Abstract
Knowledge about the clinical characteristics and prognostic factors of Talaromyces marneffei infection in children is limited, especially in HIV-positive children. We performed a retrospective study of all HIV-positive pediatric inpatients with T. marneffei infection in a tertiary hospital in Southern China between 2014 and 2019 and analyzed the related risk factors of poor prognosis using logistic regression. Overall, 28 cases were enrolled and the prevalence of talaromycosis in AIDS children was 15.3% (28/183). The median age of the onset was 8 years (range: 1–14 years). The typical manifestation of skin lesion with central umbilication was not common (21.4%). All the children had very low CD4+ cell counts (median 13.5 cells/μL, range: 3–137 cells/μL) on admission. 92.9% children were misdiagnosed and talaromycosis was only noted after positivity for HIV infection. 89.3% diagnoses of T. marneffei infections were based on positive blood cultures, with a long culture time (median 7 days, range from 3–14 days). The sensitivity of fungus 1,3-β-D-glucan assay was 63.2%. Amphotericin B was superior to itraconazole in the induction antifungal therapy of talaromycosis in HIV-positive children. A six-month follow-up revealed a 28.6% mortality. Lower ratio of CD4+/CD8+ and amphotericin B treatment not over 7 days predicted poor prognosis. Our retrospective study provided an overview and update on the current knowledge of talaromycosis in HIV-positive children. Pediatricians in endemic areas should be aware of mycoses to prevent misdiagnosis. 1,3-β-D-glucan assay did not show optimal sensitivity. Amphotericin B treatment over 7 days can improve poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Talaromyces (formerly Penicillium) marneffei is a dimorphic fungi endemic in Southeast Asia and Southern China [1, 2], which can cause life-threatening disseminated infections mainly in immunocompromised populations, such as those with HIV, organ transplantation, autoimmune diseases and hematological malignancies [3,4,5].With the increasing overseas travel, global warming and the growing use of chemotherapy and immunosuppression, talaromycosis presents a worldwide clinical challenge by disseminating to non-endemic areas [6, 7]. In 1988, talaromycosis was found to be present in HIV-positive patients [8,9,10] and became an pressing complication among those with HIV in Southeast Asia [11].
Common clinical manifestations of patients coinfected with T. marneffei and HIV were fever, lymphadenopathy, respiratory symptoms, weight loss, skin lesions and gastrointestinal complications [7]. However, since most of the clinical manifestations were nonspecific, and overlapping those HIV-positive patients or other opportunistic infections (OIs), talaromycosis has always been misdiagnosed, which potentially contributed to a high mortality rate due to the lack or delay of antifungal treatment [12]. Presumptive diagnosis of talaromycosis can be made based on microscopic findings of intramacrophage and extramacrophage yeast organisms in smears of clinical specimens. Definitive diagnosis is made by a positive culture of T. marneffei. Amphotericin B and itraconazole have been proved effectively for the treatment of disseminated T. marneffei infection [2].
Recently, endemic-systemic mycoses in children have attracted a significant attention, including talaromycosis. The children with immunodeficient disease are more susceptible to certain endemic mycoses [13]. However, studies exploring talaromycosis in children with HIV-positive have been scarce in the last decade [14,15,16], which has led to a limited knowledge about the clinical epidemiology and diagnostic and treatment strategies. Subsequently, there is a concern regarding the underestimation of this complication and poor clinical management of HIV-positive children with talaromycosis.
Guangxi province is in the south of China, with a relatively high burden of HIV/AIDS infection [17]. The cumulative number of HIV/AIDS cases in Guangxi was ranked third in China [18, 19]. It was reported that 42.8% of talaromycosis in China came from Guangxi [20]. Up to 16.1% of HIV-infected hospitalized patients were coinfected with T. marneffei in Guangxi [15]. As such the scope of the current study was to determine the epidemiological, clinical and laboratory findings, imaging results, misdiagnosis rates, treatments and prognostic factors of talaromycosis among HIV-positive children to enrich our knowledge which can potentially advances our awareness of this disease.
Materials and Methods
Study Design and Population
A retrospective study was conducted at the Fourth People’s Hospital of Nanning, the largest infectious disease hospital as well as HIV/AIDS Clinical Treatment Center in Guangxi. There were more than 2500 HIV/AIDS inpatients admitted to the hospital each year [21]. We evaluated all hospitalized children infected with T. marneffei from January 2014 to December 2019. The inclusion criteria included: (1) HIV infection, (2) talaromycosis, and (3) children.
Diagnosis of Talaromycosis
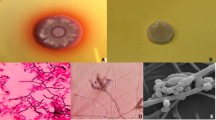
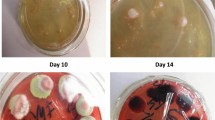
The diagnosis of talaromycosis included a positive culture of T. marneffei from blood, bone marrow, and other clinical specimens on sabouraud dextrose agar according to standard culture techniques [22]. Identification was based upon the morphology of the colonies. T. marneffei grew as a mold form at 25 °C and a yeast form at 37 °C . At 25 °C, it produced a soluble red pigment that diffused into the agar. Under the microscope, a typical broomstick with septal hyphae can be seen [7].
Definitions
HIV infection was determined by positive ELISA and western blot assays [21]. Tuberculosis was defined as having any tuberculosis symptom or tuberculosis with culture-positive sputum [7]. The diagnosis of pneumonia included bacterial pneumonia, mycoplasma pneumonia, pneumocystis pneumonia and pneumonia caused by other factors, except for tuberculosis pneumonia, which was classified as tuberculosis. Severe anemia were defined as hemoglobin < 60 g/L. Fungus 1,3-β-D-glucan (BDG) assay (Dynamiker Biotechnology (Tianjin) Co., Ltd, China) was used for the evaluation of invasive fungal infection. Values < 70 pg/mL of BDG were considered negative, while values 70–94 pg/mL and > 94 pg/mL were classified as intermediate and positive, respectively [23].
Data Collection
The data were obtained from electronic medical records according to a standardized form, including epidemiology, personal history, laboratory and imaging data, treatment, clinical manifestations, diagnoses and misdiagnoses, and outcome scored as died or survived [24]. In order to comprehensively evaluating the survival status, all patients were followed up six months after therapy. For those with multiple admissions, data from the first admission were collected.
Review of the Literature
A literature search was performed in PubMed on July 7th, 2021 to further analyze the characteristics of HIV-associated talaromycosis in children. We screened using the key words ‘‘Talaromyces marneffei’’ or ‘‘Penicillium marneffei’’ or ‘‘Penicilliosis’’ or ‘‘Talaromycosis’’. Cases including T. marneffei-infected children with HIV-positive were included. Infant cases were excluded.
Statistical Analysis
SPSS software (version 22.0, SPSS Inc., Chicago, IL, U.S.A.) was used for statistical analysis. The results were presented as number, frequency (%) or median (interquartile ranges). Student’s t-test, χ2 test and Mann–Whitney U-test were applied for continuous variables conforming to a normal distribution, categorical variables and non-normally distributed data, respectively. Univariable and multivariable logistic regression analysis with forward stepwise selection were used to predict the risk factors of poor prognosis. Variables with a P-value < 0.10 from the univariable analysis were tested in multivariable models. A P-value of < 0.05 was applied for indicating statistical significance.
Results
Demographic and Epidemiological Characteristics
A total of 183 HIV/AID children were admitted to the Fourth People's Hospital of Nanning from January 2014 to December 2019. Among them, 28 children (15.3%) were diagnosed with T. marneffei infection, including 15 girls and 13 boys (Table 1). The proportion of HIV-infected talaromycosis children among total HIV-infected talaromycosis cases was 1.7%. The median age of talaromycosis children was 8 years (range: 1–14 years). None of them had a clear history of bamboo rat exposure. Twenty-two children had either one or both parents with AIDS. Five children had been diagnosed with HIV-positive before the onset of symptoms and three of them received antiretroviral therapy (ART).
Clinical, Laboratory and Radiographic Characteristics
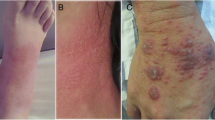
Clinical, laboratory and radiographic characteristics of HIV-positive children coinfected with T. marneffei are presented in Table 2. Recurrent fever was the most common clinical manifestation (96.4%, average 39.6 °C), followed by cough (89.3%), hepatosplenomegaly (64.3%), and abdominal pain or diarrhea (64.3%). 21.4% patients presented the manifestation of papular skin lesion with central umbilication. Only two patients had underlying diseases, including glucose-6-phosphate dehydrogenase deficiency (n = 1) and thalassemia (n = 1). All patients had complications and pneumonia (92.9%) was the major presentation.
All the T. marneffei infection in the ch ildren occurred late in the course of HIV infection with very low CD4+ lymphocyte counts (median 13.5 cells/μL, range: 3–137 cells/μL) and ratio of CD4+/CD8+ lymphocyte (median 0.05, range: 0.01–0.11). However, only 64.3% cases presented decreasing lymphocytes counts and 50.0% cases presented decreasing leukocytes on admission. Other main laboratory abnormities included increased serum C-reactive protein levels (26/28, 92.9%), decreased hemoglobin levels (24/28, 85.7%), increased aspartate aminotransferase level (23/28, 82.1%), and thrombocytopenia (15/28, 53.6%).
Lungs and pleura were the major cumulative sites of talaromycosis coinfected with HIV, and only two children showed no obvious abnormality on plain CT scan of the chest. There was a high misdiagnosis rate of talaromycosis and 26 cases (92.9%) were misdiagnosed as pneumonia or bronchial pneumonia. Talaromycosis was only noted after positivity for HIV infection.
Etiological Diagnosis of Talaromycosis
Among the five specimen types used to diagnose T. marneffei, blood was the most common specimens (n = 28), followed by sputum (n = 10), pharyngeal swab (n = 14), feces (n = 9) and bone marrow (n = 4). Most patients (25/28, 89.3%) were confirmed of T. marneffei infection by blood culture, and three by sputum, bone marrow or pharyngeal swab culture. Six patients were confirmed by two and more kinds of specimens (Table 3). Fungi culture was relatively time-consuming. The median positive culture time of T. marneffei was 7 days (range: 3–14 days). In addition to fungal culture, fungus BDG assay was conducted for the early diagnosis of invasive fungal infection. There was a sensitivity of 63.2% (12/19) in our study.
Treatment and Outcomes
Most patients (27/28, 96.4%) were treated with antifungal agents, including fluconazole for injection (Beijing Sihuan Kebao Pharmaceutical Co., Ltd., China) before confirmed talaromycosis, amphotericin B for injection (Huabei Pharmaceutical Co., Ltd., China) and itraconazole capsules (Chengdu Brilliant Pharmaceutical Co., Ltd., China). The details are available in Table 4. Fluconazole was applied on eleven patients and switched to amphotericin B (n = 7) or itraconazole (n = 3) when T. marneffei infection was suspected or confirmed. One patient died before the switch. The patients infected with T. marneffei received induction antifungal therapy with amphotericin B or itraconazole, followed by consolidation and maintenance therapy with itraconazole [21]. Of the 17 patients who were treated with amphotericin B, three (17.6%) died during hospitalization and the rest survived during 6-month follow-up. Nine patients received induction therapy with itraconazole, of whom two died at home during the followed-up and one died on readmission. Only one patient didn’t receive antifungal agents and was discharged for a deteriorative condition 24 h after admission. Adverse drug reactions (ADRs) of amphotericin B were present, including fever (n = 1) and hypokalemia (n = 1). The ADRs were relieved with symptomatic treatment after drug withdrawal. Other treatments included antibacterial therapy, anti-tuberculosis, ART, blood transfusions, anti-herpesvirus, and cotrimoxazole. ART was initiated when the condition of the patient was stabilized. Six patients didn’t receive ART for the deteriorated condition. Twenty-two patients received ART and the median initiation time was 23 days after antifungal therapy (Table 4). The overall mortality rate was 28.6% (8/28), among whom five patients died for septic shock or circulatory failure during hospitalization. The other three patients discharged against medical advice for worsening conditions were found dead in follow-up. The mortality rate in those children was much higher than that in HIV-infected talaromycosis adults (15.3%, 227/1481). The median length of stay was 24 days (range: 1–73).
Prognostic Factors
Prognostic factors were investigated for the clinicians to better predict the outcome of HIV-positive children with T. marneffei infection. The patients were divided into two groups according to outcomes as the died and survived groups. Twenty-six factors related to clinical manifestations, laboratory tests, treatment were involved (Supplementary table). According to compare the two group, we found that the ratio of CD4+/CD8+ rather than CD4+ cell counts showed statistically significant differences (P = 0.039). In addition, whether the patient received ART after antifungal therapy and was treated with amphotericin B for over 7 days also contributed to statistically significant differences.
Both the univariate and multivariate logistic regression analysis showed that the lower ratio of CD4+/CD8+ and amphotericin B treatment not over 7 days were risk factors for poor outcome (95% confidence interval [CI], P < 0.05).
Systematic Review of HIV-associated Talaromycosis in Children
For a better understanding of the characteristics of HIV-positive children coinfected with T. marneffei, a literature review was conducted in PubMed (Table 5). We found nine related articles, and one of which was abandoned for without the full text. As a result, a total of 11 cases with HIV-associated talaromycosis in children were analyzed (Table 5). Most of the cases were reported 10 years ago (9/11, 81.8%). The age of the patients ranged from 1 to 14 years. 63.6% (7/11) of the cases were from Thailand, and none was from China. Only three patients were treated with ART for a short period of time before confirmed T. marneffei infection. The common clinical manifestations included fever (10/11, 90.9%), skin lesion (9/11, 81.8%), lymphadenopathy (9/11, 81.8%), abdominal pain or diarrhea (6/11, 54.5%) and hepatosplenomegaly (6/11, 54.5%). CD4+ cell counts were available in five cases, all of which were less than 200 cells/mm3. All the patients were diagnosed by positive culture of T. marneffei and blood was the most common culture specimen (n = 8), followed by skin scraping (n = 6). Two patients did not receive antifungal treatment and died. Two patients died despite antifungal treatment.
Discussion
Despite seemingly causing a higher mortality rate among children, the talaromycosis has not been studied well among HIV-positive children [33] and the recent studies were almost conducted on HIV-negative children [15, 33,34,35]. By systemically reviewing the literatures, we found only two cases available in the last decade and none was from China. Herein, we conducted a retrospective study on HIV-positive children coinfected with T. marneffei in Southern China. To our knowledge, it is the largest number of cases in the existing literature. Our study provided a broader knowledge for the awareness of talaromycosis in children.
We found a high prevalence (15.3%) of talaromycosis among HIV admissions in children, which was in line with that in adults [7]. However, the proportion (1.7%) of HIV-infected talaromycosis children among total HIV-infected talaromycosis cases was much lower than that (7.4%) of non-HIV-infected talaromycosis children among talaromycosis patients [15]. It indicates that the status of HIV-positive children in the field of children T. marneffei infection changes.
Although bamboo rats have been considered as nature reservoirs of T. marneffei [2], none of the patients had a history of contact with bamboo rats in our study. The infection route is still mysterious.
The common clinical manifestations of children HIV-associated talaromycosis included fever, cough, abdominal pain or diarrhea, oral mucosal injury, and hepatomegaly. They were nonspecific and could be shown in adult patients with HIV-positive or related OIs [7]. However, there were different laboratory findings between HIV-positive and HIV-negative talaromycosis children. On the one hand, HIV-positive talaromycosis children showed significant elevated levels of ALT and AST together with lymphocytopenia and leukopenia [15]. On the other hand, abnormal immunoglobulin findings (mainly decreased IgG or increased IgE) were considerable in HIV-negative talaromycosis children.
The occurrence of talaromycosis in HIV-positive children in Guangxi seemed to be associated with HIV untreated or treatment failure. ART can markedly increase CD4 counts in HIV-infected children and reduce AIDS-related OIs [36]. In our study, only three children received ART before the onset and they failed to response. All the children had very low CD4+ cell counts (median 13.5 cells/μL, range: 3–137 cells/μL) on admission, which was probably the most important factor for T. marneffei infection in HIV-positive children. The same findings were present according to the literature review in our study (Table 5). In addition, ratio of CD4+/CD8+ plays an important role in the prognosis of HIV-associated children talaromycosis. A low ratio of CD4+/CD8+ provides a poor prognosis, which helps physicians to make a correct judgment of the patient’s condition.
Rapid diagnostics of T. marneffei is still lacking. All the T. marneffei infections were confirmed according to a positive culture in the present study. Fungal culture is featured with high accuracy of diagnosis and wide applicability of various specimens. Blood was the most common specimen with high positive rates (89.3%) in our study. However, fungal culture is relatively time-consuming [37]. The longest culture time was 14 days, which was not conducive to improve treatment. BDG assay was used for the early diagnosis of invasive fungal infection. However, it was nonspecific and cannot determine the species recognition. The sensitivity of BDG assay was also not high, only 63.2% in our study. Therefore, a rapid diagnostic method with high sensitivity and specificity for T. marneffei is demanded.
Amphotericin B and itraconazole are effective drugs in the treatment of T. marneffei infection, both with low minimal inhibition concentrations [38]. However, efficacy evaluation of the two drugs in HIV-positive children with talaromycosis was few. Amphotericin B was superior to itraconazole in the induction antifungal therapy of HIV-associated talaromycosis in adults with respect to 6-month mortality [39]. In line with the results in adults, amphotericin B showed a lower mortality rate than itraconazole in children at 6-month follow-up in our study. In addition, the ADRs of amphotericin B can be relieved. Therefore, amphotericin B is more recommended than itraconazole for the induction antifungal treatment of talaromycosis in HIV-positive children. Notably, considering prognostic factors, the treatment course of amphotericin B should be over 7 days.
There was a relatively high mortality (28.6%) among talaromycosis in HIV-positive children. Although it was lower than the mortality of talaromycosis in HIV-negative children [34], but higher than that in HIV-positive adults [7]. In addition to antifungal agents, the high mortality was associated with the high misdiagnosis rate of talaromycosis [12]. Approximately 93% of our cases were misdiagnosed as pneumonia or bronchial pneumonia in our study. There was a similar result in HIV-negative children infected with T. marneffei [15]. Misdiagnosis was mainly concerned with the following reasons. Firstly, pediatricians were not well aware of mycosis. Talaromycosis was only noted after positivity for HIV infection. Secondly, the common clinical manifestations of talaromycosis were nonspecific, including fever, cough, hepatosplenomegaly, and abdominal pain or diarrhea. They could be shown in patients with HIV-positive or other OIs [7]. Finally, the most typical skin lesion manifestation of talaromycosis was not common in our cases (21.4%). The percentage was much lower than that in perinatally HIV-infected children (67%) [40], in HIV-positive adults (44.5%) [7], and in the literature review of our study (81.8%), which made diagnosis more difficult. The reason for the discrepancy is unclear. Therefore, in endemic areas, pediatricians should be alerted to the possibility of talaromycosis to avoid misdiagnosis.
For the current study, the main limitation was owing to its retrospective design in a single center. Nevertheless, this study may provide an overview and update on the current knowledge of HIV-associated talaromycosis in children.
In conclusion, our findings confirmed that there was a high prevalence and mortality among talaromycosis in HIV-positive children in Guangxi. The occurrence of talaromycosis was associated with HIV untreated or treatment failure. 1,3-β-D-glucan assay did not show optimal sensitivity. In order to prevent misdiagnosis, rapid diagnostic methods of T. marneffei infection still need to be explored and pediatricians in endemic regions should be alerted to the possibility of mycoses. Ratio of CD4+/CD8+ was useful to help physicians to make a correct judgment of the patient’s condition. Induction therapy with amphotericin B over 7 days provide a good prognosis of children HIV-associated talaromycosis.
References
Supparatpinyo K, Khamwan C, Baosoung V, et al. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994. https://doi.org/10.1016/s0140-6736(94)91287-4.
Limper AH, Adenis A, Le T, et al. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017. https://doi.org/10.1016/S1473-3099(17)30303-1.
Vergidis P, Rao A, Moore CB, et al. Talaromycosis in a renal transplant recipient returning from South China. Transpl Infect Dis. 2021. https://doi.org/10.1111/tid.13447.
Beena H, Gupta M, Kindo AJ. Pulmonary infection with Penicillium citrinum in a patient with multiple myeloma. Indian J Med Microbiol. 2021. https://doi.org/10.1016/j.ijmmb.2021.03.001.
Vanittanakom N, Cooper CR Jr, Fisher MC, et al. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006. https://doi.org/10.1128/CMR.19.1.95-110.2006.
Nenoff P, Reinel D, Krüger C, et al. Tropical and travel-related dermatomycoses: part 2: cutaneous infections due to yeasts, moulds, and dimorphic fungi. Hautarzt. 2015;66(70):522–32.
Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med. 2020. https://doi.org/10.1111/hiv.13024.
Peto TE, Bull R, Millard PR, et al. Systemic mycosis due to Penicillium marneffei in a patient with antibody to human immunodeficiency virus. J Infect. 1988. https://doi.org/10.1016/s0163-4453(88)97700-6.
Ancelle T, Dupouy-Camet J, Pujol F, et al. A case of disseminated Penicillium marneffei penicilliosis in a patient with acquired immunodeficiency syndrome. Presse Med. 1988;17(21):1095–6.
Piehl MR, Kaplan RL, Haber MH. Disseminated penicilliosis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1988;112(12):1262–4.
Li PC, Tsui MC, Ma KF. Penicillium marneffei: indicator disease for AIDS in South East Asia. AIDS. 1992;6(2):240–1.
Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Med Mal Infect. 2014;44(11–12):495–501.
Yeoh DK, Butters C, Curtis N. Endemic Mycoses in Children. Pediatr Infect Dis J. 2019. https://doi.org/10.1097/INF.0000000000002324.
Zeng W, Qiu Y, Lu D, et al. A retrospective analysis of 7 human immunodeficiency virus-negative infants infected by Penicillium marneffei. Medicine (Baltimore). 2015. https://doi.org/10.1097/MD.0000000000001439.
Guo J, Li BK, Li TM, et al. Characteristics and Prognosis of Talaromyces marneffei Infection in Non-HIV-Infected Children in Southern China. Mycopathologia. 2019. https://doi.org/10.1007/s11046-019-00373-4.
Pan M, Qiu Y, Zeng W, et al. Disseminated Talaromyces marneffei infection presenting as multiple intestinal perforations and diffuse hepatic granulomatous inflammation in an infant with STAT3 mutation: a case report. BMC Infect Dis. 2020. https://doi.org/10.1186/s12879-020-05113-4.
Cui Z, Lin D, Chongsuvivatwong V, et al. Spatiotemporal patterns and ecological factors of tuberculosis notification: a spatial panel data analysis in Guangxi China. PLoS One. 2019. https://doi.org/10.1371/journal.pone.0212051.
Sun X, Yang W, Tang S, et al. Declining trend in HIV new infections in Guangxi, China: insights from linking reported HIV/AIDS cases with CD4-at-diagnosis data. BMC Public Health. 2020. https://doi.org/10.1186/s12889-020-09021-9.
Zang X, Tang H, Min JE, et al. Cost-Effectiveness of the “One4All” HIV Linkage Intervention in Guangxi Zhuang Autonomous Region. China PLoS One. 2016;11(11):e0167308.
Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013. https://doi.org/10.1007/s11046-012-9577-0.
Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019. https://doi.org/10.1016/j.cmi.2018.04.018.
Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia. 2019. https://doi.org/10.1007/s11046-019-00410-2.
White PL, Price JS, Posso RB, et al. An evaluation of the performance of the Dynamiker(R) Fungus (1–3)-beta-D-Glucan Assay to assist in the diagnosis of invasive aspergillosis, invasive candidiasis and Pneumocystis pneumonia. Med Mycol. 2017;55(8):843–50.
Larsson M, Nguyen LH, Wertheim HF, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther. 2012. https://doi.org/10.1186/1742-6405-9-24.
Sirisanthana V, Sirisanthana T. Penicillium marneffei infection in children infected with human immunodeficiency virus. Pediatr Infect Dis J. 1993;12(12):1021–5.
Chokephaibulkit K, Veerakul G, Vanprapar N, et al. Penicilliosis-associated hemophagocytic syndrome in a human immunodeficiency virus-infected child: the first case report in children. J Med Assoc Thai. 2001;84(3):426–9.
Chaiwun B, Khunamornpong S, Sirivanichai C, et al. Lymphadenopathy due to Penicillium marneffei infection: diagnosis by fine needle aspiration cytology. Mod Pathol. 2020. https://doi.org/10.1097/01.MP.0000027203.44333.95.
Othman N, Yip CW, Intan HI, et al. An abdominal mass owing to Penicillium marneffei in an HIV-infected 7-year-old boy: case report. Ann Trop Paediatr. 2006. https://doi.org/10.1179/146532806X120381.
Sharma A, Hazarika NK, Barua P, et al. Penicillium marneffei infection in a HIV infected child. Indian J Med Res. 2007;126(6):580–2.
Saikia L, Nath R, Biswanath P, et al. Penicillium marneffei infection in HIV infected patients in Nagaland & immune reconstitution after treatment. Indian J Med Res. 2009;129(3):333–4.
Sudjaritruk T, Sirisanthana T, Sirisanthana V. Immune reconstitution inflammatory syndrome from Penicillium marneffei in an HIV-infected child: a case report and review of literature. BMC Infect Dis. 2012. https://doi.org/10.1186/1471-2334-12-28.
Sethuraman N, Thirunarayan MA, Gopalakrishnan R, et al. Talaromyces marneffei outside endemic areas in India: an emerging infection with atypical clinical presentations and review of published reports from India. Mycopathologia. 2020. https://doi.org/10.1007/s11046-019-00420-0.
Lee PP, Chan KW, Lee TL, et al. Penicilliosis in children without HIV infection–are they immunodeficient? Clin Infect Dis. 2012. https://doi.org/10.1093/cid/cir754.
Zeng Q, Jin Y, Yin G, et al. Peripheral immune profile of children with Talaromyces marneffei infections: a retrospective analysis of 21 cases. BMC Infect Dis. 2021;21(1):287.
Fan JH, Luo HY, Yang LG, et al. Penicilliosis marneffei in HIV negative children three case reports. Ann Palliat Med. 2021. https://doi.org/10.21037/apm-20-2056.
Zhang LX, Jiao YM, Zhang C, et al. HIV reservoir decay and CD4 recovery associated with high CD8 counts in immune restored patients on long-term ART. Front Immunol. 2020;11:1541.
Ning C, Lai J, Wei W, et al. Accuracy of rapid diagnosis of Talaromyces marneffei: a systematic review and meta-analysis. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0195569.
Lei HL, Li LH, Chen WS, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong China. Eur J Clin Microbiol Infect Dis. 2018. https://doi.org/10.1007/s10096-018-3222-x.
Le T, Kinh NV, Cuc NTK, et al. A trial of Itraconazole or Amphotericin B for HIV-associated Talaromycosis. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1613306.
Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995. https://doi.org/10.1097/00006454-199511000-00003.
Acknowledgements
This work was supported by the Shanghai Sailing Program (19YF1448000), National Natural Science Foundation of China (31770161 and 82072257), Shanghai Science and Technology Committee (20DZ2272900), Key R&D projects in Nanning (20193008-1) and Chinese Academy of Engineering (2019-XY-33, 19-HN-XZ-06, 2021-XY-40).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics Statement
The study was approved by the Institutional Review Board of the Fourth People's Hospital of Nanning, and the need for informed consent was waived due to the retrospective nature of the study.
Additional information
Handling Editor: Cunwei Cao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xue, X., Zou, J., Fang, W. et al. Characteristics and Prognosis of Talaromyces marneffei Infection in HIV-positive Children in Southern China. Mycopathologia 187, 169–180 (2022). https://doi.org/10.1007/s11046-021-00614-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00614-5