Abstract
Talaromycosis is a disseminated disease caused by Talaromyces (Penicillium) marneffei, mainly seen in acquired immunodeficiency syndrome (AIDS) patients. Its distribution is restricted to southeast Asian countries; a small pocket of endemicity exists in the northeast Indian state of Manipur. Here, we present a series of five cases presenting to our tertiary care hospital, originating from non-endemic states neighboring Manipur. In addition to the geographical distinction, a variety of unique features were noted in our cases, including human immunodeficiency virus (HIV)-negative hosts, the absence of typical skin lesions, presentation as pneumonia and generalized lymphadenopathy. Our series highlights the importance of distinguishing this disease from histoplasmosis and tuberculosis, both endemic in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Talaromyces marneffei, previously known as Penicillium marneffei, is a dimorphic fungus which causes disseminated infection in immunosuppressed individuals and is considered as an AIDS defining illness. The fungus thrives in humid climates in close association with bamboo rats [1] and is geographically restricted to southeast Asia, especially Hong Kong, Thailand, Vietnam, Taiwan, China, Laos, Cambodia, Malaysia and Myanmar. In India, the major focus of endemicity is in Manipur, a state located in northeast India sharing a border with Myanmar, where over fifty cases have been reported to date (Fig. 1) [2, 3].
Disseminated disease in HIV-positive hosts often presents in a subacute fashion with multiple umbilicated skin lesions, fever, lymphadenopathy, hepatomegaly and splenomegaly. Disseminated talaromycosis has also been described in other groups of immunosuppressed individuals including renal transplant recipients from Hong Kong, Vietnam and Taiwan [4,5,6]. In Manipur, in the absence of classical features of this disease such as fever with skin lesions in setting of advanced AIDS, talaromycosis is generally not considered in the differential diagnosis. Outside of Manipur state, only eight cases have been reported from Assam which neighbors Manipur and none from Sikkim (Fig. 1) [7,8,9]. To the best of our knowledge, this disease has never been reported among transplant recipients in India.
Here, we report five cases: four that originated from Assam and one from Sikkim. One of our cases was a renal transplant recipient, and only two had skin lesions.
Case 1
A 56-year-old male, from Dibrugarh, Assam, underwent a live renal allograft transplant for end-stage renal failure secondary to long standing bilateral renal calculi. He received perioperative induction immunosuppression with anti-thymocyte globulin. Maintenance immunosuppression consisted of mycophenolate mofetil 360 mg twice daily, tacrolimus 3.5 mg twice daily and prednisolone 10 mg once daily.
Two years post-transplant, he complained of a cutaneous swelling over the left elbow joint. Examination of potassium hydroxide (KOH) mount of a skin biopsy from lesion revealed dematiaceous septate hyphae and culture yielded Alternaria alternata. The lesion was excised, and itraconazole therapy was given for 4 weeks. Six months later, he again presented with patchy consolidation of right middle and lower lobes. Bronchoalveolar lavage fluid was negative for acid-fast bacilli (AFB) or fungal elements. Preemptive amphotericin B was started based on galactomannan value of 0.82 and continued for 3 weeks during which the patient improved.
Six months later, he presented again with cough and shortness of breath. Computed tomography (CT) scan revealed bilateral multiple variable-sized lung nodules, two of them showing hypodensity. A CT-guided fine needle aspiration (FNA) was done, and the sample was sent for cytopathological and mycological examination. The smear showed collection of neutrophils, macrophages, lymphocytes and numerous intracellular and extracellular organisms measuring 3–5 µm, with several of them showing a transverse septation (Fig. 2a). Culture grew white mold and was identified as T. marneffei. Liposomal amphotericin B was started at a dose of 3 mg/kg/day for a week, followed by itraconazole 200 mg twice daily for 6 months. He remains asymptomatic on follow-up.
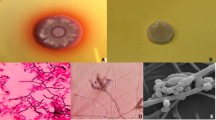
a Giemsa stain of FNAC lung nodule of case 1 showing characteristic yeast cells with transverse septations; 2b peripheral smear from case 2 showing neutrophil with intracellular yeast cells; 2c PAS stain of bone marrow biopsy from case 5 showing intracellular yeast cells, sausage forms and occasional elongated hyphal-like cells; 2d GMS stain of bone marrow biopsy from case 5 showing characteristic yeast cells with transverse septations; 2e SDA plate showing mycelial form of T. marneffei after 7 days of incubation at 25 °C. 2f LPCB mount from the colony showing typical biverticillate conidiophores of T. marneffei
Case 2
A 64-year-old HIV-positive male from Guwahati, Assam, presented with complaints of cough with expectoration for 6 months and fever and shortness of breath for 1 month. He also gave a history of decreased urine output, anorexia and > 10 kg weight loss. On physical examination, he was cachectic. White blood cell (WBC) count was 4460/mm3, platelet count was 60,000/mm3, and hemoglobin was 9.1 g %. Liver function test (LFT) revealed a cholestatic picture (total bilirubin—8.6 mg/dl, direct bilirubin—8.4 mg/dl, AST—128U/L, ALT—89U/L, ALP—429U/L, GGT—92U/L), and renal function was deranged (blood urea-115 mg/dL and serum creatinine-2.29 mg/dL). Chest X-ray showed bilateral reticulo-nodular pattern. CT chest showed bilateral ground glass opacities (Fig. 3a). Ultrasonogram of abdomen revealed ascites with splenomegaly. CD4 count was 11 cells/mm3 (8%), and HIV viral load was 42, 89,427 copies/ml. Peripheral blood smear showed neutrophils containing yeast cells (Fig. 2b). Blood culture grew T. marneffei. He was given a dose of amphotericin B deoxycholate 50 mg, but he opted for discharge against medical advice and could not be followed up.
a CT chest of case 2 showing bilateral ground glass opacities; b CT abdomen of case 3 transverse section showing multiple necrotic nodes; c sagittal section CT of case 3 showing the necrotic abdominal nodes; d chest X-ray of case 4 showing left-sided pleural effusion at presentation; e chest X-ray of case 4 showing partial resolution of lesions at 6-month follow-up
Case 3
A 37-year-old HIV-positive male from Guwahati, Assam, was admitted with vague non-colicky abdominal pain of 1-month duration and low-grade intermittent fever for 2 years with significant weight loss. He was on antiretroviral therapy (tenofovir, emtricitabine and efavirenz) for 1 year. He had been on anti-tuberculosis treatment from another clinic for 9 months for suspected tuberculous lymphadenitis with no clinical improvement. Clinical examination revealed a single, small umbilicated skin lesion over the forehead. Systemic examination was normal. Investigations showed hemoglobin of 11 g % and total WBC count of 3800/mm3. LFTs were mildly deranged (AST—126 U/L, ALT—98 U/L, GGT—270 U/L, ALP—209 U/L). CD4 count was 45 cells/mm3, and HIV viral load was 17,04,942 copies/ml. CT abdomen and chest revealed features of parenchymal liver disease with multiple necrotic intra-abdominal nodes (Fig. 3b, c) and miliary infiltrates in the lung, respectively. CT-guided needle biopsy of the abdominal node revealed necrosis, fibrosis and ill-defined granulomas with the presence of numerous yeasts on periodic acid Schiff (PAS) and Gomori’s methenamine silver (GMS) stains. AFB and fungal stains were negative. Tissue Xpert MTB/Rif test (Cepheid) was negative. Tissue fungal culture yielded growth of T. marneffei. He was started on itraconazole 200 mg twice daily and discharged but did not return for follow-up.
Case 4
A 60-year-old HIV-negative female from Gangtok, Sikkim, came with fever and left-sided pleuritic chest pain, associated with loss of appetite and loss of weight for the preceding 5 months. She had a history of diabetes of 2-year duration, a past history of multiple hospitalizations for recurrent pneumonias and a history of skin lesions that had been treated elsewhere 4 years ago with steroids for 4 months. Further questioning revealed that she was involved in gardening activities at her home. Blood investigations showed a fasting glucose of 124 mg/dl and serum creatinine level of 2.59 mg/dl. Chest X-ray revealed a left-sided pleural effusion (Fig. 3d). CT chest confirmed a well-defined mass in left lung infra-scapular region associated with loculated pleural effusion. Needle biopsy of the lung showed foci of fibroblastic proliferation with hemosiderophages and mild lymphomononuclear cell infiltration. The Xpert MTB/Rif test and mycobacterial cultures were negative. Culture of the biopsy material grew T. marneffei. She was started on itraconazole 200 mg twice daily for a year. Follow-up a year later showed significant resolution of lesions on chest X-ray (Fig. 3e).
Case 5
A 3-year-old boy from Kamrup, Assam, presented with fever of 2-month duration, vomiting, diarrhea and skin rash of 1-month duration and shortness of breath for 4 days, along with unquantified weight loss. He had seizure disorder 6 months earlier for which he was treated with levetiracetam and valproate. Three months prior, he had an episode of epistaxis associated with ecchymotic patches over right leg at which time, his platelet count was 10,000/mm3 and aPTT was deranged. He had received intravenous methylprednisolone followed by oral prednisone for 7 weeks. One month prior to presentation, he was evaluated elsewhere for fever and found be to HIV-positive with a CD4 count of 60 cells/mm3 for which antiretroviral therapy was started. On examination at presentation, he appeared toxic, tachycardic and tachypneic requiring nasal high flow oxygen. Umbilicated and crusted vesicles were noted over face, trunk and limbs (Fig. 4). His platelet count was 34,000/mm3 and serum ferritin 120,780 ng/ml; CD4 count was 135 cells/mm3 and HIV viral load was 7789 copies/ml. Serum beta D glucan was > 523 pg/ml. Bone marrow aspirate showed intracellular yeast-like cells (Fig. 2c, d) and both blood cultures and bone marrow cultures grew T. marneffei. The child was started on 3 mg/kg/day of liposomal amphotericin B. Despite initial improvement, the patient subsequently developed septic shock due to carbapenem-resistant Klebsiella bacteremia and died.
Mycological Investigations
Samples (FNA samples, needle biopsies, bone marrow and blood broth from positively flagged BACT-ALERT bottles) were cultured on 5% sheep blood agar (5SBA), two sets of Sabouraud’s dextrose agar (SDA) media, one incubated at 37 °C and the other at 25 °C, and a brain–heart infusion agar (BHIA) media supplemented with cycloheximide which was incubated at 25 °C. After 72 h of incubation, the 5SBA and SDA at 37 °C showed growth of yellowish mucoid colonies, while the SDA at 25 °C and BHIA grew greenish velvety mycelial colonies which after two further days of incubation at 25 °C developed a diffusible red pigment (Fig. 2e). A lactophenol cotton blue (LPCB) mount showed septate hyphae bearing smooth-walled hyaline conidiophores with terminal bi-verticils of 3–5 metulae, each bearing 4–5 phialides (Fig. 2f). To confirm the identification as Talaromyces marneffei, yeast conversion was done successfully in all the cases, by subculturing the mycelium on BHIA with 5% sheep blood incubated at 37 °C which yielded smooth mucoid colonies showing yeast-like cells on an LPCB mount. Molecular confirmation of identification was done for the first isolate by sequencing the internal transcribed spacer (ITS) region of ribosomal DNA, and this isolate was deposited in the National Culture Collection of Pathogenic Fungi, Chandigarh, India (NCCPF 730063).
Discussion
Talaromyces marneffei is the only species in the genus Talaromyces which exists as a dimorphic fungus and cause opportunistic infections. The earliest human cases of talaromycosis were described prior to the beginning of the AIDS era [10, 11]. The first recorded human infection was the result of a lab accident while conducting extensive animal pathogenicity studies [12]. Since the 1980s, most cases reported are in HIV-infected individuals. In India, since its original description in Manipur [2] it has also been reported from Assam and patients of northeast origin [8, 9, 13]. Bamboo rats have been shown to be the reservoir of infection, Cannomys badius being the common species in India [14].
Ecology of the fungus remains largely hypothetical. Consistent with clinical features of cough seen in many patients, inhalation of conidia is proposed as the common route of transmission. Although bamboo rats have been established as reservoirs of the fungus, they live in mountainous areas and do not come into contact with people, making direct transmission to man from bamboo rat unlikely. Reports of talaromycosis in HIV-infected infants and children also suggest that human infection from bamboo rats is unlikely [15]. However, soil exposure, especially during the rainy season, and agricultural activity have been strongly associated with higher risk of infection [16], even though search for environmental reservoir other than bamboo rats has been largely unsuccessful [17]. Case 4 in our series was involved in gardening activities around her place of residence, which may have been the source of acquisition of infection. Occurrence of all our cases between April and September, which are monsoon months in the northeast region, suggests a possible association of this disease with the rainy season.
There is also wide variation in the distribution of disease within the endemic regions. In Chiang Mai province of northern Thailand and China, it is the third most common opportunistic infection (OI) in AIDS patients after tuberculosis and cryptococcosis. However, in southern Thailand which also houses a large HIV population, the prevalence is low [18, 19]. Even in the north eastern state of Manipur where the largest case series from India is reported, talaromycosis was reported in 25% of all AIDS patients presenting to the hospital [3]. Our reports suggest that the disease may be endemic in many parts of northeast India, from Manipur state through Assam till Sikkim.
Diagnosis is challenging, especially in areas endemic for tuberculosis, histoplasmosis and talaromycosis, as the three entities share many clinical features such as fever, weight loss, cough, anemia, lymphadenopathy and hepatosplenomegaly [20]. Skin lesions may also be present in both histoplasmosis and talaromycosis, though more common in the latter wherein papulonecrotic lesions with central umbilication resembling molluscum contagiosum lesions are seen. In a Thai study, hyperbilirubinemia was more commonly associated with talaromycosis [20]. The fungus may also produce granulomas in the lung, liver, lymph nodes and subcutaneous tissue, further complicating the distinction between tuberculosis and histoplasmosis.
Definitive diagnosis can be made by culture of the fungus from blood or other involved tissue (skin nodules/lymph nodes/bone marrow), or by histopathological demonstration of the characteristic morphologic findings in biopsy material. In the Manipur study of 46 cases of talaromycosis, only 10 were culture confirmed and 36 were diagnosed based on histopathology alone [3]. The presence of oval or elongated yeast-like organisms with clear central septum is a unique feature of T. marneffei, by which it can be differentiated from Histoplasma capsulatum. This septum may be missed if not specifically looked for by the pathologist. Fungal cultures establish the diagnosis with early growth of mycelial colonies with a flat green surface and diffusible red pigment. Cultures of bone marrow have the highest sensitivity for detection. The fungus may also be visualized in peripheral blood smears in fulminant cases, as was seen in case 2. Microscopically, the fungus has typical filamentous reproductive structures of the genus Talaromyces. Mold-to-yeast conversion can be achieved by subculturing the fungus onto brain–heart infusion agar and incubating at 37 °C. This is necessary for confirmation of species identification, as there may be other Talaromyces species such as T. purpurogenus, T. minioluteus and T. atroroseus which are capable of producing the diffusible red pigment, but are usually non-pathogenic saprobes [21,22,23].
There are several unique features in our case series (Table 1). First, there have been less than 50 cases of non-HIV talaromycosis described in the world literature [24, 25] and no previous reports of non-HIV associated talaromycosis in India, with case 1 being the first transplant-associated case from India (Table 2). Table 2 lists the reported cases from India, including only reports in which the diagnosis was confirmed by culture with a demonstration of fungal dimorphism by yeast conversion or histopathology. Although exact figures are unknown, about 7500 renal transplants are performed in India across 250 centers each year [26] and clinicians need to suspect this infection in transplant recipients with a compatible clinical presentation. Talaromycosis in apparently immunocompetent patients is a rare phenomenon and not previously reported from India, except for a single culture confirmed case of fungal keratitis due to T. marneffei following trauma in an agricultural field. Case 4 of our series was immunocompetent and had no comorbidities other than diabetes and early diabetic nephropathy. She was, however, involved in gardening activities around her place of residence and may have been exposed to a high fungal inoculum. In a Thai study which included 34 HIV-uninfected patients with talaromycosis, 64.9% (n = 22) were apparently immunocompetent and 8.8% (n = 3) were diabetic [27]. Atypical presentations have been observed in HIV-negative individuals. In a study comparing clinical characteristics of talaromycosis between HIV-infected and uninfected individuals, it was seen that the time to diagnosis was significantly delayed in the non-HIV group due to atypical presentation and low index of suspicion from clinicians [25].
Second, only two of the cases had typical umbilicated cutaneous lesions which are seen in more than 80% of cases in India [3]. Cutaneous lesions are usually a feature of disseminated talaromycosis in advanced AIDS. Cases 1, 2 and 4 presented with a chronic pneumonia syndrome, without involving other systems, whereas case 3 had generalized lymphadenopathy with multiple necrotic abdominal nodes and hepatosplenomegaly syndrome, with a single doubtful cutaneous lesion.
Third, the cases were misdiagnosed as histoplasmosis on cytopathology. Following growth of T. marneffei in culture, the cytopathology slides were reviewed and the characteristic transverse septations were noted (Fig. 2a, c, d). These cells do not show budding, and the cross-walls occur due to fission of the yeast cells. Occasional elongated sausage or hyphal forms may also be seen which are absent in histoplasmosis (Fig. 2c) [28].
Fourth, only eight cases of talaromycosis have been previously reported from Assam, all of which were HIV associated (Table 2). Four of our cases were from Assam and one from Sikkim. None of them had a history of travel outside India or to neighboring states like Manipur before presentation. An increase in cases in Assam may be due either to heightened clinical suspicion and availability of improved fungal diagnostics, or due to a change in ecology favoring increased exposure to the fungus. More studies are needed to clarify this hypothesis.
Treatment of severe T. marneffei infection consists of an intensive phase with amphotericin B deoxycholate for 2 weeks (0.6 mg/kg/day) followed by oral itraconazole for 10 weeks (200 mg twice daily). Voriconazole can be used as an alternative to itraconazole. ART needs to be initiated within two to four weeks of antifungal therapy. Secondary prophylaxis with itraconazole (200 mg once daily) is required till CD4 cell count rises to ≥ 100 cells/µL for at least 6 months [29]. Only two of our cases, both HIV negative, showed clinical improvement with itraconazole on follow-up. Two HIV cases did not return for follow-up, and one child died in spite of therapy due to late presentation.
Conclusion
Talaromycosis is an emerging infection in the state of Assam, India, and perhaps in Sikkim as well. Microbiological culture of blood and involved tissue in addition to histopathology is essential for confirmation of the diagnosis. The mycelial form at room temperature produces a diffusible red pigment and may resemble other non-pathogenic Penicillium or Talaromyces species, but being the only thermally dimorphic species, mold-to-yeast conversion will establish the identification. In India, the disease must be considered not just in HIV-positive patients from Manipur but in transplant recipients, immune competent hosts and also in those without skin lesions.
References
Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110.
Narendra Singh P, Ranjana K, Indiver Singh Y, Priyokumar Singh K, Surchandra Sharma S, Kulachandra M, et al. Indigenous disseminated Penicillium marneffei infection in the state of Manipur, India: report of four autochthonous cases. J Clin Microbiol. 1999;37:2699–702.
Ranjana KH, Priyokumar K, Singh TJ, Gupta CC, Sharmila L. Disseminated Penicillium marneffei infection among HIV infected patients in Manipur state India. J Infect. 2002;45(4):268–71.
Hart J, Dyer JR, Clark BM, McLellan DG, Perera S, Ferrari P. Travel-related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl Infect Dis. 2012;14:434–9.
Stathakis A, Lim KP, Boan P, Lavender M, Wrobel J, Musk M, et al. Penicillium marneffei infection in a lung transplant recipient. Transpl Infect Dis. 2015;17:429–34.
Lin JN, Lin HH, Lai CH, Wang JL, Yu TJ. Renal transplant recipient infected with Penicillium marneffei. Lancet Infect Dis. 2010;10:138.
Gorai S, Saha M, Madhab V, Mitra S. Talaromycosis (penicilliosis): a rare, opportunistic systemic fungal infection. Indian J Dermatol. 2019;64:331.
Bordoloi P, Nath R, Borgohain M, Huda MM, Barua S, Dutta D, et al. Subcutaneous mycoses: an aetiological study of 15 cases in a tertiary care hospital at Dibrugarh, Assam Northeast India. Mycopathologia. 2015;179:425–35.
Saikia L, Nath R, Mahanta J. Penicillium marneffei infection in Assam. Indian J Dermatol Venereol Leprol. 2010;76:75–6.
Disalvo AF, Fickling AM, Ajello L. Infection caused by penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. 1973;60:259–63.
Pautler KB, Padhye AA, Ajello L. Imported penicilliosis marneffei in the United States: report of a second human infection. Sabouraudia. 1984;22:433–8.
Segretain G. Penicillium Marneffei N. Sp., une Mycose Du Système Réticulo-Endothélial. Mycopathol Mycol Appl. 1959;11(4):327–53.
Michael JS, Abraham OC, Mathai D, Mathews MS. Varied clinical manifestations of penicillium marneffei in patients with human immunodeficiency virus: a report from south India. Mycoses. 2005;48:120–1.
Gugnani H, Fisher MC, Paliwal-Johsi A, Vanittanakom N, Singh I, Yadav PS. Role of Cannomys badius as a natural animal host of Penicillium marneffei in India. J Clin Microbiol. 2004;42:5070–5.
Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:935–40.
Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NPH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City. Viet Nam Clin Infect Dis. 2011;52:945–52.
Chariyalertsak S, Vanittanakom P, Nelson KE, Sirisanthana T, Vanittanakom N. Rhizomys sumatrensis and Cannomys badius, new natural animal hosts of Penicillium marneffei. J Med Vet Mycol. 1996;34:105–10.
Chakrabarti A, Slavin MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol. 2011;49:337–44.
Supparatpinyo K, Khamwan C, Baosoung V, Sirisanthana T, Nelson KE. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–4.
Mootsikapun P, Srikulbutr S. Histoplasmosis and penicilliosis: comparison of clinical features, laboratory findings and outcome. Int J Infect Dis. 2006;10:66–71.
Frisvad JC, Yilmaz N, Thrane U, Rasmussen KB, Houbraken J, Samson RA. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE. 2013;8(12):e84102 Baker SE, editor.
Yilmaz N, Houbraken J, Hoekstra ES, Frisvad JC, Visagie CM, Samson RA. Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia. 2012;29:39–54.
Atalay A, Koc AN, Akyol G, Cakir N, Kaynar L, Ulu-Kilic A. Pulmonary infection caused by Talaromyces purpurogenus in a patient with multiple myeloma. Infez Med. 2016;24:153–7.
Joosten SA, Hannan L, Heroit G, Boerner E, Irving L. Penicillium marneffei presenting as an obstructing endobronchial lesion in an immunocompetent host. Eur Respir J. 2012;39:1540–3.
Li HR, Cai SX, Chen YS, Yu ME, Xu NL, Xie BS, et al. Comparison of Talaromyces marneffei Infection in Human Immunodeficiency Virus-positive and Human Immunodeficiency Virus-negative Patients from Fujian China. Chin Med J(Engl). 2016;129(9):1059.
Shroff S. Current trends in kidney transplantation in India. Indian J Urol. 2016;32:173–4.
Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464.
Mo W, Deng Z, Li S. Clinical blood routine and bone marrow smear manifestations of disseminated penicilliosis marneffei. Chin Med J (Engl). 2002;115(12):1892–4.
Le T, Van Kinh N, Cuc NTK, Tung NLN, Lam NT, Thuy PTT, et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N Engl J Med. 2017;376:2329–40.
Varghese GM, Pise G, Michael SJ, Jacob M, George R. Disseminated Penicillium marneffei infection in a human immunodeficiency virus-infected individual. J Postgrad Med. 2004;50:235–6.
Maniar JK, Chitale AR, Miskeen A, Shah K, Maniar A. Penicillium marneffei infection: an AIDS-defining illness. Indian J Dermatol Venereol Leprol. 2005;71:202–4.
Gupta S, Mathur P, Maskey D, Wig N, Singh S. Immune restoration syndrome with disseminated Penicillium marneffei and cytomegalovirus co infections in an AIDS patient. AIDS Res Ther BioMed Central. 2007;4(1):21.
Sharma A, Hazarika NK, Barua P, Dey I, Tudu NK. Penicillium marneffei infection in a HIV infected child. Indian J Med Res. 2007;126:580–2.
George I, Sudarsanam T, Pulimood A, Mathews M. Acute abdomen: an unusual presentation of disseminated Penicillium marneffei infection. Indian J Med Microbiol. 2008;26:180–2.
Baradkar V, Kumar S, Kulkarni SD. Penicillium marneffei: the pathogen at our doorstep. Indian J Dermatol. Venereol. Leprol. 2009;75:619–20.
Saikia L, Nath R, Biswanath P, Hazarika D, Mahanta J. Penicillium marneffei infection in HIV infected patients in Nagaland and immune reconstitution after treatment. Indian J Med Res. 2009;129:333–4.
Sood N, Gugnani HC. Disseminated Penicillium marneffei infection in a Myanmar refugee from Mizoram state. Indian J Pathol Microbiol. 2010;53:361–3.
Saikia L, Nath R, Hazarika D, Mahanta J. Atypical cutaneous lesions of Penicillium marneffei infection as a manifestation of the immune reconstitution inflammatory syndrome after highly active antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2010;76:45–8.
Yanamandra U, Anantaram J, Subramanian S, Sharma M, Hazra N, Nair V. Penicilliosis presenting as fungating skin lesion. J Infect Chemother. 2011;17:700–2.
Puri P, Ramesh V, Singh A, Muralidhar S, Capoor MR. Facial eruption in a human immunodeficiency virus (HIV)-seropositive patient. Int J Dermatol. 2012;51:777–9.
Ghalige HS, Sahoo B, Sharma S, Devi KR, TH SCS. Acute abdomen due to Penicillium Marneffei an indicator of HIV infection in Manipur state. J Clin Diagnostic Res. 2014;8(9):NDO5–6.
Vyawahare CR, Misra RN, Gandham NR, Angadi KM, Paul R. Penicillium keratitis in an immunocompetent patient from Pune, Maharashtra, India. J Clin Diagnostic Res. 2014;8:DDO1–2.
Bachaspatimayum R, Haokip T, Zamzachin G, Devi YE. Ulceronecrotic penicillosis. Indian J Dermatol. 2015;60:215.
Sunny N, Nair S, Justus L, Beena A. Total dystrophic onychomycosis caused by Talaromyces marneffei in a patient with Acquired immunodeficiency syndrome on combined anti-retroviral therapy. Indian J Dermatol Venereol Leprol. 2018;84:87–90.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nandini Sethuraman who also wrote the first draft. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Informed consent
Informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Vishnu Chaturvedi.
Rights and permissions
About this article
Cite this article
Sethuraman, N., Thirunarayan, M.A., Gopalakrishnan, R. et al. Talaromyces marneffei Outside Endemic Areas in India: an Emerging Infection with Atypical Clinical Presentations and Review of Published Reports from India. Mycopathologia 185, 893–904 (2020). https://doi.org/10.1007/s11046-019-00420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-019-00420-0