Abstract
Aluminum (Al) is a potentially toxic element causing many neuropathological and behavioral alterations such as affective and cognitive disorders. Recently, several studies described melatonin (MEL) as an effective antidepressant and anxiolytic substance. This study aimed to assess the effects of MEL and Al combination on affective and cognitive behavior and oxidative stress in male Wistar rats. Animals were injected intraperitoneally with saline (0.9% NaCl), MEL (4 mg/kg), Al (1 mg/kg) or MEL (4 mg/kg) + Al (1 mg/kg) for 8 weeks. After the treatment period, open-field, elevated plus maze, forced swimming test, Y-maze and Morris water maze were used to evaluate anxiety-like, depression-like behaviors, spatial learning, and memory ability. The hippocampus was taken for biochemical examination. The results revealed that MEL and Al combination exerts anxiolytic and antidepressant effects and shows a positive effect on working memory and spatial learning compared to the Al treated groups. Also, biochemical analysis showed that MEL co-administration played a neuroprotective role, increasing superoxide dismutase (SOD) activity and decreasing significantly nitric oxide (NO) and lipid peroxidation (LPO) levels in rat hippocampus in the MEL+Al treated groups. In conclusion, MEL in combination with Al has both anxiolytic and antidepressant effects, improves cognitive behavior and biochemical dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is a very common agent in nature and the third component of the earth’s crust (8% of its weight) after oxygen (47%) and silicon (28%). It is the most used metal after iron in various fields and the most abundant known for its neurotoxicity [1]. It easily accesses the central nervous system and accumulates in different regions and could be a potential factor influencing affective and cognitive disorders in both humans and animals, it induces oxidative stress (OS) and loss of synapses and neurons in the hippocampal and cerebral cortical regions [2, 3].
In the context of prevention and/or control of the neurotoxicity effects of heavy metals in general and Al in particular, several researches in the recent years have been focusing on a pineal hormone, melatonin (MEL), as a molecule that attenuates and prevent effects on affective and cognitive disorders. In addition to its anxiolytic, antidepressant, anti-inflammatory, regulatory and immunomodulatory properties, which have already been shown in humans and rodents, this hormone and its metabolites have properties that can combat oxidative stress and stimulate the activation of molecules that help synaptic plasticity in the nervous system [4,5,6].
The objective of our study is to demonstrate in Wistar rats the possible neuroprotective effect of melatonin against the neurotoxicity of Al administered chronically by intraperitoneal injection and its negative impact on behavioral and biochemical capacities.
Materials and methods
Animals and experimental conditions
This study was performed on twenty male Wistar rats initially weighing 120 ± 20g. Animals, from the breeding of the Ibn Tofaïl University, were maintained under LD 12/12 (12 h Light/12 h Darkness) and at a standard temperature (21 ± 1°C). Water and food were provided ad libitum. All animal procedures were carried out in accordance with the Animal Scientific procedure and approved by the University Ethics Committee for Animal Experiments.
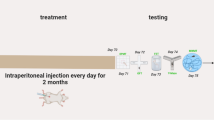
The rats are divided into four groups of five animals in each who received intraperitoneally and chronically one injection per day, between 16:00 and 17:00 during eight weeks, of physiological saline, AlCl3 (SEGMA-ALDRICH) or MEL (SIGMA France) as following:
Groups | Drugs and dose |
|---|---|
Group I (control) | NaCl 0,9% |
Group II (MEL) | MEL 4 mg/kg |
Group III (Al) | AlCl3 1mg/kg |
Group IV (Al + MEL) | AlCl3 1mg/kg + MEL 4 mg/kg |
Neurobehavioral tests
Anxiety-Like measurement
Open Field Test
The OFT is used to measure the anxiety-like behavior in rodents [7, 8]. The animal is placed in the arena and allowed to move freely for 10 minutes while being recorded by an overhead camera for subsequent analysis. The parameters measured are: The time spent in the center area (TCA), the number of returns to the center (NRC) and number of total squares (NTS). The entries in the central area and the time spent in this area by the rats are inversely proportional to the anxiety level. The time spent in the central area will be higher than the anxiety level of the animal will be lower. While the number of total squares is a reliable index of general locomotors activity. The apparatus was cleaned between each examination using 7% ethanol solution.
Elevated Plus Maze (EPM)
The EPM is used to assess anxiety-related behavior in rodent models of CNS disorders [9]. The rat ware placed on the central area of the maze facing an open arm and left to explore the EPM for 5 min and its movements while being recorded by a camera for later analysis. The parameters measured are: The time spent on the open arms (TOA), the entries into open arms (EOA) and the number of full entries into the arms (TAE). The TOA and EOA parameters are inversely correlated with the level of anxiety, whereas the TAE parameter is a reliable index of general locomotors activity. Reduced anxiety behavior is shown by a statistically significant increase in open-arm parameters (time and/or entries). The apparatus was cleaned between each examination using 7% ethanol solution.
Depression-like measurement
Forced Swimming Test (FST)
The FST is designed to evaluate the depressive-like behavior in rodent [10]. The rat are forced to swim for 5 min in glass cylinders (height = 50 cm; diameter = 30 cm) containing 30 cm of water at (23°C ± 2°C) while the camera is recording it for later analysis to determine the immobility times (TIM). Depressive-like behavior is characterized by an increase in immobility time (TIM) [10, 11].
Cognitive Measurement
Y-maze test
Is a behavioral test for measuring the willingness of rodents to explore new environments [12]. Rat ware placed on the central area of the Y-maze and left to explore the arena for 8 min. Rats typically prefer to investigate a new arm of the maze rather than returning to one that was previously visited. A rat would be making a triad when it visited all 3 arms consecutively. As a measure for working memory, the percentage of alternations that the rat made was calculated, being the number of triads divided by the maximum possible alternations (i.e. the total number of entries minus 2)*100. If a rat scored significantly above 50% alternations, this was indicative of functional working memory. The apparatus was cleaned between each examination using 7% ethanol solution.
Morris Water Maze Test (MWM)
The MWM is the most commonly test used to evaluate cognitive functions related with learning and memory in rodents [13]. Tempera paint is added into the water (22◦C) until it becomes opaque. A hidden platform, from a Plexiglas cylinder (13.75 cm high, 9 cm in diameter), is placed about 0.5 cm below the water surface making it invisible. The MWM The water maze was in a room with posters and furniture around the walls, which served as additional visual cues. Rats were tested in two phases: acquisition and probe trial test [14].
During acquisition (4 trials/day for 4 days), rats were trained to swim to a hidden platform located in the northeast quadrant. Each rat was released from a randomly assigned starting point (East, North, South or West). If the rat did not find the platform within 60 s, it was guided to the platform and allowed to remain there for approximately 10 s. On the fifth day, the rats spatial memory was tested in a 60 s probe trial with no platform present [15].
Biochemical Examination
One day after the end of the behavioral tests, all animals were firstly anesthetized and then sacrificed by decapitation. Brains were quickly removed and maintained at low temperature on ice. The hippocampus was rapidly and gently removed and separated from surrounding tissues and homogenized in phosphate buffer at PH: 7.4 (w/v), centrifuged at 1500 rpm for 10 min, and the resulting supernatant was used in the biochemical assays.
Lipid peroxidation assay
The formation of lipid peroxides during lipid peroxidation process was analyzed by measuring the thiobarbituric-acid-reacting substances (TBARS) in cells, as previously described by Draper and Hardley [16]. The TBARS levels in hippocampus is presented as nmol/g of tissue and were determined by the absorbance at 535 nm [17].
Nitrite/nitrate assay
The nitric oxide (NO) activity was assayed by the method of [18]. Tissue nitrite levels is expressed as µmol/g of tissue.
Determination of Superoxide Dismutase (SOD) activity
The superoxide dismutase (SOD) activity was determined according to the method described by Beauchamp and Fridovich [19]. SOD activity is reported as U/g of hippocampal tissue.
Statistical analysis
All data are expressed as the means ± standard error of the means (S.E.M.). To determine the differences between experimental groups in behavioral data and biochemical parameters, statistical analysis was performed by two-way ANOVA using SPSS (version 22 SPSS). Post hoc comparisons were made using the Tukey’s test. ANOVA repeat measures were used for the Morris water maze test. Differences were considered significant when p<0.05, very significant when p<0.01 and highly significant when p<0.001.
Results
Effect of Al and MEL on the levels of anxiety-like measured in the OFT (Fig. 1)
Effect of Aluminum, Melatonin and their combination on (A): Total amount time spent in the center (TCA), (B): Number of return into center area (NRC) and (C): Number of total squares (NTS) measured in OFT in male rats treated with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+ MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
Statistical analysis showed that the TCA and NRC parameters was significantly affected by Al treatment compared to their controls (p<0.001). Moreover, MEL administration did not induce any significant change in this parameter compared with control group (p>0.05). Additionally, rats treated with the combination of Al and MEL (Al+MEL) show a significantly elevated in those parameters compared to Al-treated groups (p<0.01 and p<0.05 respectively). Furthermore, locomotors activity represented by the NTS was unaffected by any treatment (p>0.05).
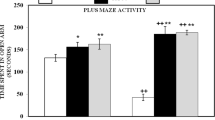
Effect of Al an MEL on anxiety levels measured in EPM (Fig. 2)
Effect of Aluminum, Melatonin and their combination on (A): Total amount of time spent in exposed arms (TOA); (B): Number of entries in exposed arms (EOA) and (C): Total number of arms entries (TEA) measured in EPM test in male rats treated with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
The results obtained showed that TOA parameter was significantly affected by Al treatment compared to control group (p<0.001) and no difference was noted between Al+MEL-treated groups and Al-treated groups (p>0.05). In addition, the EOA and TEA parameters was unaffected by any treatment. No statistically significant difference was found between the control and treated groups (p>0.05).
Effect of Al and MEL on depressive-like performances measured by FST (Fig. 3)
Effect of Aluminum, Melatonin and their combination on depression-like behaviors parameters measured in FST in male rats treated with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+ MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
For the TIM parameter, statistical analysis showed that chronic injection of Al significantly increased the immobility time compared to control group (p<0.001). For the Al+MEL-treated groups, a significant decrease in TIM was observed compared to Al-treated groups (p<0.01).
Al and MEL effect on memory
Y-maze test (Fig. 4)
Spontaneous alternation percentage evaluated in Y-maze test, in male rats treated with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+ MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
The spontaneous alternation percentage was significantly affected by Al treatment compared to control groups (p<0.01). In the same, the statistical analysis shows that treatment with MEL alone did not significantly increase this parameter compared to control groups (p>0.05). Similarly, a significant increase in this parameter is observed in Al+MEL-treated groups compared to Al-treated groups (p<0.01).
Morris water maze
Acquisition phase (Fig. 5A)
Fig. 5(A) shows that during acquisition phase, the latency to reach the hidden platform on each of the 4 days is decreased in all treated groups. Statistical analysis indicates a highly significant difference between Al-treated groups compared to control rats (p<0.001). Paradoxically to Al-treated groups, the statistical analysis for MEL groups indicates a non-significant decrease in rats compared to their controls (p>0.05). With respect to Al+MEL groups, statistical analysis shows a significant decrease in this parameter compared to Al-treated groups (p<0.05).
A): Latency to reach the hidden platform on each of the 4 days of learning phase, (B): Percentage of time spent in the correct quadrant measured in MWM tests in male rats after 8 weeks of treatment with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+ MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
Percentage time spent in the correct quadrant (Fig. 5B)
The percentage of time spent in the correct quadrant was significantly affected by Al treatment. It is observed that Al group rats spend significantly less time in the correct quadrant (NO) than control rats (p<0.01), with an estimated decrease of 50%. A significant increase in the percentage of time spent in the correct quadrant is noted in MEL-treated groups compared to Al-treated groups (p<0.05), as well as in Al+MEL-treated groups compared to Al-treated groups (p<0.01).
Al and MEL effect on oxidative stress (Fig. 6)
A) the lipid peroxidation levels in hippocampus, TBARS levels expressed in nmol/g of tissue; (B) The nitric oxide level in hippocampus, expressed in µmol/g of tissue; (C) Changes in Superoxide Dismutase (SOD) activity in hippocampus, expressed in U/g of tissue in male rats after 8 weeks of treatment with NaCl 0.9% (Control), 4 mg/kg of melatonin (MEL), 1 mg/Kg of AlCl3 (Al) and simultaneously with 4mg/kg of MEL and 1mg/kg of AlCl3 (Al+ MEL). Results are represented as mean ± SEM. The significance level is 0.05. *p<0.05, **p<0.01, ***p<0.001. *P, significant change with respect to control; $P, significant change with respect to Al
Lipid Peroxidation (LPO) in Hippocampus reflected by TBARS levels was significantly affected by Al treatment compared with control group (p<0.01). Even though it is statistically not significant, the treatment with MEL reduced this parameter compared to control (p>0.05). For the Al+MEL combination, we note a decrease in TBARS levels in hippocampus compared to Al-treated groups, but no significant difference was observed.
NO concentrations in hippocampus was significantly affected by treatment. The chronic injection of Al significantly increased the NO levels compared to control group (p<0.001). As well, chronic injection of MEL reduced this parameter compared to control, but no statistically significant difference was observed (p>0.05). For the Al+MEL combination, a significant decrease in NO concentrations in hippocampus was observed compared to Al-treated groups (p<0.001).
Superoxide Dismutase (SOD) Activity in Hippocampus was affected by treatment. The results summarized in Fig. 6(C) showed the following: Al affect SOD activity in rat hippocampus compared with the control group (p<0.01). In addition, the treatment with Al+MEL combination significantly increased the SOD activity in rat hippocampus compared with Al-treated groups (p<0.05).
Discussion
The aim of this study was to demonstrate, in addition to the neurotoxic effect of chronic Al administration, the neuroprotective impact of MEL via chronic injections on neurobehavioral functions in male rats.
Analysis of the different parameters showed that chronic exposure of male rats to Al causes:
-
Anxiety-like disorders. This anxiogenic effect is based on the fact that Al decreases the TCA and NRC parameters in the OFT, and EBO and TOA parameters in EPM.
-
Depressive-like disorders evaluated by the FST, whose results clearly show a very significant increase in TIM parameter.
-
Cognitive disorders characterized by the impairment of working memory due to the decrease in the percentage of alternations (Y-maze test) and the affection of spatial learning performance causing a deficit in retention of spatial memory (MWM).
The behavioral outcomes presented here are consistent with current literature. These observations corroborate with findings from our previous paper that investigated the effect of chronic Al injection at different doses for 8 weeks on affective and cognitive disorders in male Wistar rat [20]. Another study by Tair et al., [21] evaluated the protective effect of an aqueous extract of Hamda scoparia against the neurotoxic effects of chronic intraperitoneal Al exposure for 90 days in rats, reported that Al intake causes anxiety in the OFT. Also, in adult Wistar rats, decreased TOA in the EPM was obtained after oral exposition to AlC3 for 8 weeks [22]. Regarding the depressive effect of Al assessed by the FST, similar effects were observed in mice after chronic exposure to Al [23]. The same results were observed in male Prague Dawley rats who received for 5 weeks an intraperitoneal injection of AlCl3 at 70 mg/kg [24].
Several studies on memory disorders showing that Al causes cognitive dysfunction and negatively affects spatial learning and memory capacities of rats [25]. Our data was consistent with results obtained in Wistar rats treated with Al lactate intragastrically for 12 weeks [26]. As well as in albino mice treated with AlCl3 in distilled water for 3 months at a dose of 50 mg/kg/d [23]. In addition, Ribes et al., [27] study showed that low oral exposure of Al (Al lactate at 0 and 1 mg/g feed for 120 days) altered spatial learning and memory in transgenic mice of Alzheimer's disease.
The results of our study, supported with the works mentioned above, confirmed that Al induces depression-like and anxiety-like behaviors, as well as memory and learning disorders. Since no single mechanism of Al neurotoxicity has been identified, some interconnected hypotheses have been suggested. These hypotheses include the alteration of intracellular signal transduction pathways and exacerbation of OS.
Several investigators have suggested a link between OS and the etiology of cognitive and affective impairments [28,29,30,31]. OS can alter brain activity including neurotransmission, provokes neuronal cell death, leading to an altered behavior [32]. In this context, several studies reported that behavioral abnormality in rats treated with Al is associated to enhanced OS in the brain, especially in the hippocampus [33,34,35] which plays a crucial role in emotion regulation and in learning and memory processes [36, 37].
In our work, chronic exposure to Al showed a marked increase in OS in the hippocampus, which was indicated by a decrease in SOD activity, and an increase in TBARS (LPO) levels. These findings are consistent with recent reports showing similar results [33, 34, 38]. Under OS, the LPO provoked is followed by alterations in membrane permeability and consequently deleterious effects on neuronal function [39]. The increased LPO level is, at least in part, due to an inhibition of SOD and CAT enzymes in the hippocampus following Al exposure. These two antioxidant enzymes can control the harmful effects of free radicals [40]. Al was found to induce decline in the mRNA expression of endogenous antioxidants [41].
On the other hand, the reduction in SOD activity may indicate inability of this enzyme to cope with the influx of free radicals such NO generated due to administration of Al as shown in this study. The reports of Al-Amin et al., are in line with our results; Al treatment enhanced the level of LPO and NO in the hippocampus as well as a decrease in antioxidant status [35, 42]. Since Al is implicated in free radicals generation, it is possible that it does execute its effects by impairing mitochondrial functions [43]. As known, the changes in mitochondrial functions are responsible for generation of OS. Mitochondria are considered to be the major sites producing free radicals. High levels of free radicals such as NO will induce oxidative damage to unsaturated fatty acids, proteins, and DNA in the mitochondria [44, 45], leading to cell damage and death [46]. It has been demonstrated that chronic Al exposure (10 mg/kg) for 3 months increases free radicals generation and is implicated in the impairment of mitochondrial functions in the various regions of rat brain [43]. These findings may explain the increased level of NO and LPO observed in this experiment following Al administration. In addition, Al is likely capable of inducing NO production in the rat brain [47], which may be due to the ability of this metal to enhance the expression of NO synthase (NOS) [48]. At high levels, NO can reacts with the superoxide ion (O2-) to form the ion peroxynitrite (ONOO-), a reactive toxic molecule which can elicit cellular damages by initiating LPO formation, leading to neuronal cell damage [49], and consequently, an altered behavior.
To reduce the neurotoxic effects of Al, our research relied on the chronic administration of MEL in combination with Al. In our study, in rats treated with MEL in combination with Al, levels of anxiety and depression were significantly reduced compared to rats treated with Al alone. Similar results have been observed in other studies [50, 51]. In addition, the anxiolytic and antidepressant effects of MEL have also been reported in recent years [52,53,54]. Thus, learning and memory performances are high in the Y-Maze and the MWM tests. Some recent data indicate that MEL may reduce hippocampal-dependent memory deficit in mice [53, 55, 56]. Additionally, MEL improves spatial memory in ovariectomized adult rats with cognitive impairment [57]. The results thus obtained confirmed that MEL as a neuroprotective substance has both anxiolytic and antidepressant effects, as well as a positive effect on memory and learning in rat.
Because OS and neurobehavioral changes are strongly correlated [58,59,60,61], the anti-OS property of MEL provides one possible mechanism by which this molecule abolishes Al-induced impairment of emotional process and learning and memory [62]. In the present study, chronic administration of MEL was found to improve not only the affective and cognitive functions but also reduced Al induced-oxidative damage in the hippocampus by attenuating the rise in LPO and NO levels and also by restoring the SOD activity. These findings supported earlier observation about attenuation of brain OS by MEL in Al treated animals [62]. Al-Olayan et al. also found that treatment with MEL (10 mg/kg) for 7 days, decreased LPO and NO levels and increased antioxidant enzymes activities in brains of Al treated rats [48]. MEL might protect against the Al induced neurotoxicity through reducing OS and improving mitochondrial function. This indolamine and its metabolites provide on-site protection to mitochondria from OS, thus, preserving optimal functions of mitochondria and preventing the cell death [63]. Generally, the on-site production of Mel could provide local protection of the mitochondria [64]. Interestingly, exogenously administered MEL can quickly penetrate the blood-brain-barrier and accumulate at high levels in each neuron compartment [65]. Compared to conventional antioxidants such as vitamin C and E, MEL is more effective in reducing OS in every neuronal compartment [64, 65]. MEL can act at two levels; firstly, as a direct antioxidant by its scavenging actions, MEL detoxify free radicals and their derivatives such ONOO– [66, 67], and secondly as an indirect antioxidant, this hormone up-regulates gene expression of antioxidant enzymes, such as SOD [68, 69]. Collectively, these reports confirmed the protective effect of MEL against oxidative damage.
Conclusions
In summary, our results confirmed some previous findings of the neurotoxic effect of Al and here we revealed that chronic administration of Al induced anxiety-like, depression-like, memory impairment and hippocampal OS in male rats. Moreover, MEL attenuates affective and cognitive disorders induced via Al intoxication possibly by inhibiting OS in the hippocampus. This investigation reinforces the neuroprotective potential of MEL.
Data availability
My manuscript has no associated data.
References
Gourier-Fréry C, Fréry N (2004) Aluminium. EMC - Toxicologie-Pathologie 1:79–95. https://doi.org/10.1016/j.emctp.2004.04.002
Maya S, Prakash T, Madhu K Das, Goli D (2016) Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomedicine and Pharmacotherapy 83:746–754. https://doi.org/10.1016/j.biopha.2016.07.035
Walton JR (2006) Aluminum in hippocampal neurons from humans with Alzheimer’s disease. NeuroToxicology 27:385–394. https://doi.org/10.1016/j.neuro.2005.11.007
Tan D, Reiter RJ (2019) Mitochondria : the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Research 2:44–66. https://doi.org/10.32794/mr11250011
Ding K, Xu J, Wang H, et al (2015) Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochemistry International 91:46–54. https://doi.org/10.1016/j.neuint.2015.10.008
Favero G, Franceschetti L, Bonomini F, et al (2017) Review Article Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. 2017:17–19. https://doi.org/10.1155/2017/1835195
Carola V, D’Olimpio F, Brunamonti E, et al (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behavioural Brain Research 134:49–57. https://doi.org/10.1016/S0166-4328(01)00452-1
Gentsch C, Lichtsteiner M, Feer H (1987) Open field and elevated plus-maze: A behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behavioural Brain Research 25:101–107. https://doi.org/10.1016/0166-4328(87)90003-9
Naranjo-Rodriguez EB, Osornio AO, Hernandez-Avitia E, et al (2000) Anxiolytic-like actions of melatonin, 5-metoxytryptophol, 5-hydroxytryptophol and benzodiazepines on a conflict procedure. Progress in Neuro-Psychopharmacology and Biological Psychiatry 24:117–129. https://doi.org/10.1016/S0278-5846(99)00075-5
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology 47:379–391. https://doi.org/10.1016/0014-2999(78)90118-8
Benabid N, Mesfioui A, Ouichou A (2008) Effects of photoperiod regimen on emotional behaviour in two tests for anxiolytic activity in Wistar rat. Brain Research Bulletin 75:53–59. https://doi.org/10.1016/j.brainresbull.2007.07.016
Sierksma ASR, Van Den Hove DLA, Pfau F, et al (2014) Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology 77:120–130. https://doi.org/10.1016/j.neuropharm.2013.09.015
Morris R (2008) Developments of a water1maze procedure for studying spatial learning in the rat. Search 11:7336–7336. https://doi.org/10.1016/016510270(84)9000714
Wong AA, Brown RE (1984) Age-related changes in visual acuity, learning and memory in C57BL/6J and DBA/2J mice. Neurobiology of Aging 11:47–60. https://doi.org/10.1016/0165-0270(84)90007-4
Kahloula K, Eddine D, Adli H, et al (2014) Effet de l ’ exposition chronique au nickel sur les fonctions neurocomportementales chez les rats Wistar pendant la période de développement. Toxicologie Analytique & Clinique 26:186–192. https://doi.org/10.1016/j.toxac.2014.09.056
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods in enzymology 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-i
Freitas RM, Sousa FCF, Vasconcelos SMM, et al (2004) Pilocarpine-induced status epilepticus in rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacology, biochemistry, and behavior 78:327–332. https://doi.org/10.1016/j.pbb.2004.04.004
Chao CC, Hu S, Molitor TW, et al (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. Journal of immunology (Baltimore, Md : 1950) 149:2736–2741. https://doi.org/10.4049/jimmunol.149.8.2736
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Zghari O, Rezqaoui A, Ouakki S, et al (2018) Effect of Chronic Aluminum Administration on Affective and Cognitive Behavior in Male and Female Rats. 179–196. https://doi.org/10.4236/jbbs.2018.84012
Taïr K, Kharoubi O, Taïr OA, et al (2016) Aluminium-induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammada scoparia). Journal of Acute Disease 5:470–482. https://doi.org/10.1016/j.joad.2016.08.028
Buraimoh AA, Ojo SA, Hambolu JO, et al (2011) Effects of Aluminium Chloride on Anxiety-Related Behaviour. Department of Human Anatomy, Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. 2:65–69
Rebai O, Djebli NE (2008) Chronic Exposure to Aluminum Chloride in Mice : Exploratory Behaviors and Spatial Learning. 2:26–33
Ali AA, Ahmed HI, El-samea HAA, El-demerdash E (2016) Alzheimer ’ s Disease & Parkinsonism The Potential Effect of Caffeine and Nicotine Co-administration against Aluminum-induced Alzheimer ’ s disease in Rats. 6:. https://doi.org/10.4172/2161-0460.1000236
Sethi P, Jyoti A, Singh R, et al (2008) NeuroToxicology Aluminium-induced electrophysiological , biochemical and cognitive modifications in the hippocampus of aging rats. 29:1069–1079. https://doi.org/10.1016/j.neuro.2008.08.005
Sharma DR, Wani WY, Sunkaria A, et al (2013) Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotoxicity Research 23:336–357. https://doi.org/10.1007/s12640-012-9351-6
Ribes D, Colomina MT, Vicens P, Domingo JL (2008) Effects of oral aluminum exposure on behavior and neurogenesis in a transgenic mouse model of Alzheimer’s disease. 214:293–300. https://doi.org/10.1016/j.expneurol.2008.08.017
Zghari O, Lamtai M, Azirar S, et al (2023) Neuroprotective Effects of Melatonin Against Neurotoxicity Induced by Intrahippocampal Injection of Aluminum in Male Wistar Rats: Possible Involvement of Oxidative Stress Pathway. Advances in Animal and Veterinary Sciences 11:711–719. https://doi.org/10.17582/journal.aavs/2023/11.5.711.719
Zghari O, Azirar S, Lamtai M, et al (2023) Intrahippocampal dose-dependent effects of aluminum injection on affective and cognitive response in male Wistar rat: potential role of oxidative stress. Egyptian Journal of Basic and Applied Sciences 10:460–475. https://doi.org/10.1080/2314808X.2023.2229623
El Brouzi MY, Lamtai M, Zghari O, et al (2020) Intrahippocampal Effects of Nickel Injection on the Affective and Cognitive Response in Wistar Rat: Potential Role of Oxidative Stress. Biological Trace Element Research. https://doi.org/10.1007/s12011-020-02457-5
Naila N, Makthar W, Lamtai M, et al (2021) Effect of intra-hippocampal lead injection on affective and cognitive disorders in male WISTAR rats: Possible involvement of oxidative stress. E3S Web Conf 319:2017. https://doi.org/10.1051/e3sconf/202131902017
Salim S (2017) Oxidative stress and the central nervous system. Journal of Pharmacology and Experimental Therapeutics 360:201–205. https://doi.org/10.1124/jpet.116.237503
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Frontiers in Neurology 10:. https://doi.org/10.3389/fneur.2019.00399
Shaik A, Shenoy S, Anupama V, et al (2019) Antidepressants modulate behavioral, biochemical, and histological alterations induced by chronic aluminum chloride administration in wistar rats. Journal of Pharmacology and Pharmacotherapeutics 10:16–21. https://doi.org/10.4103/jpp.JPP_135_18
Al-Amin MM, Chowdury MIA, Saifullah ARM, et al (2019) Levocarnitine Improves AlCl3-Induced Spatial Working Memory Impairment in Swiss albino Mice. Frontiers in Neuroscience 13:1–11. https://doi.org/10.3389/fnins.2019.00278
Jangra A, Lukhi MM, Sulakhiya K, et al (2014) Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. European Journal of Pharmacology 740:337–345. https://doi.org/10.1016/j.ejphar.2014.07.031
Sulakhiya K, Kumar P, Jangra A, et al (2014) Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. European Journal of Pharmacology 744:124–131. https://doi.org/10.1016/j.ejphar.2014.09.049
Benyettou I, Kharoubi O, Hallal N, et al (2017) Aluminium-induced behavioral changes and oxidative stress in developing rat brain and the possible ameliorating role of omega-6/omega-3 ratio. Journal of Biological Sciences 17:106–117. https://doi.org/10.3923/jbs.2017.106.117
Albendea CD, Gómez-Trullén EM, Fuentes-Broto L, et al (2007) Melatonin reduces lipid and protein oxidative damage in synaptosomes due to aluminium. Journal of Trace Elements in Medicine and Biology 21:261–268. https://doi.org/10.1016/j.jtemb.2007.04.002
El-Missiry MA, Shalaby F (2000) Role of β-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. Journal of Biochemical and Molecular Toxicology 14:238–243. https://doi.org/10.1002/1099-0461(2000)14:5<238::AID-JBT2>3.0.CO;2-X
Gonzalez MA, Alvarez MDL, Pisani GB, et al (2007) Involvement of oxidative stress in the impairment in biliary secretory function induced by intraperitoneal administration of aluminum to rats. Biological Trace Element Research 116:329–348. https://doi.org/10.1007/BF02698017
Al-Amin MM, Reza HM, Saadi HM, et al (2016) Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. European Journal of Pharmacology 777:60–69. https://doi.org/10.1016/j.ejphar.2016.02.062
Kumar V, Dip K (2014) NeuroToxicology Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration : A review. 41:154–166
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283:1482–1488
Xu SC, He M Di, Zhong M, et al (2010) Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. Journal of Pineal Research 49:86–94. https://doi.org/10.1111/j.1600-079X.2010.00770.x
Zaky A, Mohammad B, Moftah M, et al (2013) Apurinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation. BMC Neuroscience 14:1–12. https://doi.org/10.1186/1471-2202-14-26
Al-olayan EM, El-khadragy MF, Moneim AEA (2015) The protective properties of melatonin against aluminium-induced neuronal injury. 196–202. https://doi.org/10.1111/iep.12122
Moncada S, Bolaños JP (2006) Nitric oxide, cell bioenergetics and neurodegeneration. Journal of Neurochemistry 97:1676–1689. https://doi.org/10.1111/j.1471-4159.2006.03988.x
El Mrabet F., Ouakki S, Mesfioui A, et al (2012) Pinealectomy and Exogenous Melatonin Regulate Anxiety-Like and Depressive-Like Behaviors in Male and Female Wistar Rats. Neuroscience and Medicine 03:394–403. https://doi.org/10.4236/nm.2012.34049
Ouakki S, El Mrabet FZ, Lagbouri I, et al (2013) Melatonin and Diazepam Affect Anxiety-Like and Depression-Like Behavior in Wistar Rats : Possible Interaction with Central GABA Neurotransmission. Journal of Behavioral and Brain Science 3:522–533
Corrales A, Martínez P, García S, et al (2013) Long-term oral administration of melatonin improves spatial learning and memory and protects against cholinergic degeneration in middle-aged Ts65Dn mice, a model of Down syndrome. Journal of Pineal Research 54:346–358. https://doi.org/10.1111/jpi.12037
García T, Ribes D, Colomina MT, et al (2009) Evaluation of the protective role of melatonin on the behavioral effects of aluminum in a mouse model of Alzheimer’s disease. Toxicology 265:49–55. https://doi.org/10.1016/j.tox.2009.09.009
Olcese JM, Cao C, Mori T, et al (2009) Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. Journal of Pineal Research 47:82–96. https://doi.org/10.1111/j.1600-079X.2009.00692.x
McKenna JT, Christie MA, Jeffrey BA, et al (2012) Chronic ramelteon treatment in a mouse model of Alzheimer’s disease. Archives italiennes de biologie 150:5–14. https://doi.org/10.4449/aib.v149i5.1375
Silva AF, Socorro M, Aguiar S, et al (2013) Hippocampal neuronal loss , decreased GFAP immunoreactivity and cognitive impairment following experimental intoxication of rats with aluminum citrate. 1491:23–33. https://doi.org/10.1016/j.brainres.2012.10.063
Garcia-Santos G, Martin V, Rodriguez-Blanco J, et al (2012) Fas/Fas ligand regulation mediates cell death in human Ewing’s sarcoma cells treated with melatonin. British journal of cancer 106:1288–1296. https://doi.org/10.1038/bjc.2012.66
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Progress in Neuro-Psychopharmacology and Biological Psychiatry 35:676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
Rahman MF, Wang J, Patterson TA, et al (2009) Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicology Letters 187:15–21. https://doi.org/10.1016/j.toxlet.2009.01.020
Lima FD, Souza MA, Furian AF, et al (2008) Na+,K+-ATPase activity impairment after experimental traumatic brain injury: Relationship to spatial learning deficits and oxidative stress. Behavioural Brain Research 193:306–310. https://doi.org/10.1016/j.bbr.2008.05.013
Kucukatay V, Aǧar A, Gumuslu S, Yargiçoǧlu P (2007) Effect of sulfur dioxide on active and passive avoidance in experimental diabetes mellitus: Relation to oxidant stress and antioxidant enzymes. International Journal of Neuroscience 117:1091–1107. https://doi.org/10.1080/00207450600934531
Allagui MS, Feriani A, Saoudi M, et al (2014) Effects of melatonin on aluminium-induced neurobehavioral and neurochemical changes in aging rats. Food and Chemical Toxicology 70:84–93. https://doi.org/10.1016/j.fct.2014.03.043
Reiter RJ, Tan DX, Rosales-Corral S, et al (2018) Mitochondria: Central organelles for melatonins antioxidant and anti-Aging actions. Molecules 23
Tan DX, Manchester LC, Terron MP, et al (2007) One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of Pineal Research 42:28–42. https://doi.org/10.1111/j.1600-079X.2006.00407.x
Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Annals of the New York Academy of Sciences 939:200–15. https://doi.org/10.1111/j.1749-6632.2001.tb03627.x
Koh PO (2008) Melatonin regulates nitric oxide synthase expression in ischemic brain injury. Journal of Veterinary Medical Science 70:747–750. https://doi.org/10.1292/jvms.70.747
Ucar M, Korkmaz A, Reiter RJ, et al (2007) Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicology Letters 173:124–131. https://doi.org/10.1016/j.toxlet.2007.07.005
Rodriguez C, Mayo JC, Sainz RM, et al (2004) Regulation of antioxidant enzymes: A significant role for melatonin. Journal of Pineal Research 36:1–9. https://doi.org/10.1046/j.1600-079X.2003.00092.x
Reiter R, Tan D-X, Rosales-Corral S, C. Manchester L (2013) The Universal Nature, Unequal Distribution and Antioxidant Functions of Melatonin and Its Derivatives. Mini-Reviews in Medicinal Chemistry 13:373–384. https://doi.org/10.2174/1389557511313030006
Acknowledgements
The authors would like to express their heartfelt and deepest condolences to family and friends of Prof. Ali OUICHOU. The neuroscience family in Morocco will miss one of its significant members, and the University Ibn Tofail has lost a great academic professor, who has contributed to the growth of the neuroscience community in Morocco. May his soul rest in peace.
Author information
Authors and Affiliations
Contributions
Oussama Zghari and Sofia Azirar performed the experiments, analyzed the data and wrote the paper. Mouloud Lamtai participated in behavioral analysis and statistical significance. Aboubaker El Hessni reviewed and provided comments on the content and interpretation of the manuscript. Ali Ouichou and Abdelhalem Mesfioui supervised the work, revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interests with authors or other organizations.
Ethics approval
The experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee (Local Institutional Research Committee).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zghari, O., Azirar, S., Lamtai, M. et al. Melatonin counteracts aluminum-induced affective and cognitive disorders and oxidative damage in male wistar rats. Neurosci Behav Physi 53, 917–928 (2023). https://doi.org/10.1007/s11055-023-01465-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01465-x