Abstract
Background
Talaromyces marneffei (T. Marneffei) infection is considered as an indicator of immunosuppression in immunocompromised individuals, leading to multiple organ damage. Our study aimed to evaluate both the clinical characteristics and immunological features of pediatric patients infected with T. marneffei from our institute, providing novel insights into diagnosis and treatment for this life-threatening disease.
Method
Thirteen pediatric patients with T. marneffei infection were enrolled in Guangzhou Women and Children’s Medical Center during 2012 to 2020. Clinical data and laboratory findings were collected and further analyzed. Pearson correlation coefficient was calculated to determine the relationship between serum immunoglobulins (Igs) levels and white blood cell count, or the absolute lymphocyte count.
Results
Patients were diagnosed as having T. Marneffei infection mainly based on the results of fungal culture and Gram stain of specimens. The most common presentations were fever (69%), pneumonia (38%) and immunodeficiency (38%). The total levels of Igs (IgE, IgA, and IgM) were positively correlated with both white blood cell count and absolute lymphocyte count.
Conclusion
Serum Ig expression Pattern in patients diagnosed with T. marneffei infection might serve as an effective prognostic marker which would help with the development of early interventions for children with this fatal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Talaromyces marneffei (T. marneffei) is a thermally dimorphic fungus that causes severe systemic infection, especially in individuals infected with human immunodeficiency virus (HIV) [1, 2]. T. marneffei was first isolated from wild bamboo rats in Vietnam in 1956, and this infection is mainly prevalent in Southeast Asia, including South China, Thailand, Laos, Vietnam, and Northeast India [3, 4]. Although T. marneffei infection is relatively more common in people with acquired immunodeficiency syndrome (AIDS), reports have shown an increase in this disease even in HIV-negative patients in endemic areas, causing life-threatening syndromes [5,6,7].
Talaromyces marneffei infection is usually associated with a high mortality rate. The most common clinical manifestation of this infection includes fever, anemia, and pneumonia, eventually decreasing the quality of life and shortening the lifespan of patients [8, 9]. Therefore, it is important to identify useful prognostic markers for the diagnosis and prediction of the disease's future outcomes.
In this study, we retrospectively examined 13 pediatric patients with T. marneffei infection and compared their clinical characteristics, as well as laboratory findings. Furthermore, we investigated the correlation between serum immunoglobulins (Igs) and other peripheral factors such as white blood cell count and lymphocyte count. Our findings provide more evidence for the diagnosis and treatment of pediatric patients with T. marneffei infection, which will hopefully contribute to improved prognosis and a reduced mortality rate.
Methods
Study Population and Data Collection
Patients diagnosed with T. marneffei infection from March 2012 to February 2020, at Guangzhou Women and Children’s Medical Center, Guangzhou Medical University were enrolled in the study. The patients’ medical histories, ages and genders were collected. Laboratory tests were performed for each of the patients, including HIV testing, routine blood examination, serum Ig test, absolute count of lymphocytes and biochemical test including globulin, albumin, bile acid, bilirubin, aspartate aminotransferase and alanine aminotransferase.
The study was conducted according to the principles outlined in the Declaration of Helsinki and approved by the Institutional Review Board of Guangzhou Women and Children’s Medical Center of Guangzhou Medical University, Guangzhou, China (ethics number: 2022-206B00).
Diagnostic Criteria for T. marneffei
Specimens from bone marrow, blood, throat, or alveolar lavage fluid were cultured on Sabouraud dextrose agar at 25 °C. Detection of T. marneffei was identified by growth of the mold-like form. In addition, Wright’s-stained bone marrow smears were observed under an optical microscopy. For T. marneffei-positive samples, numerous yeast-like cells were examined inside and outside host cells, appearing as small, basophilic, round or oval to sausage-shaped inclusions and contained eccentric or central dot-like structures [10].
Statistical Analysis
Associations between different Igs levels and inflammatory markers or lymphocyte cell count were calculated using linear correlation with the Pearson correlation coefficient (Graphpad Prism 8.0). Statistical significance was determined as P < 0.05.
Results
Clinical Characteristics
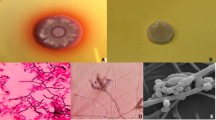
A total of 13 pediatric patients were diagnosed with T. marneffei infection between 2012 and 2020. All patients were diagnosed based on the results of Gram stain of their bone marrow, as well as a fungal culture of different specimens, Including blood, bone marrow, skin lesions, alveolar lavage fluid, and throat swabs. After culturing T. marneffei at 25 °C, the colonies became visible on the plate after 3 days, which then turned green and granular, developing a characteristic red diffusible pigment by Day 14 (Fig. 1). Furthermore, Wright-Giemsa staining of bone marrow revealed hemophagocytosis and abundant oval- to sausage-shaped pathogens (Fig. 2).
Analysis of patients’ clinical data revealed that the most common manifestations were fever, pneumonia and immunodeficiency, followed by erythra. In addition, other symptoms included ulcers, abdominal distension, cough and sepsis (Table 1). Moreover, most patients involved in the current study were presented with hemophagocytosis, further confirming the diagnosis of T. Marneffei infection.
Laboratory findings
Based on the complete blood count test, 10 out of all 13 patients had abnormal white blood cell (WBC) counts, with a mean WBC count of 6.7 × 109 cells/L (SD = 6.69). 10 patients showed abnormal neutrophil numbers, with a mean neutrophil count of 4.7 × 109 cells/L (SD = 4.23). 8 patients had abnormal lymphocyte counts (CD3+), with a mean absolute lymphocyte count of 1436 × 109 cells/L (SD = 2093). Moreover, the Ig test showed overall high levels of serum Igs, mainly IgM, IgA and IgE, with a mean level of 1.31, 1.51 and 212.36 g/L (SD = 0.79, 1.74 and 302.78), respectively. The detailed results of laboratory testing are shown in Table 2.
Treatment and Outcome
The most commonly used antifungal treatment strategies in our study include amphotericin B, voriconazole, itraconazole, and intravenous Ig (IVIg), with amphotericin B being the most recommended primary treatment for people with disseminated T. marneffei infection. Patient 1 had respiratory failure and severe pneumonia, as well as diarrhea before he visited our institute. After he was diagnosed with T. marneffei infection, he received caspofungin, latamoxef, IVIg and plasma exchange; unfortunately, he died due to septic shock.
Patients 8 and 10 were found to be HIV-positive, both of whom were automatically transferred to another institute without any antifungal treatment. Patient 2 was treated with amphotericin B, caspofungin and IVIg, after which, chest and abdomen CT was recommended by the physicians. However, although the family members had been informed of the impending danger to the child’s life, they insisted that the child be discharged. Patient 11 was admitted to our institute with a long-term cough and pneumonia. His parents claimed that the boy had been suffering from X-linked hyper IgM syndrome (XHIGM) caused by the CD40 ligand gene [11]. After diagnosis with T. marneffei infection, he received latamoxef, voriconazole (8 mg/kg), and IVIg (2 g/kg), and eventually achieved a significant improvement. Patient 12 was treated with amoxicillin clavulanate, voriconazole (8 mg/kg), clarithromycin, amphotericin B (1 mg/kg), sulfamethoxazole and itraconazole (400 mg/day), followed by IVIg (2 g/kg). After the abovementioned treatment, the patient showed no fever, cough or abdominal-related symptoms and was discharged from the hospital. After admission, Patient 13 showed a good response to IVIg (2 g/kg), as well as voriconazole (8 mg/kg), and was discharged eventually (Table 3).
Serum Ig Levels are Closely Related to Immune Cell Count Among Patients with T. marneffei
Igs are known to play a critical role in initiating host–pathogen interaction. Thus, serum Ig levels can reflect the immune status to a large extent [12, 13]. To investigate whether the elevated levels of serum Igs in patients with T. marneffei infection have any clinical relevance, we performed a correlation among WBC, lymphocyte count and serum Ig levels (IgE, IgA and IgM) by calculating the Pearson correlation coefficient (Pearson’s r). As a result, we found a positive correlation between serum IgE levels and total white cell count, neutrophil count, as well as natural killer cell count. The same trends were observed when we analyzed the correlation between IgA/IgM and the abovementioned parameters (Figs. 3A, 4A and 5A). These results suggest that elevated serum Ig is closely associated with elevated inflammatory responses or more vigorous infection, as characterized by increased WBC counts in patients with T. marneffei infection.
Pearson correlation between the serum levels of IgE with WBC and lymphocyte count in patients with T. marneffei infection. A Correlation between serum IgE and white-cell count, absolute neutrophil count or absolute natural killer cell count. B Correlation between serum IgE and the serum CD3+, CD4+, CD8+ T lymphocyte counts, as well as CD19+ B cell count
Pearson correlation between the serum levels of IgA with WBC and lymphocyte count in patients with T. marneffei infection. A Correlation between serum IgA and white-cell count, absolute neutrophil count or absolute natural killer cell count. B Correlation between serum IgA and the serum CD3+, CD4+, CD8+ T lymphocyte counts, as well as CD19+ B cell count
Pearson correlation between the serum levels of IgM with WBC and lymphocyte count in patients with T. marneffei infection. A Correlation between serum IgM and white-cell count, absolute neutrophil count or absolute natural killer cell count. B Correlation between serum IgM and the serum CD3+, CD4+, CD8+ T lymphocyte counts, as well as CD19+ B cell count
To further understand the correlation between the levels of Igs and the immune status of patients, we continued to perform Pearson correlation analysis by including lymphocyte count. We found that there was an apparent positive correlation between Ig levels and T lymphocyte (CD3+, CD3+CD4+ and CD8+), as well as B lymphocyte (CD19+) count, indicating that blood levels of Igs strongly reflect the host’s immune response to T. marneffei infection (Figs. 3B, 4B and 5B). Further, we observed that Patients 11, 12, and 13, who were successfully treated, had relatively lower IgE levels (6.9, 0.55 and 0.98 g/L respectively), as compared to patients 1, 3 and 5 (15, 740 and 850 g/L respectively) who eventually died of this disease. These results imply that circulating IgE in the serum of T. marneffei infected-patients may be associated with a poorer prognosis (Supplemental Fig. 1).
Discussion
T. marneffei infection is a global health problem, especially in Southeast Asia. Despite adequate antifungal treatment, the outcome remains poor with mortality rates of 20.7% and 29.4% among HIV infected and uninfected patients, respectively [14]. Moreover, studies have shown that the prevalence of T. marneffei infection keeps increasing primarily due to an increased number of HIV cases [9, 15, 16]. Furthermore, there is no standard antifungal treatment for patients infected with T. marneffei infections [17, 18]. Hence, it is important to further investigate the clinical characteristics of this infectious disease to enhance patient outcomes in the future.
A recent study showed that the development of post-acute COVID-19 syndrome correlates with a distinct pattern of total Ig levels in patients, highlighting the importance of measuring Igs to predict disease risk [19]. Another study conducted by Mintoff et al. found that serum IgG in patients with hidradenitis suppurativa correlates closely with both dynamic and static hidradenitis suppurativa severity scoring systems, suggesting that the serum IgG could be used as a biomarker of disease severity as well as an adjunct to clinical severity scoring [20]. Therefore, serological parameters could represent promising therapeutic markers for infectious diseases. In our study, we first found that the levels of IgE, IgA, IgG and IgM were elevated in the patients. Further analysis revealed a positive correlation between levels of Igs and inflammatory indices as marked by elevated WBC and lymphocyte counts. Finally, we found that the levels of IgE in patients who were eventually cured appeared to be relatively lower than those in patients who died after treatment. Thus, our results indicate that serum Igs may represent a strong indicator of disease severity. To our knowledge, this is the first study to evaluate the relationship between the levels of serum Igs and WBC, as well as lymphocytes in pediatric patients infected with T. marneffei. Additional studies are needed to clarify the mechanism underlying the biological or physiological effects of serum Igs in different individuals.
Our study also found that different lymphocyte subsets were correlated with serum Ig levels in patients infected with T. marneffei, including CD3+, CD3+CD4+, CD3+CD8+ T lymphocyte and CD19+ B lymphocyte count. CD4+ T lymphocytes are known to play a key role in reducing infection, while CD8+ T lymphocytes are effector cytotoxic cells. Moreover, B lymphocytes differentiate into Ig-secreting plasma cells [21,22,23]. Therefore, further research on the function and biological activities of these lymphocytes, such as proliferation and activation in the context of P. marneffei infection is needed. A previous study revealed that CD8+ lymphocyte count correlated with serum IgA levels in HIV patients, based on an 8-year prospective follow-up [24]. More recent research reported that in primary Sjögren’s syndrome, an autoimmune disease, serum Ig levels were positively correlated with the percentage of T cells [25]. Another study proved that CD8+ T lymphocytes from the germinal centers of lymph nodes from HIV patients could promote B cells to produce Igs, eventually eradicating virus-infected target cells, which suggests that T lymphocytes are promising candidate cells for immune therapies [26].
Patient 11 was the only one with T. marneffei infection who had XHIGM among all cases reported here. XHIGM is a rare primary immunodeficiency disorder caused by mutations in the CD40 ligand molecule on the surface of T cells in the pediatric population, which usually makes patients more susceptible to infection [11, 27]. Li et al. reported a case with XHIGM who also had severe eosinophilia and fungal infection. After receiving anti-fungal treatment and IVIg replacement, the patient underwent allogeneic stem cell transplantation, eventually being discharged in good condition [28]. Another study by Fan et al. showed that children with XHIGM were more vulnerable to respiratory infections, including T. marneffei, which in turn would help pediatricians to identify XHIGM [29]. Hence, in order to avoid life-threatening infection, allogeneic stem cell transplantation should be carried out once the patient is diagnosed with XHIGM. More importantly, it is necessary to perform genetic testing on patients to check whether they have XHIGM before making decisions about the treatment of T. marneffei.
However, we acknowledge several limitations to our study. Firstly, only 13 patients were included in our study; therefore, the sample size was considerably small. Thus, in the future, we should increase the sample size as much as possible to improve the validity and credibility of our findings. Secondly, the post-treatment follow-up period for all patients enrolled was short and limited. Hence, a long-term follow-up would allow us to fully understand the effects of primary treatments. Lastly, some patients described in the current study had been suffering from different underlying medical conditions, such as different stages of pneumonia or thalassemia (data not shown), which may influence conclusions of the research.
In conclusion, our results highlight the benefit of measuring Igs for the early identification of patients at high risk of death, which facilitates the study of developing more effective targeted treatment for T. marneffei infection.
References
Si Z, Qiao J. Talaromyces marneffei Infection. New Engl J Med. 2017;377(26):2580. https://doi.org/10.1056/NEJMicm1704164.
Ustianowski AP, Sieu TP, Day JN. Penicillium marneffei infection in HIV. Curr Opin Infect Dis. 2008;21(1):31–6. https://doi.org/10.1097/QCO.0b013e3282f406ae.
Capponi M, Segretain G, Sureau P. [Penicillosis from Rhizomys sinensis]. Bulletin de la Societe de Pathologie Exotique et de Ses Filiales. 1956;49(3):418–21
Stathakis A, Lim KP, Boan P, et al. Penicillium marneffei infection in a lung transplant recipient. Transpl Infect Dis Official J Transpl Soc. 2015;17(3):429–34. https://doi.org/10.1111/tid.12377.
Li Y, Lin Z, Shi X, et al. Retrospective analysis of 15 cases of Penicillium marneffei infection in HIV-positive and HIV-negative patients. Microb Pathog. 2017;105:321–5. https://doi.org/10.1016/j.micpath.2017.01.026.
Qiu Y, Zhang J, Liu G, et al. Retrospective analysis of 14 cases of disseminated Penicillium marneffei infection with osteolytic lesions. BMC Infect Dis. 2015;15:47. https://doi.org/10.1186/s12879-015-0782-6.
You CY, Hu F, Lu SW, et al. Talaromyces Marneffei Infection in an HIV-negative child with a CARD9 Mutation in China: a case report and review of the literature. Mycopathologia. 2021;186(4):553–61. https://doi.org/10.1007/s11046-021-00576-8.
Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis Official Publ Infect Dis Soc Am. 1996;23(1):125–30. https://doi.org/10.1093/clinids/23.1.125.
Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. https://doi.org/10.1128/cmr.19.1.95-110.2006.
Wong KF. Marrow Penicilliosis: a readily missed diagnosis. Am J Clin Pathol. 2010;134(2):214–8. https://doi.org/10.1309/ajcpwvbqcw13djlo.
Etzioni A, Ochs HD. The hyper IgM syndrome–an evolving story. Pediatr Res. 2004;56(4):519–25. https://doi.org/10.1203/01.pdr.0000139318.65842.4a.
Aretha D, Leukaditou K, Fligou F, et al. Correlation of immunoglobulins and lymphocytes levels with the clinical and microbiological response of septic patients with gram-negative Bacteremia. J Clin Med Res. 2021;13(1):64–72. https://doi.org/10.14740/jocmr4409.
Gonzalez-Quintela A, Alende R, Gude F, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151(1):42–50. https://doi.org/10.1111/j.1365-2249.2007.03545.x.
Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13(1):464. https://doi.org/10.1186/1471-2334-13-464.
Hu F, Liu S, Liu Y, Li X, Pang R, Wang F. The decreased number and function of lymphocytes is associated with Penicillium marneffei infection in HIV-negative patients. J Microbiol Immunol Infect. 2021;54(3):457–65. https://doi.org/10.1016/j.jmii.2020.02.007.
Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. https://doi.org/10.1007/s11046-012-9577-0.
Yousukh A, Jutavijittum P, Pisetpongsa P, et al. Clinicopathologic study of hepatic Penicillium marneffei in Northern Thailand. Arch Pathol Lab Med. 2004;128(2):191–4. https://doi.org/10.5858/2004-128-191-csohpm.
Mo D, Li X, Wei L, Sun C, Liang H, Cao C. In vitro interactions of calcineurin inhibitors with conventional antifungal agents against the yeast form of penicillium marneffei. Mycopathologia. 2014;178(3–4):217–20. https://doi.org/10.1007/s11046-014-9787-8.
Cervia C, Zurbuchen Y, Taeschler P, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13(1):446. https://doi.org/10.1038/s41467-021-27797-1.
Mintoff D, Borg I, Pace NP. Serum immunoglobulin G is a marker of hidradenitis suppurativa disease severity. Int J Mol Sci. 2022;23(22):13800.
Aljabr W, Al-Amari A, Abbas B, et al. Evaluation of the levels of peripheral CD3(+), CD4(+), and CD8(+) T cells and IgG and IgM antibodies in COVID-19 patients at different stages of infection. Microbiol Spectr. 2022;10(1):e0084521. https://doi.org/10.1128/spectrum.00845-21.
Bernardo I, Mancebo E, Aguiló I, et al. Phenotypic and functional evaluation of CD3+CD4-CD8- T cells in human CD8 immunodeficiency. Haematologica. 2011;96(8):1195–203. https://doi.org/10.3324/haematol.2011.041301.
LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–80. https://doi.org/10.1182/blood-2008-02-078071.
Phillips AN, Sabin CA, Elford J, Bofill M, Lee CA, Janossy G. CD8 lymphocyte counts and serum immunoglobulin A levels early in HIV infection as predictors of CD4 lymphocyte depletion during 8 years of follow-up. AIDS. 1993;7(7):975–80. https://doi.org/10.1097/00002030-199307000-00011.
Zhou H, Yang J, Tian J, Wang S. CD8+ T lymphocytes: crucial players in Sjögren’s syndrome. Rev Front Immunol. 2021;11:602. https://doi.org/10.3389/fimmu.2020.602823.
Shen J, Luo X, Wu Q, et al. A Subset of CXCR5(+)CD8(+) T cells in the germinal centers from human tonsils and lymph nodes help B cells produce Immunoglobulins. Front Immunol. 2018;9:2287. https://doi.org/10.3389/fimmu.2018.02287.
Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol Rev. 2005;203:48–66. https://doi.org/10.1111/j.0105-2896.2005.00229.x.
Li H, Cao Y, Ma J, Li C. X-linked hyper IgM syndrome with severe eosinophilia: a case report and review of the literature. BMC Pediatr. 2022;22(1):178. https://doi.org/10.1186/s12887-022-03251-z.
Fan H, Huang L, Yang D, et al. Respiratory infections in X-linked hyper-IgM syndrome with CD40LG mutation: a case series of seven children in China. BMC Pediatr. 2022;22(1):675. https://doi.org/10.1186/s12887-022-03726-z.
Acknowledgements
This work was supported by Grants from National Natural Science Foundation of China (82200186, 82271858) and Guangzhou Municipal Science and Technology Project (202102021021, 202102010513).
Author information
Authors and Affiliations
Contributions
Yiqian Wang and Huasong Zeng conceived of and designed the study. Huishan Chen, Muxia Yan, Haowei He and Li Zhang performed most of the experiments and analyzed data. Yiqian Wang wrote the manuscript. All authors reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethics Approval
Studies involving human participants were approved by Guangzhou Women and Children’s Medical Center.
Additional information
Handling Editor: Xiao Meng.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Yan, M., He, H. et al. A Retrospective Study of Clinical and Immunological Features of a Pediatric Population with Talaromyces marneffei Infection. Mycopathologia 188, 221–230 (2023). https://doi.org/10.1007/s11046-023-00724-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-023-00724-2