Abstract
Stroke is the leading cause of adult disability in Westernized societies with increased incidence along ageing and it represents a major health and economical threat. Inactive lifestyle, smoking, hypertension, atherosclerosis, obesity and diabetes all dramatically increase the risk of stroke. While preventive strategies based on lifestyle changes and risk factor management can delay or decrease the likelihood of having a stroke, post stroke pharmacological strategies aimed at minimizing stroke-induced brain damage are highly needed. Unfortunately, several candidate drugs that have shown significant preclinical neuroprotective efficacy, have failed in clinical trials and no treatment for stroke based on neuroprotection is available today. Glucagon-like peptide 1 (GLP-1) is a peptide originating in the enteroendocrine L-cells of the intestine and secreted upon nutrient ingestion. The activation of the GLP-1R by GLP-1 enhances glucose-dependent insulin secretion, suppresses glucagon secretion and exerts multifarious extrapancreatic effects. Stable GLP-1 analogues and inhibitors of the proteolytic enzyme dipeptidyl peptidase 4 (DPP-4) (which counteract endogenous GLP-1 degradation) have been developed clinically for the treatment of type 2 diabetes. Besides their antidiabetic properties, experimental evidence has shown neurotrophic and neuroprotective effects of GLP-1R agonists and DPP-4 inhibitors in animal models of neurological disorders. Herein, we review recent experimental data on the neuroprotective effects mediated by GLP-1R activation in stroke. Due to the good safety profile of the drugs targeting the GLP-1R, we also discuss the high potential of GLP-1R stimulation in view of developing a safe clinical treatment against stroke based on neuroprotection in both diabetic and non-diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Stroke is the second most common cause of death and chronic disability in adults according to the World Health Organization (http://who.int/mediacentre/factsheets/fs310/en/). Approximately 85 % of all stroke cases are ischemic. Ischemic stroke results from the occlusion of a cerebral artery by a blood clot, which consequently results in irreversible neural tissue damage and various neurological impairments and disabilities. Although the relative rate of stroke deaths has decreased over the last decade, due to better clinical management, it still remains one of the most costly and devastating diseases in the World with annual direct and indirect healthcare costs estimated in hundreds of billions of US dollars [1].
Acute therapeutic interventions for stroke comprise chemical and surgical removal of the thrombotic clot [2, 3]. However these treatment strategies are most effective within 3–4.5 h after stroke and are not available for the vast majority of stroke patients, due to either late arrival to the hospital, delayed diagnosis, or contraindications (e.g. hypertension) [4–6]. Additionally, thrombolytic treatments increase the risk of hemorrhage, the incidence of which is typically elevated when patients have common stroke comorbidities, such as atrial fibrillation, hypertension and diabetes mellitus [7, 8].
The main goal of an ideal acute therapeutic intervention against stroke is to rescue endangered neural tissue. The thrombolytic treatments rely solely on their ability to restore the blood circulation to the stroke-affected brain areas, provide oxygen and nutrients to the tissue and let the tissue recover by itself. Although this approach is effective within a short time-window after the insult, the substantial portion of the injured tissue will remain damaged. There is also, like in the heart, the problem of reperfusion injury, proposed to be mediated by oxygen radicals, once an ischemic area rapidly becomes oxygenated upon clot dissolution [9].
The stroke-damaged tissue can be classified into two categories-ischemic core and the penumbra [10, 11]. Whereas the core area is irreversibly damaged as neurons in this area die before therapeutic intervention, the penumbra region surrounding the ischemic core can be salvaged by timely intervention and is most responsive to acute neuroprotective treatment in different preclinical paradigms [12, 13]. If no intervention takes place, the penumbra region will slowly progress into ischemic core [14]. The concept of pharmacological neuroprotection in stroke has been intensively studied during the past few decades aiming at reducing pharmacologically stroke-induced neuronal death in the penumbra region. The neural death mechanisms of stroke within the ischemic penumbra have been thoroughly characterized and many molecular targets have been identified that could induce neuroprotection after pharmacological intervention [15, 16]. This approach together with thrombolytic treatment could potentially yield the most favorable stroke outcome. However, the neuroprotective strategies that were very successful in animal models have failed to succeed in the clinic [17–19]. The main reasons for such failure might be the discrepancies in the timing of neuroprotective intervention between preclinical and clinical studies [20]. Additionally the dosage and routes of administration of candidate drugs between preclinical studies and the translational attempts have differed largely. Moreover, many stroke patients have comorbidities and conditions such as high age, hypertension, atherosclerosis, atrial fibrillation, obesity and diabetes mellitus [1]. These factors significantly impair stroke outcome in general and may also influence the outcome or the efficacy of neuroprotective agents [20]. Thus, future preclinical stroke research needs to focus on more closely modeling the potential clinical setting when evaluating the efficacy of neuroprotective substances. This can be accomplished by using animal models with common stroke comorbidities and by experimenting using clinically relevant pharmacological doses and treatment paradigms.
Recent preclinical stroke studies have identified neuroprotective properties mediated by several molecules targeting the glucagon-like peptide-1 receptor (GLP-1R) [21–25]. Interestingly, these substances also show significant neuroprotective effects in experimental stroke models when tested under comorbid conditions (e.g. obesity and diabetes) and at clinically relevant doses and routs of administration [26, 27]. Since some of these substances are already in clinical use for the treatment of type 2 diabetes (T2D), the time required to re-profiling them as means of anti-stroke neuroprotective intervention could likely be significantly reduced.
2 The GLP-1 system
2.1 GLP-1 physiology and function
GLP-1 is a small incretin peptide hormone, released from enteroendocrine intestinal L-cells which exerts numerous pleiotropic effects in various tissues [28]. The metabolically active forms of GLP-1 are GLP-1 (7–37) and GLP-1 (7–36) NH2 (see below). In contrast, while not metabolically active, the DPP-4 degradation product GLP-1 (9–36) appears to exert effects on the cardiovascular system [29].
Incretin hormones enhance meal-stimulated insulin secretion from pancreatic β-cells in a glucose-dependent manner [30] and accounts for 20 to 60 % of the overall postprandial insulin secretion in healthy subjects [31]. GLP-1 also decreases glucagon secretion from the pancreas in the presence of hyperglycemia, an effect that ceases at glucose levels < 4 mmol/l [32].
Defective GLP-1 secretion has been reported in T2D [33–35], indicated to result from defective secretion of the hormone and not from a transcriptional defect, as increased intestinal proglucagon mRNA has been observed [36, 37] . However, some studies have failed to show defective GLP-1 secretion in T2D, and a generalized association of defective GLP-1 secretion and T2D as such cannot be made [38]. Decreased levels of circulating GLP-1 in response to meal ingestion have been linked to impaired glucose tolerance [39, 40] and increasing body mass index (BMI) [39]. Further, decreased GLP-1 levels have been observed with increased BMI and obesity in patients with or without T2D [41], where defective GLP-1 secretion has also been correlated to insulin resistance [42]. These data are further supported by the observation of defective GLP-1 secretion in T2D but not in type 1 diabetes [43].
Plasma GLP-1 levels rise within 10–15 min of food ingestion and reach peak levels of 15–50 pmol/l by 40 min [44]. Further, GLP-1 -- like insulin-is secreted in a pulsatile manner with a frequency comparable to that of pancreatic hormones [45].
2.2 GLP-1R activation pathways
The GLP-1R was first isolated by transient expression of a rat pancreatic islet cDNA library into COS cells followed by binding of radiolabeled GLP-1 [46]. The gene for the human GLP-1R is localized to chromosome 6p21 [47], and the receptor consists of a functional, 463 amino-acid, G-protein-coupled receptor with a seven-span trans-membrane domain to which GLP-1 and its analogues bind [48, 49]. GLP-1R is expressed in a wide range of tissues including the pancreatic islets, lung, kidney, heart, vessels, stomach, intestine, pituitary, skin and several regions in the brain [50]. For the latter, a high expression is demonstrated in hypothalamus and the brain stem [51], areas known to be involved in the central control of cardiovascular function [52, 53]. Intriguingly, cardiovascular actions by GLP-1R agonists have been observed in GLP-1R−/− mice and by blocking the receptor with the GLP-1R inhibitor exendin (9–39) [29]. This reflects a non-classical action from GLP-1 actions partly due from the split product GLP-1 (9–36) without any affinity to the GLP-1R [29]. There are also reports of physiological actions on liver, skeletal muscle and fat cells by GLP-1, tissues that lack of expression of GLP-1R. One explanation for this complexity might be due to a not yet known second GLP-1R [54]. Importantly, it was recently shown that evidence for the presence of GLP-1R is ambiguous due to the low sensitivity and specificity of GLP-1R antisera commonly used to show the presence of the GLP-1R [55]. Thus, there is a high need for validated methods detecting the GLP-1R [54].
The signal transduction pathways of GLP-1 and its analogues in the pancreatic β-cell are mediated through adenylate cyclase and the cAMP/PKA pathways to potentiate glucose-induced closure of ATP-sensitive K+ channels, thereby generating cellular depolarization, activation of voltage-dependent Ca2+ channels (VDCCs) and influx of Ca2+ that sets in motion insulin exocytosis [48]. GLP-1 may also directly stimulate entry of Ca2+ into the cell through activation of dihydropyridine-sensitive VDCCs [56], and by mobilizing Ca2+ from intracellular stores [57]. GLP-1R agonists also evoke several other biological actions in the rodent pancreas, including enhanced β-cell proliferation, neogenesis and inhibition of apoptosis, thereby increasing pancreatic β-cell mass [58–61]. These signaling pathway actions evoked by GLP-1 and its analogues are the same as for other tissues, since they regulate proliferation, neogenesis and anti-apoptotic actions in endothelial [62, 63] and neuronal cells [64].
GLP-1 and the GLP-1R agonist exenatide can both pass the blood brain barrier (bbb [51]). Whether other GLP-1 analogues can enter the brain remains elusive. On the other hand, indirect mechanisms may also contribute to CNS beneficial effects from GLP-1R activation. Interestingly, a role for central nervous system GLP-1R signaling in the control of glucose homeostasis, as well as blood pressure and heart rate, has been proposed by peripheral communication with the brain activating sensory afferent vagal neurons [52, 65, 66]. Some earlier studies clearly demonstrate that GLP-1 and its analogues protect against neuronal cell damages through the adenylate cyclase and the cAMP/PKA pathways that are activated by GLP-1 also in in the pancreatic β-cell [67]. However, more results are needed in order to characterize the molecular mechanisms at the basis of GLP-1R activation in neural cells.
2.3 GLP-1 mimetics/GLP-1R agonists
Due to the very short half-life of GLP-1, caused by DPP-4 degradation, GLP-1R agonists resistant to degradation have been developed. These subcutaneously administered compounds exert a transient -- predominantly prandial -- rise in insulin secretion and a suppression of glucagon, both of which are glucose dependent and therefore carry a low risk of hypoglycemia [68]. Here below we will briefly discuss the main features of these molecules, including clinical aspects in terms of glycemic control, cardiovascular parameters and tolerability.
2.3.1 Exendin-4
Exendin-4 (Ex-4) was originally found in the saliva of the lizard Heloderma suspectum [69]. It shares a 53 % amino acid sequence homology with that of native GLP-1, giving it a circulating plasma half-life of 60–90 min [70]. Ex-4 was the first GLP-1R agonist, resistant to DPP-4 degradation, developed in clinical use as exenatide (synthetic form of Ex-4) for the treatment of T2D [71]. In clinical trials with T2D patients, addition of exenatide on top of metformin, sulfonylurea or both reduces HbA1c with 0.8-1.0 % and gives a weight reduction of 0.9 - 2.5 kg in overweight patients [72–74]. Furthermore, exenatide has been associated with beneficial effects on systolic blood pressure [52] and found to reduce total cholesterol and LDL cholesterol [75–77]. The most common side effects of the exenatide treatment are nausea (8-44 %) and vomiting (4-13 %) [78, 79]. In a large retrospective study, patients treated with exenatide were found to be less likely to have cardiovascular disease (CVD) events than those not treated with exenatide [80]. Interestingly, CVD was in this study defined as a composite endpoint including ischemic stroke [80].
2.3.2 Other GLP-1R agonists
Liraglutide is a GLP-1 analogue with ~ 97 % homology to the native GLP-1 [81, 82] that binds to albumin with a plasma half-life of 10–14 [81, 82]. Liraglutide reduces both fasting and postprandial glycemia. The Liraglutide Effect and Action in Diabetes (LEAD) program was designed to compare the efficacy and tolerability of once-daily liraglutide alone or in combination with other commonly used oral agents, for T2D [83–86]. In these studies, reductions of HbA1c of 1.0-1.5 % were observed with liraglutide [83–86]. The incidence of nausea caused by liraglutide is estimated to 8-35 % and vomiting affects 7-12 % of the treated patients [87, 88]. Liraglutide also improves surrogate measures of β-cell function, reduces systolic blood pressure and has been associated with a mean weight loss up to 3.2 kg after 26 weeks of treatment [83–86].
Lixisenatide is the most recent GLP-1R agonist to be approved by the European Medicines Agency [89, 90]. Dulaglutide and Albiglutide are long-acting once weekly administered GLP-1R agonists in development but not yet approved for clinical use [91, 92].
2.4 DPP-4 inhibitors
DPP-4 is a 766 amino acid, dimeric, transmembrane glycoprotein and ubiquitously expressed enzyme that plays major roles in metabolism, in the immune and endocrine systems, but also in cancer growth and cell adhesion. There is also a soluble form of DPP-4, which originates from membrane shedding. Known also as adenosine deaminase complexing protein 2 or CD26, DPP-4 is a serine aminopeptidase enzyme that inactivates the incretin hormones GLP-1 and GIP -- as well as several other peptides -- via dipeptide cleavage of the penultimate N-terminal amino acid [82]. Inhibition of DPP-4 increases the half-life and bioavailability of active incretin hormones [93].
The inhibitors of DPP-4 currently available in Europe or US for the treatment of T2D include vildagliptin, sitagliptin, saxagliptin, linagliptin and alogliptin.
Inhibitors of DPP-4 exert their most overt pharmacological effects in humans through their inhibition of GLP-1 degradation. In doing this, they mimic some of the effects of GLP-1 and GLP-1 analogs, including stimulation of insulin secretion, inhibition of glucagon secretion, and enhancement of β-cell mass (the latter only shown in animal models) [82]. Conversely, DPP-4 inhibitors seem to have only a marginal slowing effect on the rate of gastric emptying and no obvious effect on satiety or weight loss [82]. Although DPP-4 inhibition has been associated with an enhancement of β-cell survival and neogenesis, e.g. in streptozotocin-treated diabetic rats [94], this effect has not yet been demonstrated in humans. DPP-4 substrates also include several proline or alanine containing peptides, such as growth factors, chemokines, neuropeptides and vasoactive peptides [94–96]. Inhibition of the DPP-4 enzyme also modulates the activity of several cardioactive factors, neuropeptide Y and stromal cell derived factor-1 (SDF-1) [94]. Finally, as for the GLP-1R agonists, DPP-4 inhibitors-mediated effects via the vagus nerve have been reported [97]. In contrast to GLP-1 analogs which are all injectable drugs, DPP-4 inhibitors have an oral application route.
3 GLP-1R activation for the treatment of stroke
3.1 Exendin-4 for the treatment of stroke
GLP-1R is broadly expressed in the adult brain [98, 99], with its main expression in neurons [22, 24, 26, 100]. In addition, adult neural stem cells/progenitors are positive for GLP-1R [101]. Glia cells seem not to express GLP-1R unless following inflammation [102], in response to stroke [24] or to a mechanical lesion [103]. In the past few years, several studies have provided proof of concept data showing GLP-1R-mediated neuroprotection in animal models of neurological disorders. The topic of this review specifically focuses on GLP-1R and stroke, although excellent reviews extending to other neurological brain disorders/diseases have been recently published [104–107].
Within the stroke research field, experimental evidence supporting the potential use of the GLP-1R agonist Ex-4 as therapeutic is growing. The first work showing that the activation of GLP-1R by Ex-4 led to neuroprotection against stroke was provided by Li et al. in 2001 [21]. In this work, the authors demonstrated that intracerebral administration of Ex-4 decreased the infarct size and enhanced locomotor activity at 48 h after a stroke in the rat. The authors also showed that the effect was indeed mediated by GLP-1R since Ex-4 was ineffective in GLP-1R knock-out mice. Ex-4 has been shown to pass the bbb [108] and Teramoto et al. recently showed that peripheral administration of Ex-4 could induce neuroprotection against stroke in rodents [22]. The authors demonstrated that intravenous Ex-4 at the time of stroke or 1 h after stroke onset reduced the infarct volume and improved functional deficit in the mouse and that the effect was independent of glycemic regulation. The authors could not detect infarct volume reduction when Ex-4 was given at 3 h after stroke. The results of this work are robust. However, the doses employed in this study (approx. 400 μg/kg) are far higher than the used clinical dose of Ex-4 to treat T2D (0.1 μg/kg). To study the potential efficacy of Ex-4 against stroke, a transient cerebral ischemia model targeting the CA1 region of the hippocampus in the gerbil was also employed [24]. Ex-4 delivered 2 h before stroke (intraperitoneally at the dose of 1–3 μg/kg) decreased brain damage and microglia activation. However, no effect was shown at the dose 0.3 μg/kg. The authors also showed that Ex-4 treatment transiently enhanced GLP-1R expression in CA1 hippocampal neurons 24 h after stroke. GLP-1R expression decreased at 4 days after stroke and then increasing again several days after stroke; this time with its main localization in astrocytes and interneurons. Whether these cells can play a role in the protective effect against stroke by Ex-4 remains to be investigated.
In an attempt to mimic the clinical situation of a diabetic patient at high risk of stroke, our research group showed that 4 weeks pretreatment with Ex-4 before transient MCAO followed by another 4 weeks of Ex-4 treatment decreased the stroke-induced brain damage in T2D rats [26]. Ex-4 was administered intraperitoneally and it induced a dose-dependent effect already significant at the clinical dose (0.1 μg/kg). However, the protective effect was stronger at 1 μg and 5 μg/kg. Briyal et al. employed a similar strategy based on chronic pretreatment in non-diabetic rats in a model of permanent MCA occlusion [25]. The results showed that intraperitoneal Ex-4 at the dose of 0.5 μg/kg was efficacious in reducing infarct volume and oxidative stress parameters as well as in improving neurological deficits.
Not all effects of GLP-1R activation, however, may necessarily be beneficial for the ischemic brain. The reduction in glucagon, with the attendant decrease in ketosis, may cause detriment as it will likely reduce neuronal tolerance to hypoxia. Notwithstanding this, the majority of effects conveyed through GLP-1R activation would be expected to promote neuroprotection.
In conclusion, extensive preclinical work has been performed showing proof of concept of Ex-4 for the acute treatment of stroke. However, further experimental evidence is needed by using other GLP-1R agonists. This point is of particular importance since not all the effects mediated by Ex-4 seem to occur through the canonical GLP-1R [29]. Interestingly in a study published meantime this review was in progress, Sato et al. showed that liraglutide administered 90 min after stroke reperfusion induced neuroprotection in the rat [109].
The neuroprotective effect of Ex-4 seems to occur independently of the glycemic effects of the drug. However, the molecular mechanisms at the basis of Ex-4-mediated neuroprotection are still largely unknown and will need to be determined. Furthermore, the Ex-4 neuroprotective window, as well as the most efficacious doses and route of administration in relation to anti-stroke efficacy, remain to be determined. Needless to say, clinical work is needed to assess the safety of a potential Ex-4 treatment in stroke patients (some studies are ongoing, see https://www.clinicaltrialsregister.eu/ctr-search/search?query = exenatide + stroke). Interestingly, a recent clinical feasibility study in eleven stroke patients with a history of diabetes showed that exenatide treatment seemed safe and tolerable and did not cause any serious adverse events but mild nausea and vomiting [110].
GLP-1 receptor activation has been reported to be beneficial for behavioral recovery and to improve learning and memory in animal models of neurodegenerative disorders [111]. Furthermore, GLP-1R activation stimulates brain regeneration in normal rodents [101] as well as in response to neurodegeneration [64] or stroke [26]. Finally, GLP-1R activation promotes synaptic plasticity, neurite outgrow and rearrangement [112, 113], which are all important factors for stroke recovery [114]. Altogether, these data suggest a potential use of a GLP-1R-mediated therapy to also treat patients in the long-term recovery phase after stroke. In this perspective, experimental evidence is almost entirely lacking and -- consequently -- future preclinical work is urgently needed. Considering the proliferative action of GLP-1R activation, careful surveillance of any oncogenic or growth-promoting effects of preneoplastic lesions in the regenerating tissue is highly warranted.
3.2 DPP-4 inhibitors for the treatment of stroke
As with GLP-1R agonists, there is growing evidence showing neuroprotective effects mediated by DPP-4 inhibitors. However, it is still unclear whether this is secondary to their effects on blood glucose control or by independent mechanisms [115, 116]. Expression of DPP-4 in the human brain was originally thought to be limited [117]. However, tissue specific investigation found strong DPP-4 protein expression in cortical astrocytes via monoclonal antibodies and immunohistochemistry [118]. DPP-4 is also expressed on the endothelium of the bbb and data from animal studies support possible direct effects of DPP-4 inhibitors on the endothelium and vascular tissue [119]. These effects appear to be independent of the glucose-lowering properties of DPP-4 inhibitors and specific inhibitor properties may differ between the different molecules. For example, linagliptin has shown pleiotropic antioxidant and anti-inflammatory properties not shared by other DPP-4 inhibitors [120]. There is evidence for an association of inflammation and expression of proline- and alanine-specific proteases (such as DPP-4, DPP-8 and aminopeptidase N) within the brain following ischemic episodes [121]. Inhibition of these enzymes with the dual DPP-4/APN inhibitor, IPC1755, (introduced to the intracerebral-ventricular space), reduces cortical lesions after transient cerebral ischemia in rat models [121]. It was also observed that 7 days (but not 3 days) after unilateral cerebral ischemia DPP-4 activity was significantly up-regulated but only in cortex ipsilateral to stroke. Infarct size was also reduced by sitagliptin, although to a lesser extent than with IPC1755.
Under high-fat diet (HFD) feeding, shown to cause systemic and neuronal insulin resistance as well as brain mitochondrial dysfunction in rats, DPP-4 inhibition with vildagliptin counteracted neuronal insulin resistance, brain mitochondrial dysfunction and learning and memory deficit [122]. In a similar study, both sitagliptin and vildagliptin significantly improved metabolic parameters and decreased brain oxidative stress levels. In addition, both drugs completely prevented brain and hippocampal mitochondrial dysfunction and improved HFD-impaired learning behaviors [123].
The potential anti-stroke efficacy of the DPP-4 inhibitor alogliptin (7.5, 15 and 30 μg/kg) was determined after 3 weeks pretreatment before inducing stroke in normal mice [23]. The results showed that infarct size and neurological deficits were significantly reduced in one of the dose groups (15 μg). The neuroprotective efficacy of alogliptin was associated with an increase in the potent neuroprotectant brain-derived neurotrophic factor (BDNF). Of note, efficacy when the treatment started after stroke was not achieved. Our research group showed that 4 weeks pretreatment with clinical doses of the DPP-4 inhibitor linagliptin in T2D and obese mice was associated with reduced neuronal loss [27]. Although these effects were accompanied by increased GLP-1 plasma levels, they appeared to be independent of glycemic control. In this model, neuronal salvage in T2D mice was greater in linagliptin-treated animals than in mice treated with the sulfonylurea, glimepiride, even though linagliptin provided less effective glycemic control. In contrast, linagliptin and glimepiride demonstrated similar neuroprotective effects in normal mice. Together, these data provide thought-provoking supportive evidence for benefits of exposure to DPP-4 inhibitors in stroke. However, the underlying mechanisms responsible for these effects remain unclear. Unlike GLP-1 and some GLP-1 analogues, DPP-4 inhibitors do not cross the blood brain barrier. Therefore these neuroprotective effects are likely to be related to their systemic actions or through the vagus nerve (see below) rather than actions directly on the CNS. On the other hand, stroke-mediated damage has been reported to increase the permeability of the bbb and further studies aimed to investigate potential neuroprotective effect of DPP-4 inhibitors will be needed to clarify this important aspect. Although these actions might be expected to be through effects on GLP-1 and GIP, there are many other substrates for DPP-4 with neuroprotective properties (see above), some of which (e.g. SDF1α) are even more sensitive to DPP-4 than GLP-1 and GIP [96] .
With regard to clinical trials, meta-analysis data suggest that there may be a lower risk of major cardiovascular events, including stroke, in diabetic patients receiving DPP-4 inhibitor therapy than comparator drugs [124, 125]. However, the findings of these studies are equivocal and the results are largely limited by the short duration of studies and low number of events in addition to their non-uniform and incomplete determination of cardiovascular endpoints. A larger 2-year efficacy and safety study comparing linagliptin with glimepiride in patients with T2D inadequately controlled on metformin suggest that linagliptin has a beneficial action on cardiovascular outcomes [126]. However, no superiority of DPP-4 inhibition on cardiovascular endpoints (such as CV death and non-fatal stroke) was apparent from results of two recently published large cardiovascular outcome trials for saxagliptin [127] or alogliptin [128]. It remains to be shown by the up-coming CV outcome trials of sitagliptin (http://clinicaltrials.gov/ct2/results?term = TECOS) and linagliptin (http://clinicaltrials.gov/ct2/show/NCT01243424?term = CARdiOvascular + Safety + of + LINAgliptin & rank = 1 ) whether DPP-4 inhibitors may lower stroke incidence or rather improve stroke outcome. Furthermore, future preclinical and clinical studies are needed to clarify whether there are neuroprotective differences among different DPP-4 inhibitors.
4 Potential mechanisms at the basis of GLP-1R activation against stroke
In the past few years it has been broadly demonstrated that the activation of GLP-1R can produce beneficial effects in several animal models of neurological disorders including stroke. Clinical studies are under way and will be essential to understand whether new therapies can be developed by using this strategy (see above). However, the molecular mechanisms at the basis of GLP-1R-mediated neuroprotection are still largely unknown. Also, it is not known whether neuroprotection mediated by GLP-1R agonists and DPP-4 inhibitors occur through the same mechanism of action. Recently it has been proposed that at least part of the effects of GLP-1R agonists and DPP-4 inhibitors can occur through the activation of the vagus nerve (see above and refs [52, 65, 66, 129–133]). Indeed, recent work showed that the effect of exogenous GLP-1 on food intake is lost in vagotomized subjects [134]. Interestingly, neuroprotective effects against stroke via the vagus nerve have been reported in the past few years (reviewed by Cheyuo et al.[135]) suggesting that vagus nerve stimulation by GLP-1R activation could indirectly mediate neuroprotection in the CNS. This type of mechanism could even more likely occur following a treatment based on DPP-4 inhibitors since low doses of these drugs have been shown to reduce intestinal, but not systemic, DPP-4 activity and to increase vagal nerve activity [97].
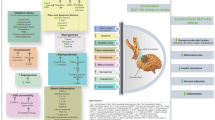
GLP-1R-mediated neuroprotection could occur as summarized in Fig. 1. We hypothesize that pharmacological doses of GLP-1R agonists with the ability to pass the bbb could stimulate neuroprotection both directly and indirectly via the vagus nerve. On the contrary, DPP-4 inhibitors not crossing the bbb would unlikely increase GLP-1 levels in the brain. Also, DPP-4 inhibitors would unlikely provide high levels (in nM range) of systemic active GLP-1 able to pass the bbb to exert direct neuroprotection. Thus, DPP-4 inhibitors could mediate neuroprotection mainly through the activation of the vagus nerve or independently of GLP-1 by regulating other factors (see above).
Potential neuroprotective mechanisms induced by GLP-1R agonists and DPP-4 inhibitors. GLP-1R agonists and DPP-4 inhibitors may have a variety of targets in the brain including neuronal cells, different glia cells and neural stem cells. Evidence is accumulating that pharmacological concentrations of GLP-1R agonists could induce both direct neuroprotection from the systemic circulation (via the bbb; red line) and indirectly by stimulating the vagus nerve that via the nucleus of the tractus solitaries (NTS) projects to the forebrain (blue line). Alternatively, increased GLP-1 levels by DPP-4 inhibitors in the intestine and in the portal vein could stimulate mainly the vagus nerve (blue line) thus inducing neuroprotection indirectly. DPP-4 inhibitors could also modulate systemically the activity of other neuroprotective factors (red line). The mechanisms of action of GLP-1R agonists and DPP-4 inhibitors are discussed in more detail in the text
Important preclinical studies will be needed in the near future to test these hypotheses thus developing our knowledge within this very interesting research area.
5 Conclusions and future directions
Increasing experimental evidence suggests that the treatment with GLP-1R agonists and DPP-4 inhibitors induces beneficial neuroprotective effects against stroke. A number of these molecules are already in clinical use for the treatment of T2D. Thus, their potential repositioning into also anti-stroke treatments would present several advantages since these molecules are rather safe and well tolerated.
The molecular mechanisms at the basis of GLP-1R-mediated neuroprotection are still largely unknown and future preclinical efforts aimed at identifying these mechanisms are needed in this respect. In particular, studies aimed at determining whether GLP-1R agonists/mimetics and DPP-4 inhibitors act via the same mechanism of action are highly required.
In conclusion, the results of preclinical studies addressing the molecular mechanisms at the basis of GLP-1R-mediated neuroprotection and of on-going clinical studies will be crucial in the near future to understand whether a therapy based on GLP-1R activation can be translated into the clinic.
References
Go AS et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245.
Taqi MA et al. Past, present, and future of endovascular stroke therapies. Neurology. 2012;79(13 Suppl 1):S213–20.
Diener HC et al. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol. 2013;12(7):677–88.
Herlitz J et al. Early identification and delay to treatment in myocardial infarction and stroke: differences and similarities. Scand J Trauma Resusc Emerg Med. 2010;18:48.
Crimmins DS et al. Acute stroke and transient ischaemic attack management–time to act fast. Intern Med J. 2009;39(5):325–31.
Kurz MW, Kurz KD, Farbu E. Acute ischemic stroke–from symptom recognition to thrombolysis. Acta Neurol Scand Suppl. 2013;196:57–64.
Whiteley WN et al. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43(11):2904–9.
Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10.
Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17(11):1391–401.
Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–65.
Macdonald RL, Stoodley M. Pathophysiology of cerebral ischemia. Neurol Med Chir (Tokyo). 1998;38(1):1–11.
Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62(4):329–39.
Liu R et al. Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurol Res. 2012;34(4):331–7.
Heiss WD. The ischemic penumbra: How does tissue injury evolve? Ann N Y Acad Sci. 2012;1268:26–34.
Sacco RL et al. Experimental treatments for acute ischaemic stroke. Lancet. 2007;369(9558):331–41.
O'Collins VE et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–77.
Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1(1):36–45.
Endres M et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25(3):268–78.
Gladstone DJ et al. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–36.
Sena E et al. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30(9):433–9.
Li Y et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–90.
Teramoto, S., et al., Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab, 2011.
Yang D et al. Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res. 2013;1517:104–13.
Lee CH et al. Ischemia-Induced Changes in Glucagon-Like Peptide-1 Receptor and Neuroprotective Effect of Its Agonist, Exendin-4, in Experimental Transient Cerebral Ischemia. Journal of Neuroscience Research. 2011;89(7):1103–13.
Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012;1427:23–34.
Darsalia V et al. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond). 2012;122(10):473–83.
Darsalia V et al. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes. 2013;62(4):1289–96.
Gupta, V., Pleiotropic effects of incretins. Indian J Endocrinol Metab, 2012. 16 (Suppl1): p. S47-56.
Ban K et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–50.
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117(1):24–32.
Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21(2):91–117.
Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–66.
Mannucci E et al. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with Type 2 diabetes mellitus. Diabet Med. 2000;17(10):713–9.
Vilsboll T et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–13.
Lugari R et al. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (non insulin-dependent) diabetes. Horm Metab Res. 2002;34(3):150–4.
Jin T. Why diabetes patients are more prone to the development of colon cancer? Med Hypotheses. 2008;71(2):241–4.
Yi F et al. Cross talk between the insulin and Wnt signaling pathways: evidence from intestinal endocrine L cells. Endocrinology. 2008;149(5):2341–51.
Ahren B, Carr RD, Deacon CF. Incretin hormone secretion over the day. Vitam Horm. 2010;84:203–20.
Toft-Nielsen MB et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86(8):3717–23.
Nathanson D et al. Reduced plasma levels of glucagon-like peptide-1 in elderly men are associated with impaired glucose tolerance but not with coronary heart disease. Diabetologia. 2010;53(2):277–80.
Muscelli E et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57(5):1340–8.
Rask E et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24(9):1640–5.
Vilsboll T et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–13.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Balks HJ et al. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82(3):786–90.
Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89(18):8641–5.
Stoffel M et al. Human glucagon-like peptide-1 receptor gene. Localization to chromosome band 6p21 by fluorescence in situ hybridization and linkage of a highly polymorphic simple tandem repeat DNA polymorphism to other markers on chromosome 6. Diabetes. 1993;42(8):1215–8.
Goke R et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268(26):19650–5.
Thorens B et al. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42(11):1678–82.
Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20(6):876–913.
Orskov C et al. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45(6):832–5.
Barragan JM et al. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277(5 Pt 1):E784–91.
Yamamoto H et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52.
Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor–or not? Endocrinology. 2013;154(1):4–8.
Panjwani N et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE (−/−) mice. Endocrinology. 2013;154(1):127–39.
Fehmann HC et al. Ligand-specificity of the rat GLP-I receptor recombinantly expressed in Chinese hamster ovary (CHO-) cells. Z Gastroenterol. 1994;32(4):203–7.
Holz, G.G.t., W.M. Kuhtreiber, and J.F. Habener, Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37). Nature, 1993;361:(6410): p. 362–5.
Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435(5):583–94.
Lu M et al. The role of the free cytosolic calcium level in beta-cell signal transduction by gastric inhibitory polypeptide and glucagon-like peptide I (7–37). Endocrinology. 1993;132(1):94–100.
Xu G et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–6.
Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37.
Erdogdu O et al. Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI3K, eNOS, p38 MAPK, and JNK pathways. J Mol Endocrinol. 2013;50(2):229–41.
Erdogdu O et al. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325(1–2):26–35.
Parthsarathy V, Holscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One. 2013;8(3):e58784.
Baraboi ED et al. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1011–24.
Knauf C et al. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57(10):2603–12.
Holscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26(10):871–82.
Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur J Endocrinol. 2009;160(6):909–17.
Raufman JP et al. Actions of Gila monster venom on dispersed acini from guinea pig pancreas. Am J Physiol. 1982;242(5):G470–4.
Kolterman OG et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62(2):173–81.
AMYLIN PHARMACEUTICALS, I.B.e.i.p.i.A.P., Inc. San Diego (CA) USA . 2005.
Buse JB et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–35.
DeFronzo RA et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–100.
Kendall DM et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–91.
Blevins T et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–10.
Diamant M et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375(9733):2234–43.
Drucker DJ et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–50.
Byetta: exenatide injection [package insert]. San Diego, C., Amylin Pharmaceuticals, 2005. Available from http://pi.lilly.com/us/byetta-pi.pdf., Assessed 27 September 2010.
Moretto TJ et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30(8):1448–60.
Best JH et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–5.
Agerso H et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45(2):195–202.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Buse JB et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47.
Garber A et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81.
Russell-Jones D et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55.
Zinman B et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care. 2009;32(7):1224–30.
Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther. 2009;31(11):2472–88.
Victoza: liraglutide (rDNA origin) injection [package insert]. Princetown, N., Novo Nordisk, 2010. Available from http://www.victozapro.com/pdf/Victoza_ComboPI_5.24.pdf. Assessed 27 September 2010, 2010.
Christensen M, Knop FK. Once-weekly GLP-1 agonists: How do they differ from exenatide and liraglutide? Curr Diab Rep. 2010;10(2):124–32.
Werner U. Preclinical pharmacology of the new GLP-1 receptor agonist AVE0010. Ann Endocrinol (Paris). 2008;69(2):164–5.
Jimenez-Solem E et al. Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther. 2010;12(6):790–7.
St Onge, E.L. and S.A. Miller, Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Expert Opin Biol Ther, 2010;10:(5): p. 801–6.
Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60(11):1454–70.
Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–43.
Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55(1–3):10–6.
Lambeir AM et al. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209–94.
Waget A et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152(8):3018–29.
Goke R et al. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7(11):2294–300.
Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–80.
Hamilton, A. and C. Holscher, Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport, 2009.
Bertilsson G et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86(2):326–38.
Iwai T et al. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–60.
Chowen JA et al. Increased glucagon-like peptide-1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides. 1999;33(3):212–5.
Holscher, C., Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol, 2013.
Harkavyi A, Whitton PS. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol. 2010;159(3):495–501.
Salcedo I et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. British Journal of Pharmacology. 2012;166(5):1586–99.
Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin. 2011;27(3):547–58.
Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313–8.
Sato K et al. Neuroprotective effects of liraglutide for stroke model of rats. Int J Mol Sci. 2013;14(11):21513–24.
Daly SC et al. Exenatide in acute ischemic stroke. Int J Stroke. 2013;8(7):E44.
McIntyre RS et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res. 2013;237:164–71.
Luciani P et al. Differentiating effects of the glucagon-like peptide-1 analogue exendin-4 in a human neuronal cell model. Cell Mol Life Sci. 2010;67(21):3711–23.
Perry T et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300(3):958–66.
Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72.
Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33(2):187–215.
Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res. 2012;9(2):109–16.
Abbott CA et al. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40(5):331–8.
Mentzel S et al. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44(5):445–61.
Takasawa W et al. Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem Biophys Res Commun. 2010;401(1):7–12.
Kroller-Schon S et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 2012;96(1):140–9.
Rohnert P et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J Neuroinflammation. 2012;9:44.
Pipatpiboon N et al. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37(5):839–49.
Pintana, H., et al., DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin resistant rats. J Endocrinol, 2013.
Johansen OE et al. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: A pre-specified, prospective, and adjudicated meta-analysis of a Phase 3 programme. Cardiovasc Diabetol. 2012;11(1):3.
Monami M et al. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27 Suppl 3:57–64.
Gallwitz B et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83.
Scirica, B.M., et al., Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med, 2013.
White, W.B., et al., Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med, 2013.
Nauck MA et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273(5 Pt 1):E981–8.
Tolessa T et al. Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig Dis Sci. 1998;43(10):2284–90.
Wettergren A et al. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7–36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut. 1997;40(5):597–601.
Wishart JM et al. Relation between gastric emptying of glucose and plasma concentrations of glucagon-like peptide-1. Peptides. 1998;19(6):1049–53.
Burcelin R. The gut-brain axis: a major glucoregulatory player. Diabetes Metab. 2010;36 Suppl 3:S54–8.
Plamboeck A et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1117–27.
Cheyuo C et al. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31(5):1187–95.
Acknowledgments
Work in our laboratories is supported by grants from the Novo Nordisk foundation, the European Foundation for the Study of Diabetes (EFSD)/Lillly, Diabetesfonden, the Swedish Heart and Lung Foundation, AFA Insurance, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Diabetes Research & Wellness Foundation, Avtal om Läkarutbildning och Forskning (ALF) and by the foundations Magnus Bergvall, Fredrik and Ingrid Thuring, Axel and Signe Lagerman’s Donation, Loo and Hans Osterman, Stohne, Åhlén, STROKE Riksförbundet, Tornspiran, Gamla Tjänarinnor and Syskonen Svensson.
Conflict of interest
Work in CP’s laboratory is supported by Boehringer Ingelheim Pharma GmbH & Co. Thomas Klein is an employee of Boehringer Ingelheim Pharma GmbH & Co. ÅS has received research grants, consultancy fees, lecture honoraria, and fees for expert testimony from most companies involved in incretin-based therapy, and is on the national/Nordic/European/global advisory boards of Eli Lilly, Merck, Boehringer Ingelheim, AstraZeneca, Sanofiaventis, and Novartis. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darsalia, V., Nathanson, D., Nyström, T. et al. GLP-1R activation for the treatment of stroke: Updating and future perspectives. Rev Endocr Metab Disord 15, 233–242 (2014). https://doi.org/10.1007/s11154-014-9285-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-014-9285-9