features. In our study, five cases were diagnosed as PMGCTB by histology, while the clinical and radiographic features suggested a diagnosis of benign GCTB.
As in another study, we found MGCTB often to involve the distal femur and tibia [23]. The preponderance of gender is controversial in different cohorts. In our study and in another report [4], there is a male preponderance, but in other reports, a female preponderance was described [23]. The age distribution in MGCTB reports also varies. In our study, the mean age was younger than what was reported earlier [4].
The prognosis of MGCTB is still indefinite because of its rareness and short follow-up of MGCTB. Most reports consider the prognosis as poor, and most of the patients die rapidly [24]. Anract et al. [6] reported poor prognosis of MGCTB with a 5-year survival of 50%, despite the combination of surgery and chemotherapy. Similar to the report of Nascimento et al. [25], we found PMGCTB to have a better prognosis than SMGCTB. However, Anract et al. [6] observed equally poor outcomes in PMGCTB and SMGCTB.
In summary, we report a series of cases of MGCTB and describe its histological characteristics. Because of its rareness, the diagnosis should be made carefully, and the lesion should be differentiated from other primary malignant sarcomas. Genetic abnormalities and other factors related to malignant transformation should be further investigated.
References
Reed R, Bareyce SS, Sciot R (2002) Giant cell tumour. In: Fletcher CD, Unni KK, Mertens F (eds) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC, Lyon, pp 309–312
Unni KK (1996) Dalin’s bone tumors-general aspects and data on 11,087 cases, 5th edn. Lippincott-Raven, Philadelphia, pp 263–287
Marui T, Yamamoto T, Yoshihara H et al (2001) De novo malignant transformation of giant cell tumor of bone. Skeletal Radiol 30:104–108
Bertoni F, Bacchini P, Staals EL (2003) Malignancy in giant cell tumor of bone. Cancer 97:2520–2529
Rock MG, Sim FH, Unni KK et al (1986) Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am 68:1073–1079
Anract P, De Pinieux G, Cottias P et al (1998) Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop 22:19–26
Jaffe HL, Lichtenstein L, Portis RB (1940) Giant cell tumor of bone. Its pathologic appearance, grading, supposed variants and treatment. Arch Pathol 30:993–1031
Lichtenstein L (1972) Bone tumors, 4th edn. Mosby, St. Louis, pp 135–165
Sanerkin NG (1980) Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer 46:1641–1649
Dahlin DC, Cupps RE, Johnson EW Jr (1970) Giant-cell tumor: a study of 195 cases. Cancer 25:1061–1070
Hutter RV, Worcester JN Jr, Francis KC et al (1962) Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer 15:653–690
Mirra MJ (1989) De novo malignant transformation of giant cell tumor of bone. Bone tumous: clinical, radiologic, and pathologic correlations. Lea & Febiger, Philadelphia, pp 941–1020
Campanacci M, Baldini N, Boriani S et al (1987) Giant-cell tumor of bone. J Bone Joint Surg Am 69:106–114
Present D, Bertoni F, Hudson T et al (1986) The correlation between the radiologic staging studies and histopathologic findings in aggressive stage 3 giant cell tumor of bone. Cancer 57:237–244
Boriani S, Sudanese A, Baldini N et al (1986) Sarcomatous degeneration of giant cell tumors. Ital J Orthop Traumatol 12:191–199
Gitelis S, Wang JW, Quast M et al (1989) Recurrence of a giant-cell tumor with malignant transformation to a fibrosarcoma twenty-five years after primary treatment. A case report. J Bone Joint Surg Am 71:757–761
Kenan S, Abdelwahab IF, Klein MJ et al (1995) Case report 863. Osteosarcoma associated with giant cell tumor. Skeletal Radiol 24:55–58
Brien EW, Mirra JM, Kessler S et al (1997) Benign giant cell tumor of bone with osteosarcomatous transformation (“dedifferentiated” primary malignant GCT): report of two cases. Skeletal Radiol 26:246–255
Ortiz-Cruz EJ, Quinn RH, Fanburg JC et al (1995) Late development of a malignant fibrous histiocytoma at the site of a giant cell tumor. Clin Orthop Relat Res 318:199–204
Zhu XZ, Steiner GC (1990) Malignant giant cell tumor of bone: malignant transformation of a benign giant cell tumor treated by surgery. Bull Hosp Jt Dis Orthop Inst 50:169–176
Sakkers RJ, van der Heul RO, Kroon HM et al (1997) Late malignant transformation of a benign giant-cell tumor of bone. A case report. J Bone Joint Surg Am 79:259–262
Layfield LJ, Bentley RC, Mirra JM (1999) Pseudoanaplastic giant cell tumor of bone. Arch Pathol Lab Med 123:163–166
Domovitov SV, Healey JH (2010) Primary malignant giant-cell tumor of bone has high survival rate. Ann Surg Oncol 17:694–701
Karamanakos PN, Jaaskelainen JE, Alafuzoff I et al (2010) Malignant giant cell tumor in the posterior fossa of a neonate. J Neurosurg Pediatr 5:277–282
Nascimento AG, Huvos AG (1979) Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer 44:1393–1402
Forsyth RG, De Boeck G, Baelde JJ et al (2009) CD33(+)CD14(−) Phenotype is characteristic of multinuclear osteoclastlike cells in giant cell tumor of bone. J Bone Miner Res 24:70–77
Maggiani F, Forsyth R, Hogendoorn PCW et al (2011) The immunophenotype of osteoclasts and macrophage polykaryons. J Clin Pathol 64:701–705
Zheng MH, Robbins P, Xu J et al (2001) The histogenesis of giant cell tumour of bone: A model of interaction between neoplastic cells and osteoclasts. Histol Histopathol 16:297–307
Lau YS, Sabokbar A, Gibbons CL et al (2005) Phenotypic and molecular studies of giant cell tumours of bone and soft tissue. Hum Pathol 36:945–954
Forsyth RG, De Boeck G, Bekaert S et al (2008) Telomere biology in giant cell tumour of bone. J Pathol 214:555–563
Moskovszky L, Szuhai K, Krenács T et al (2009) Genomic instability in giant cell tumor of bone. A study of 52 cases using DNA ploidy, relocalization FISH, and array-CGH analysis GENES. Chromosomes Cancer 48:468–479
Alberghini M, Kliskey K, Krenacs T et al (2010) Morphological and immunophenotypic featuresof primary and metastatic giant cell tumour of bone. Virchows Arch 456:97–103
Conflict of interest
There is no conflict of interest in our study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, L., Liu, W., Sun, X. et al. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch 460, 327–334 (2012). https://doi.org/10.1007/s00428-012-1198-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1198-y