Abstract
Nannizzia praecox, formerly known as Microsporum praecox, is a geophilic dermatophyte. Up to now 31 cases of human tinea have been reported in the literature, most of them with an inflammatory course. Three recent cases diagnosed in Germany within 1 year suggest that the fungus might be a more common cause of human dermatophytosis than reported so far. This might be based on the fact that N. praecox is often found in an equine environment and that horse riding is becoming more popular recently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most recently, based on a compilation of molecular data the taxonomy of the dermatophytes has been revised. Nearly all anthropophilic dermatophytes are now classified in the genera Trichophyton and Epidermophyton, along with some zoophilic species that regularly infect humans. Microsporum is now restricted to some species around M. canis, while the geophilic species and zoophilic species that are more remote from the human–environment are divided over Arthroderma, Lophophyton and Nannizzia [1]. But, also in these genera, there are some dermatophytes of importance in clinical medicine. Nannizzia (N.) praecox (Padhye, Ajello and McGinnis) Graeser and de Hoog, comb. nov., formerly known as Microsporum praecox, is a geophilic dermatophyte present in soil and equine environments (saddles, straw, stables, etc.). It is rarely reported as a cause of human tinea, particularly after contact with horses. N. praecox can be isolated from horse hair in the absence of clinical lesions [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].
The following article summarizes three recent cases of inflammatory N. praecox infections in Germany and gives a review of the literature.
Case Reports
-
1.
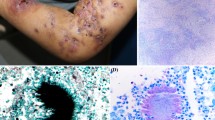
A 15-year-old female horse rider suffered from scaling, itching and small blistering at her right hand with subsequent spreading to the left one. Initially, under the preliminary diagnosis of allergic contact dermatitis a topical and oral therapy with glucocorticosteroids was started. At that time, an erythema with desquamation and tiny blisters on both hands was complicated by a severe itch (Fig. 1). Direct microscopy was positive, and N. praecox was found in culture. The macro- and micromorphological identification was confirmed by sequencing of internal transcribed spacer region (ITS). Brush cultures of 10 horses in the riding stable remained negative. The lesions cleared completely after a systemic antifungal treatment with terbinafine 250 mg/d for 21 days.
-
2.
A 10-year-old girl suffered from tinea corporis with erythematosquamous and centrifugal growing, sparse itching lesions of her right lower arm. Source of infection was horses at a horse-riding centre where the girl regularly was riding. Fluorescence optical Blancophor® preparation from skin scrapings revealed fungal hyphae. On Sabouraud’s dextrose agar, the fast growing dermatophyte formed flat, peripheral radiating and convolved colonies with white, slightly yellowish to beige-brown-stained granular and powdery surface (Fig. 2a, b). The reverse side of the colonies was smooth with luminous yellow colour (Fig. 2c). In the subculture, according to Ito and Refai the strain developed flat colonies with white–yellowish and granular surface with a conspicuous annular morphology (Fig. 2d). Microscopically, a multitude of thin-walled spindle-shaped and echinulate (with small spins) and lanceolate macroconidia appeared (Fig. 2e–g). Urease activity was positive. First, sequence analysis of the ribosomal ITS region (18S rRNA, ITS1, 5.8S rRNA, ITS2, 28S rRNA) and of the translation elongation factor 1-alpha (TEF 1-α) gene revealed the dermatophyte species M. praecox. Topical treatment was done using ciclopirox olamine cream.
Fig. 2 a Primary culture of Nannizzia praecox (strain 2) on Sabouraud’s dextrose agar. On tubes with tilted agar from skin scrapings developed flat colonies with white powdery and granular surface. b Three-week-old subculture of strain 2 on Sabouraud’s dextrose agar without cycloheximide. Slightly yellowish colonies showing with brownish pigmentation of the granular surface. c The reverse side of the colonies showed brightening yellow pigmentation. d Subculture according to Ito and Refai: Nannizzia praecox develops flat colonies with white-yellowish and granular surface with conspicuous annular morphology. e Urease activity was positive at Christensen-Agar (Becton Dickinson, Heidelberg, Germany. f Microscopically, a multitude of row-walled, spiny, echinulate (with small spins) and lanceolate macroconidia were observed. Spearhead-like in the centre raised macroconidia are typical for this dermatophyte. Lactophenol cotton blue preparation. g The spindle-like macroconidia form three to six or seven cross septae. Lactophenol cotton blue preparation. h Round and irregular formed chlamydospores—occurring single or in chains—were found in a hugh scale. Lactophenol cotton blue preparation. (Color figure online)
-
3.
A 17-year-old girl presented with a scaly plaque on the right lower leg existing since 2 weeks. There was no objective evidence for an animal source of infection. From skin scrapings, M. praecox was isolated (Fig. 3a–e). Suspicion diagnosis of M. praecox was based on results of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis of the strain. Species identification has been confirmed by sequencing the ITS region of the rDNA. Topical treatment by fusidic acid ointment was ineffective. Clotrimazole 1% plus triamcinolone in basic ointment lead to improvement in the skin lesion.
Fig. 3 a Primary culture of Nannizzia praecox (strain 3) on Sabouraud’s dextrose agar. On tubes with tilted agar developed flat colonies with white to beige powdery and granular surface. b Yellow-stained reverse side of the primary culture. c Three-week-old subculture of strain 3 on Sabouraud’s dextrose agar without cycloheximide. White granular colonies with annular brownish pigmentation. d The reverse side of the colonies showed brightening yellow pigmentation. e Microscopically, a multitude of row-walled, spiny and lanceolate macroconidia appeared. Lactophenol cotton blue preparation. (Color figure online)
Table 1 summarizes the clinical and mycological data of all three N. praecox infections.
Identification of N. praecox by Morphologic and Physiologic Parameters
On Sabouraud’s dextrose agar, the fast growing dermatophyte formed flat, peripheral radiating and convolved colonies with white, slightly yellowish to beige-brown-stained granular and powdery surface (Figs. 2a, b, d, 3a, c). The reverse side of the colonies is smooth with luminous yellow colour (Figs. 2c, 3b, d). Microscopically, a multitude of thin-walled spindle-shaped and echinulate (with small spins) and lanceolate macroconidia appeared [18, 19]. The small based macroconidia are raised in the middle and end part, however, pointy at the end (“spearhead”) (Figs. 2f-h, 3e). They are 6–9 celled and up to 65 × 9 µm. The small piriform microconidia, when present, have an orthotropic arrangement. Chlamydospores are also formed. Urease activity is positive (Fig. 2e).
Identification of M. praecox by DNA Sequence Analysis
The sequence analysis of the ITS of the ribosomal DNA and of the TEF 1-α gene was performed for the three N. praecox strains [22]. The respective DNA fragment was amplified with the universal fungal primer pair V9D and LSU266. Sequences of the amplification products were determined by Sanger sequencing using these oligonucleotide primers. For species determination, sequences were compared to the National Center for Biotechnology (NCBI) database, Bethesda, Maryland, using standard nucleotide blast.
Sequence analysis of the ribosomal ITS region (18S rRNA, ITS1, 5.8S rRNA, ITS2, 28S rRNA) and of the TEF 1-α gene revealed the dermatophyte species N. praecox (formerly M. praecox). Comparison was made with the known sequences of in the NCBI-deposited N. praecox strains (M. praecox AJ970148.1 [3] and M. praecox/N. praecox U984573.1 [4]). Moreover, DNA sequences of the here described three wild strains of N. praecox matched 99% with the M. praecox/N. praecox isolate JX122206.1 [6]. Sequence analysis using the TEF 1-α gene revealed a 100% concordance of all three wild strains with at the NCBI-deposited sequences of M. praecox/N. praecox [10].
The phylogenetic tree or the dendrogram of N. praecox and further Nannizzia species is shown in Fig. 4. N. praecox—in the upper part of the dendrogram—can be clearly distinguished from N. persicolor, N. fulva and N. gypsea (bootstrap values were over 90%) [23]. Sequencing of the ITS region (Fig. 4a) and of TEF 1-α gene (Fig. 4b) of the DNA allowed clear discrimination of N. praecox from any other Nannizzia species.
Nannizzia praecox: Phylogenetic tree (dendrogram) based on sequencing of the ITS and TEF 1-α gene region, Mega5, statistical method: neighbour-joining, 1000 bootstrap replicates, bootstrap branch supports above 70%. NCBI—National Center for Biotechnology Information (NCBI), Bethesda, Maryland; CBS—Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; DSM—German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen) Braunschweig, Germany. a Sequencing of the ITS region, used dermatophyte strains. Nannizzia praecox: AJ970148.1—NCBI database, CBS 468.74; GU984573.1—NCBI database, 009.10 (Austria); 219101_16—University Clinics Gießen and Marburg, Prof. P. Mayser, Gießen, DSM strain number 109798; 214069_15—Laboratory for Medical Microbiology, Mölbis; DSM strain number 103445; 216135_16—Laboratory Dr. Stein + Colleagues, Mönchengladbach, DSM strain number 104497; JX122206.1—NCBI database, bM 137 (Switzerland). Nannizzia persicolor: KP132834.1—NCBI database, ISHAM-ITS: MITS2651 (accession number); 108934_16—Laboratory for Medical Microbiology, Mölbis; HG518405.1—NCBI database, CCF<CZE>: 4541; 112044_16—Laboratory for Medical Microbiology, Mölbis; 213059_16—Laboratory for Medical Microbiology, Mölbis. Nannizzia fulva: AJ00627.1—NCBI, CBS 287.55 (accession number); KP132451.1—NCBI ISHAM-ITS: MITS2051 (accession number); AM000035.1—NCBI, CBS 529.71 (accession number); 211784_16—Laboratory for Medical Microbiology, Mölbis; 212343_15—Laboratory for Medical Microbiology, Mölbis. Nannizzia gypsea: AJ853774.1—NCBI database, ISHAM-ITS: ID MITS2061 (accession number); NR_13127.1—NCBI database, CBS database strain number 258.61; 111388_15—Laboratory for Medical Microbiology, Mölbis; 213183_15—Laboratory for Medical Microbiology, Mölbis; 208435_16—Laboratory for Medical Microbiology, Mölbis; KP132459.1—NCBI database, ISHAM-ITS database MITS2060 (accession number). b Sequencing of the TEF 1-α gene region. Nannizzia persicolor: 108934_16—Laboratory for Medical Microbiology, Mölbis; 213059_16—Laboratory For Medical Microbiology, Mölbis; KM678092.1—NCBI database, CBS strain number 422.74; 112044_16—Laboratory for Medical Microbiology, Mölbis; KM678091.1—NCBI database, CBS strain number 421.74. Nannizzia fulva: KM678079.1—NCBI database, CBS strain number 287.55; 211784_16—Laboratory for Medical Microbiology, Mölbis. Nannizzia gypsea: 208435_16—Laboratory for Medical Microbiology, Mölbis; KM678161.1—NCBI database, CBS strain number 130820; KM678057.1—NCBI database, IFO 8228 (accession number). Nannizzia praecox: 214069_15—Laboratory for Medical Microbiology, Mölbis; DSM strain number 103445; 216135_16—Laboratory Dr. Stein + Colleagues, Mönchengladbach; DSM strain number 104497; 219103_16—University Clinics Gießen and Marburg, Prof. P. Mayser, Gießen; DSM strain number 109798; KM678080.1—NCBI database, CBS strain number 288.55
All three isolates (culture material) were deposited at the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen, DSMZ, https://www.dsmz.de/) at Braunschweig, Germany (Table 2).
The DNA sequences of the three strains have been deposited at the NCBI database (ITS and TEF 1-α gene) and additional at the database of the International Society for Human and Animal Mycology (ISHAM) (Table 2).
Discussion
Nannizzia (N.) praecox (Padhye, Ajello and McGinnis) Graeser and de Hoog, comb. nov., formerly known as M. praecox, a geophilic dermatophyte, is present in soil and equine environments. It was described first in 1954 by Rivalier as Sabouraudites praecox [24]. In 1978, the nomenclature changed to M. praecox [25]. However, in the new classification published in 2017 this geophilic species is now classified in the genus Nannizzia, as it is more remote from the human–environment [1].
Since 1954, less than 40 cases (Table 3) of human dermatophytosis due to N. praecox have been described in the literature, mainly from France [3, 4, 7,8,9,10,11,12,13,14, 22], Belgium [6] and the USA [5, 12]. The culture morphology of N. praecox, however, is not very specific. The white, slightly yellowish to beige-brown-stained granular and powdery surface might resemble T. mentagrophytes at the first look. By microscopic features, e.g. by the shape of macroconidia, the geophilic species N. gypsea (formerly M. gypseum) and N. fulva (formerly M. fulvum) should be excluded. The hair perforation test may be a useful tool, as it is negative in case of N. praecox, but positive with N. gypsea. In contrast, urease activity is not very valid as it is positive for most of the Microsporum and Nannizzia species. Therefore, molecular methods are currently considered as reference for species identification.
For these problems of identification in particular, with conventional methods, N. praecox might be underestimated in clinical medicine underlined by the fact that we were able to detect three cases in Germany within a period of 5 years.
In humans, N. praecox can cause tinea corporis as well as tinea capitis, the majority of cases being related to contact with horses (Table 3). In our first case, however, we were not successful with the isolation of N. praecox direct from horses which could be asymptomatic carriers. The geophilic N. praecox therefore might be more associated with equine environments (saddles, straw, stables, etc.), but data are sparse (2).
In circumscribed lesions, topical antifungal therapy is often successful. As the infections by geophilic fungi are often very inflammatory and pruritic, topical combination therapy with topical steroids might be of advantage in the first days. Extended and/or high inflammatory lesions as well as tinea capitis may require systemic antifungal therapy with terbinafine, griseofulvin or one of the azoles.
However, as eczema is the main differential diagnosis single use of corticosteroids as in our first case should be avoided. To confirm or to rule out a diagnosis of tinea direct microscopy of skin scrapings from the lesions is therefore an essential tool. For the identification of the aetiologic agent, phenotypic and genomic methods should be combined. If a direct or indirect contact to horses is reported in a case of dermatophytosis, T. verrucosum, T. mentagrophytes, T. equinum, T. bullosum and, in particular, N. praecox should be taken into consideration.
References
De Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31.
Alanio A, Romand S, Penso-Assathiany D, Foulet F, Botterel F. Microsporum praecox: molecular identification of a new case and review of the literature. Mycopathologia. 2011;171:61–5.
Badillet G, Castillo S, Ghouti C. Isolement à Paris de Microsporum praecox. Bull Soc Fr Mycol Med. 1978;7:199–304.
Badillet G, Rush-Munro FM, Ouaknine D. Quelques précisions sur Microsporum praecox. Bulletin de la Société Française de Mycologie Médicale. 1980;9:5–9.
Weitzman I, McMillen S. Isolation in the United States of a culture resembling M. praecox. Mycopathologia. 1980;70:181–6.
De Vroey C, Wuytack-Raes C, Fossoul F. Isolation of saprophytic Microsporum praecox Rivalier from sites associated with horses. Sabouraudia. 1983;21:255–7.
Basset M, Kremer M, Koenig H, Ball C, Ismail MT, Waller J. Les champignons de la peau et des phanéres en Alsace. Bull Soc Fr Mycol Med. 1984;13:329–32.
Peyron F, Lebeau B, Grillot R, Aguilaniu F, Goullier A, Badillet G. Epidermatophytie à Microsporum praecox e´voquant un eczema de contact. Bull Soc Fr Mycol Med. 1986;15:197–200.
Contet-Audonneau N, Boniatsi L, Teillac D, Bordigoni C, Cherrier P, Badillet G. Trois nouvelles souches de Microsporum praecox isole´es en France. Bull Soc Fr Mycol Med. 1988;17:159–62.
Phelippot R, Feuilhade de Chauvin M, Michel Y, Pietrini P, Teillac D, Boniatsi L, Badillet G. Microsporum praecox: apropos of 4 cases. Ann Dermatol Venereol. 1988;115:1154–6.
Avram A, Gauchy O, Blanchet P, Buot G. Trois cas de Dermatophytie à Microsporum praecox. Bull Soc Fr Mycol Méd. 1988;17:329–34.
Padhye AA, Detweiler JG, Frumkin A, Bulmer GS, Ajello L, McGinnis MR. Tinea capitis caused by Microsporum praecox in a patient with sickle cell anaemia. J Med Vet Mycol. 1989;27:313–7.
Badillet G. Dermatophytes et pseudo-dermatophytes. Biopathologiste. 1993;27:11–6.
Degeilh B, Contet-Audonneau N, Chevrier S, Guiguen C. A propos de trois nouveaux cas de dermatophytie à Microsporum praecox, revue des cas de la litterature des cas humains. J Mycol Med. 1994;4:175–8.
Buzina W, Ginter-Hanselmayer G. Microsporum praecox in Austria. Unpublished, submitted to NCBI. http://www.ncbi.nlm.nih.gov/genomes/MICROBES/microbial_taxtree.html, 21 June 2016, 20.00 Uhr
Mayser P, Handrick W, Nenoff P. Sport-assoziierte Dermatophytosen—ein Überblick. Hautarzt. 2016;67:680–8.
Nenoff P, Overbeck C, Uhrlaß S, Krüger C, Gräser Y. Tinea corporis due to the rare geophilic dermatophyte Microsporum praecox. Hautarzt. 2017;68(5):396–402.
De Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of clinical fungi. 4rd Edition, USB Version, Centraalbureau voor Schimmelcultures. Reus: Universitat Rovira i Virgili; 2014.
Land GA. The genus Microsporum. In: Kane J, Summerbell R, Sigler L, Krajden S, Land GA, editors. Laboratory handbook of dermatophytes. A clinical guide and handbook of dermatophytes and other filamentous fungi from skin, hair, and nails. Belmont: Star Publishing Company; 1997. p. 193–211.
Kanbe T, Suzuki Y, Kamiya A, Mochizuki T, Fujihiro M, Kikuchi A. PCR-based identification of common dermatophyte species using primer sets specific for the DNA topoisomerase II genes. J Dermatol Sci. 2003;32:151–61.
Wiegand C, Mugisha P, Mulyowa GK, Elsner P, Hipler UC, Gräser Y, Uhrlaß S, Nenoff P. Identification of the causative dermatophyte of tinea capitis in children attending Mbarara Regional Referral Hospital in Uganda by PCR-ELISA and comparison with conventional mycological diagnostic methods. Med Mycol. 2017;55(6):660–8.
Mirhendi H, Makimura K, de Hoog GS, Rezaei-Matehkolaei A, Najafzadeh MJ, Umeda Y, Ahmadi B. Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol. 2015;53:215–24.
Makimura K, Tamura Y, Mochizuki T, Hasegawa A, Tajiri Y, Hanazawa R, Uchida K, Saito H, Yamaguchi H. Phylogenetic classification and species identification of dermatophyte strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1999;37:920–4.
Rivalier E. Description de Sabouraudites praecox nova species suivie de remarques sur le genre Sabouraudites [Description of Sabouraudites praecox n.sp. and remarks on the genus Sabouraudites]. Ann Inst Pasteur. 1954;86:276–84.
Rivalier E. Microsporum praecox. Bulletin de la Société Française de Mycologie Médicale. 1978;7:297.
Acknowledgements
We thank Boris Coonen, Mönchengladbach, for careful assistance in microscopic and cultural characterization of the strains and for MALDI-TOF MS analysis. Daniela Winkens, Mönchengladbach, has done DNA sequencing of M. praecox strain number three. We are grateful to Uwe Schossig, photographer from Leipzig, for excellent macroscopic pictures of the fungal colonies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors including co-authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Uhrlaß, S., Mayser, P., Schwarz, R. et al. Dermatomycoses Due to Nannizzia praecox (Formerly Microsporum praecox) in Germany: Case Reports and Review of the Literature. Mycopathologia 183, 391–398 (2018). https://doi.org/10.1007/s11046-017-0213-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0213-x