Abstract
Background
The behavior of Abscisic acid (ABA) as a stress phytohormone may be involved in mechanisms leading to tolerance and survival in adverse environmental conditions such as drought stress.
Methods
Here, we evaluated ABA-mediated responses at physio-biochemical and molecular levels in drought-stressed seedlings of two different Desi-type chickpea genotypes (10 as a tolerant genotype and 247 as a sensitive one).
Results
Under drought stress, two chickpea genotypes showed a decrease in their relative water content (RWC), and the intense decrease was related to the sensitive genotype (73.9%) in severe stress. Hydrogen peroxide (H2O2) and malondialdehyde (MDA) concomitant with the severity of stress increased in genotypes and the higher increase was in the sensitive genotype (5.8-fold and 3.43-fold, respectively). In the tolerant genotype, the enhanced accumulation of total phenolic content (1.75-fold) and radical scavenging action, based on 1,1-diphenyl-2-picrylhydrazyl test (DPPH), (1.69-fold) were simultaneous with ABA accumulation (1.53-fold). In the tolerant genotype, transcriptional analysis presented upregulation of Zeaxanthin epoxidase (ZEP) (1.35-fold), 9-cis-epoxycarotenoid dioxygenase (NCED) (5.16-fold), and Abscisic aldehyde oxidase (AAO) (1.52-fold compared to control conditions) genes in severe stress in comparison with mild stress. The sensitive genotype had a declining trend in total chlorophyll (up to 70%) and carotenoid contents (36%). The main conclusion to be drawn from this investigation is that ABA with its regulatory effects can affect drought tolerance mechanisms to alleviate adverse effects of unsatisfactory environmental conditions.
Conclusions
In this paper, we tried to indicate that drought stress induces overexpression of genes triggering ABA-mediated drought responses simultaneously in two genotypes while more increment expression was related to the tolerant genotype. At first thought, it seems that the tolerant genotype compared to the sensitive genotype has a genetically inherent ability to cope with and drop adverse effects of drought stress through over-accumulation of ABA as drought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is drought stress that is the most deleterious abiotic stress as a major constraint negatively affects food security. Chickpea (Cicer arietinum L.) is a self-pollinated and diploid plant that is the world’s second substantial food legume crop as a source of easily digested protein and minerals planting in arid and semi-arid regions [1, 2]. Generally, two common types of chickpea genotypes are Desi and Kabuli, which the dark coated, small size, and angular-seed shape are considerable morphological characteristics of Desi, distinguishing between Desi and Kabuli [1]. Not only chickpea is valuable as a protein source for human food and animal feed, but also influences to improve soil fertility because of its nitrogen-fixing ability [3].
Drought severely threatens chickpea life in all growth stages, affecting morphological, physiological, biochemical, and molecular aspects such that it impairs water status, nutrient relations, photosynthesis, assimilate partitioning respiration, oxidative damage, and stomatal movement in the plants [4]. The plants encounter drought-induced injuries while these damages are measurable. Therefore, some damage indices informing us about the intracellular situation are relative water content (RWC), hydrogen peroxide (H2O2), and malondialdehyde (MDA) [5]. Because RWC as a tolerance marker can express the whole amount of water in plants, it can be a useful indicator of water balance in plants [6]. ROS accumulation creates oxidative damage in drought-subjected plants while their production is related to the severity of drought stress [7]. H2O2 as a long-living member of this intracellular chemical species reacts with some molecules like, lipid, protein, and DNA and causes disruption in cell function [8]. Membrane damage occurs a consequence of lipid peroxidation due to the overaccumulation of MDA, the main product in the membrane leakiness process [9]. In parallel with the importance of damage, Plants have strategies to adapt and survive through various morphological, biochemical, and physiological responses [10]. So, understanding the intracellular state of plants undergoing drought stress helps us to classify genotypes in terms of toleration and sensitivity. Physio-biochemical alterations that are followed by reprogramming of gene expression and metabolism are the most important occurrence in drought-subjected plants [11]. Among phytohormones, ABA, an stress hormone, plays important roles in survive from environmental stress such as drought [12]. It is necessary to note that ABA can act as a plant regulator in stomatal closure and stress-responsive gene expression through induction and suppression of gene expression [13]. So, first of all, ABA biosynthesis seems to be quite important. In response to drought stress, the changes in the expression of specific genes such as ZEP (Zeaxanthin epoxidase), NCED (9-cis-epoxycarotenoid dioxygenase), AAO (Abscisic aldehyde oxidase), upregulated in water stress are remarkable [12]. The investigation of the expression of ABA biosynthesis-related genes guides to understand the cellular response in stressful conditions. Plants are equipped with an array of non-enzymatic antioxidants considered as the first line of defense system. Among non-enzymatic antioxidants, phenolic compounds participate in stress tolerance both through indirect (photoprotection), and direct approaches, as free radical scavengers by hydrogen or electron-donating, singlet oxygen quenchers and metal chelators [14].
A non-enzymatic, and reliable method for evaluating radical scavenging action is the DPPH (1,1-diphenyl-2-picrylhydrazyl) test [15]. Responding to stress conditions concomitant with physiological changes in plants due to a re-established homeostasis demand a new energy source. This constructive energy makes through photosynthesis. And also, photosynthetic efficiency is a suitable criterion to inform us about intracellular situation. Drought stress affects chlorophyll a, b, and carotenoids by changing their ratio [16]. Carotenoids which are pigment and anti-ROS have vital defensive activity and physiological roles in photoprotection and light harvesting in plants [17].
Previous research on the efficiency of screening criteria for drought tolerance in chickpea in a greenhouse confirmed that a large variation and responses to different physiological indices can use for selection between genotypes to form lines with new tolerance architecture in chickpea [18]. In this study, we investigated the role of the ABA biosynthesis pathway and its drought-induced genes in two genotypes in response to drought stress. Understanding the intracellular state of plants undergoing drought stress helps us to classify genotypes in terms of tolerance and sensitivity.
Materials and methods
Plant material and drought treatment
The seeds of two Desi-type chickpea genotypes, drought tolerant and sensitive based on an agronomical study [19], were obtained from the Gene bank of the College of Agricultural and Natural Resources, University of Tehran, Karaj, Iran). The seeds sterilized with 10% sodium hypochlorite for 5 min were washed three times with distilled water and planted in plastic pots in a growth chamber at 25 °C, 30% humidity, 16 h light, and 8 h darkness for every day [20]. Before drought treatment, pots containing seedlings regularly were watered with distilled water at field capacity (FC). For determination FC level of the soil, pots containing 0.5 kg of dry soil and fine sand in a ratio of 2:1 (v/v) were weighed (W1). These pots were watered to saturation and excess water flows under gravity. Pots were covered by plastic bags to prevent evaporation and after 48 h pots were weighed (W2). The difference between the two weights (W2-W1) was the amount of soil saturation point (100% FC). For the determination of irrigation volumes, following formulae were used [21]:
For the drought treatment, the 14-day-old seedlings were subjected to three irrigation regimes, including 100% FC (as watering control), 50% FC (mild stress), and 25% FC (severe stress) conditions for two weeks [22]. After two weeks of drought treatment, plant leaves were individually taken and stored in − 80 °C.
Relative water content (RWC)
The similar fresh leaves of each genotype were cut and weighed (FW). For 24 h, the leaves were soaked in distilled water at the darkness for the turgid weight (TW). To measure dry weight (DW), samples were oven-dried overnight at 70 °C and weighed [23]. The percentage of RWC was calculated by using the following formula;
H2O2 and MDA assay
H2O2 content was estimated according to the following method [24]. 0.3 g of fresh leaves were homogenized in an ice bath with 3 mL 0.1% (w/v) trichloroacetic acid (TCA). After centrifuge (at 12,000 × g, for 15 min at 4° C), 0.5 mL 10 mM potassium phosphate buffer (pH 7.0) and 1 mL 1 M KI in a new tube were added to 0.5 mL of the supernatant. The absorbance was read at 390 nm and the content of H2O2 was used for the standard curve. The results were indicated as µmol g− 1 Fresh Weight (FW). The thiobarbituric acid (TBA) test used for the measurement of lipid peroxidation in leaf tissues was determined according to the method of [25]. 0.5 g of fresh material was homogenized with 0.1% (w/v) TCA solution. After centrifuge at 10,000 × g, for 20 min at 4° C, 2 mL of 0.5% (w/v) thiobarbituric acid (TBA) dissolved in 20% (w/v) TCA was added to 1 mL homogenate and mixed. After maintaining the mixture at 95 °C for 30 min, it was cooled and centrifuged at 10,000 × g for 5 min at 4° C. The absorbance was read by wavelength 532 nm and the result was indicated as µmol g− 1 FW.
Total phenolic content
The total phenolic content was estimated using the Folin-Ciocalteu assay method [26] expressed in mg g− 1 FW. 0.5 g of each leaf sample was soaked in 3 mL methanol (95%) in the ice bath and incubated in darkness for 24 h. After incubation, 100 µL supernatant of each sample centrifuged at 13,000 × g, for 10 min at 4 °C was mixed with the reagent, Folin-Ciocalteu, and sodium carbonate 7%, so the absorbance of samples was determined with a plate reader at 760 nm. The different concentration of Gallic acid solutions was used as standard.
Radical scavenging capacity
The non-enzymatic antioxidant capacity of the extracts was determined following the method of [27]. 2 mL ethanolic solution of plant extracts were mixed with 1 mL of 0.5 mM DPPH ethanol solution and 2 mL of 0.1 M acetate buffer (pH 5.5) and was shaken. After 30 min incubation in a dark place at room temperature, the absorbance (A) of the mixture was measured at 517 nm. The following formula was used to calculate;
Endogenous ABA quantification (HPLC)
Quantification of ABA in chickpea leaflets was performed using the method of [28]. In order to determination of the ABA levels, 1 g of frozen leaves were ground in liquid nitrogen with a mortar and pestle. 10 mL of 80% methanol was added together with 0.01 g of ascorbic acid. To prevent oxidation reactions during extraction, 0.01 g polyvinylpyrrolidone (PVP) was added and the homogenate was stirred overnight at 4 °C. The recovered supernatant was transferred to a new tube after centrifuge (at 4000 × g, 15 min at 4° C) for pH adjustment (to pH 8.0). The aqueous methanol evaporated under reduced pressure at 35 °C. The residue was dissolved in 5 mL of deionized water. For three cycles, the solution was frozen and thawed. The supernatant was recovered and adjusted to pH 2.5 after centrifuge (at 4000 × g, 15 min at 4° C), and partitioned against ethyl acetate. For three times, the solution containing free ABA in ethyl acetate was collected. So, the collection sample was adjusted to pH 8.0 and dried. The dried precipitate was dissolved in a solution, containing 1 mL of 3% methanol and 0.1 M acetic acids. Then a 0.45 mm membrane filter was used for filtration. The extract (100 µL) was automatically injected in the reverse phase column (4.6 × 250 mm Diamonsic C18, 5 μm) of the High-Performance Liquid Chromatography (HPLC) apparatus (Unicam-Crystal-200, UK). A linear gradient of methanol (3–97%), containing 0.01% acetic acid was used for elution at a flow rate of 4 mL min− 1. The detection was run at 260 nm with a diode array detector. The retention time of ABA was 36.4 min and shifted from 0.1 to 0.5 min. Quantification of ABA was obtained by comparing the peak areas with those of known amounts of ABA (Sigma-Aldrich, with 99.97% purity).
Photosynthetic pigments determination
For determination of chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoid content, 20 mg of fresh leaves were ground in liquid nitrogen and suspended in 5 mL 80% acetone and centrifuged at 12,000 × g, for 15 min at 4° C. The supernatant was used for photosynthetic pigment assay. The absorbance (A) of samples was measured at 663, 646, and 470 nm. The content of photosynthetic pigments expressed in mg g− 1 FW which was calculated according to the following formulae [29];
Gene expression analysis by quantitative real-time PCR (qRT-PCR)
For RNA isolation, 100 mg leaf of each sample was ground in liquid nitrogen. RNA of samples was extracted by using a plant DENAzist Column RNA Isolation Kit (DENAzist Asia Co., Mashhad, Iran) in RNAase-free condition. The quantity and quality of extracted RNA were respectively checked by Nano drop and gel electrophoresis. The first strand of complementary DNA (cDNA) was synthesized after removing all DNA contaminations by DNase treatment according to the manufacturer’s protocol diluted (1:20) and was utilized as a template for qRT-PCR analysis. The following pair primer sequences were used for amplification processes during qRT-PCR: 5ʹ- TGCTATAAGAGGGGAGGGGCA-3ʹ and 5ʹ- CGCGTTCTGCAAGACCCAGA-3ʹ for Zeaxanthin epoxidase (XM_027337622.1); 5ʹ- AGACGGTATGGTCCACGCTG-3ʹ and 5ʹ- CCAAACGGTGGGTTTCGGTG-3ʹ for 9-cis-epoxycarotenoid dioxygenase (XM_004488662.3); 5ʹ- CCGCCACTCGGTTTGGAAAG − 3ʹ and 5ʹ- GAGGTCGAGACGAAGCTCGG-3ʹ for Abscisic-aldehyde oxidase (XM_004491094.3); 5ʹ- ACCACAGACGCGGGTACTAAC- 3ʹ and 5ʹ-GGGAACACTGCTCTTGGTGC-3ʹ for Actin as a reference gene (XM_004493535.3). The qRT-PCR reactions (20 µL) were prepared by using 10 µL master mix 2x (SYBR® Green Real Time PCR Master Mix), 4 µL of 1:20 diluted template DNA, and 0.8 µL of each gene-specific primer (10 pmol). The gene amplification was carried out at in 10 min at 95 °C and 40 cycles of denaturation at 95 °C for 20 s, annealing at 61 °C for 15 s, and extension at 72 °C for 10 min. Two biological and three technical replicates were used for each sample. In this analysis, the reference gene was used as an internal standard of ABA biosynthesis genes. To analyze gene expression, we used the method base on 2–ΔΔCT [30].
Statistical analysis
The factorial experiments were repeated two times based on a Completely Randomized Design (CRD) and analyzed by using SAS Ver 9.4. To determine the significant difference, Duncan’s multiple range tests were used. Based on variance analysis, because the interaction was significant, all of the data represented and discussed according to the interaction.
Results
RWC
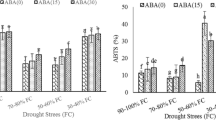
The results showed a significant decrease in two genotypes in all three irrigation regimes but the declining trend in the sensitive genotype was more intense than tolerant. In response to mild stress, RWC content decreased by 47.8% and 13.5% in sensitive and tolerant plants, respectively. In severe stress conditions, RWC content decreased in sensitive plants (by 73.9%) and tolerant plants (by 47.3%) compared to control conditions.
H2O2 content
In control conditions, H2O2 content had similar patterns in the two genotypes. During the increasing trend of drought stress, H2O2 content significantly increased in two genotypes but an overall higher increase was related to the sensitive plant. In mild stress, the sensitive plant showed 151% whereas the tolerant one showed a 41.8% increase in H2O2 content. In severe stress, the increment of H2O2 content in sensitive plants was 5.88-fold but tolerant plant showed a lower increase (1.9-fold) compared to control conditions (Fig. 1).
Effect of drought stress (control: 100% field capacity, mild stress: 50% field capacity, and severe stress: 25% field capacity) on RWC (A), H2O2 (B), and MDA (C) in drought-tolerant (black columns) and drought-sensitive (gray columns) chickpea genotypes. Columns show mean ± standard deviation. Here different letters indicate significant differences according to Duncan’s multiple range test
MDA content
In normal conditions, the MDA content of tolerant and sensitive genotypes was lower than stress conditions. In mild stress, it started to rise in two genotypes but the trend of increment in the sensitive plant was higher than in the tolerant genotype (up to 132% and 36%, respectively). In severe stress, two genotypes had the same pattern as mild stress and showed an increase in their MDA content (in sensitive 243% and in tolerant 63%) while the strength of the increase was far higher than mild stress.
Total phenolic content
In non-stressed conditions, the total phenolic content in tolerant was more than the sensitive genotype (by 1.38-fold). During the increasing trend of drought stress, total phenolic content showed a different pattern in the two genotypes. Interestingly, total phenolic content increased in the tolerant plant while it decreased in the sensitive one. Sensitive plants in mild and severe stress had a 14% and 28.6% decrease, respectively, but tolerant plants demonstrated an increase in total phenolic content in mild (20%) and severe stress (76%) in comparison with control conditions.
Antioxidant capacity
Evaluation of antioxidant capacity through DPPH assay showed a significant increase during drought stress than non-stress conditions. In both mild and severe stress, a high level of antioxidant capacity was related to the tolerant genotype (up to 1.40-fold and 1.69-fold respectively). The maximum antioxidant capacity of the sensitive genotype was in severe stress (1.3-fold) although it was less than the tolerant genotype in mild stress (Fig. 2).
Effect of drought stress (control: 100% field capacity, mild stress: 50% field capacity, and severe stress: 25% field capacity) on Total phenolic content (A), DPPH (B), and ABA content (C) in drought-tolerant (black columns) and drought-sensitive (gray columns) chickpea genotypes. Columns show mean ± standard deviation. Here different letters indicate significant differences according to Duncan’s multiple range test
ABA content
According to our results, ABA content increased in two genotypes by the intensifying trend of drought stress but the higher ABA content was presented in the tolerant genotype compared to the sensitive one under both control and stress conditions. In control, ABA content in the tolerant plant was more (51.7 ng mL− 1) than the sensitive genotype (26.5 ng mL− 1). In mild stress, ABA content was raised in both tolerant and sensitive genotypes (up to 29% and 19.6% respectively). The maximum amount of ABA was observed in the tolerant genotype under severe stress (by 53.6%) compared to control conditions.
Expression patterns of ABA biosynthesis genes
The increasing progress of drought stress upregulated ABA biosynthesis genes in two Desi-type chickpeas in particular tolerant genotype. The translational process of ZEP gene showed more expression in severe stress than mild stress. Tolerant plant presented higher expression in severe than mild stress (1.53-fold) also it showed 13.20-fold overexpression than sensitive one. In tolerant plants, NCED gene showed 145-fold transcriptional level in severe stress, while sensitive plants showed 33.5-fold expression compared to non-stress conditions. In mild stres, the transcriptional level of AAO gene constantly increased in tolerant (by 25.1-fold) and sensitive (by 2.8-fold) genotypes. In severe stress, the tolerant plant showed 38.3-fold while sensitive one had 6.63-fold gene expression compared to control conditions (Fig. 3).
Effect of drought stress (control: 100% field capacity, mild stress: 50% field capacity, and severe stress: 25% field capacity) on relative expression of Zeaxanthin epoxidase (ZEP) (A), 9-cis-epoxycarotenoid dioxygenase (NCED) (B), and Abscisic-aldehyde oxidase (AAO) (C) in drought-tolerant (black columns) and drought-sensitive (gray columns) chickpea genotypes. Columns show mean ± standard deviation. Here different letters indicate significant differences according to Duncan’s multiple range test
Photosynthetic pigments contents
In this study, as expected, the increasing severity of drought stress started a declining trend in chlorophyll content in two chickpea genotypes. The maximum decrease in chlorophyll content was observed in the sensitive genotype (70%) whereas the tolerant one had 30% decrease in severe stress compared to normal conditions. Under non-stressed conditions, the carotenoid content in sensitive genotype was near to tolerant. In mild stress, carotenoid content of the tolerant genotype remained unchanged but in severe stress, it started to rise (12.5%) compared to normal conditions. The sensitive plants showed a different pattern than the tolerant ones and displayed a declining trend with increasing severity of drought stress (by 35% decrease in severe stress compared to control) (Fig. 4).
Effect of drought stress (control: 100% field capacity, mild stress: 50% field capacity, and severe stress: 25% field capacity) on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoid (D) in drought-tolerant (black columns) and drought-sensitive (gray columns) chickpea genotypes. Columns show mean ± standard deviation. Here different letters indicate significant differences according to Duncan’s multiple range test
Discussion
As RWC indicated, drought tolerant genotype showed higher maintained water in tissues compared to the sensitive one during the stressful conditions. It seems that RWC as a cell osmosis reflection may be has a close relation with drought tolerance. Therefore, RWC measurement seems to be an appropriate criterion to classify genotypes into tolerant or sensitive groups.
The increase of ROS content in dehydrated plants may affect membrane integrity. So, H2O2 content as a cellular damage index may be a signal molecule in measurement of drought tolerance. At first thought, it can be a suitable reason for the lower increase of H2O2 accumulation in the tolerant genotype than the sensitive one. An evaluation for tolerance responses in two landrace wheat cultivars (Bolani and Sistan) revealed that Bolani or tolerant plants had lower increases in H2O2 content compared to sensitive plants [20]. According to our results, an overall higher MDA content was related to sensitive plants. In agreement with our findings, the previous report on Mentha pippeita L. documented an increase in MDA content under severe drought stress conditions (in 25% FC) in comparison to the control [31]. It may justify the drought sensitivity of sensitive plants due to maximum oxidative injury under drought stress. H2O2 increment probably simulates MDA accumulation in stressful conditions. H2O2 upsurge results in membrane damage consequence of lipid peroxidation [32]. The lower increase of MDA and H2O2 presumably be due to the strong non-enzymatic antioxidant activity system that accumulation of phenolic compounds facilitates endurance of adverse effects in tissues undergoing low water content in tolerant genotype. As a matter of fact, in abiotic stresses, the plant’s capacity to produce antioxidant agents such as phenolic compounds controls degree of cellular oxidative injury by inactivating lipid free radicals or preventing decomposition of hydro peroxides into free radicals [33]. Here, DPPH measurement was used to inform us about the ability of cells for antioxidant activity or free radical scavenging in plants A previous report referred to the higher DPPH-radical scavenging activity corresponding with the severe level of drought stress in Amaranthus [34]. Different responses of two genotypes may be related to distinct increment of ABA accumulation. ABA as a signaling compound probably causes cellular responses to dehydration and possibly can affect plant antioxidant status in two genotypes. A previous research indicated that ABA treatment increased DPPH radical scavenging activity in Gladius, Drysdale, and Kharchia in stress conditions [35]. Probably, cell readiness in response to stresses such as drought be the main cause for higher ABA accumulation in tolerant genotype compared to sensitive one and higher amount of total phenolic content in tolerant genotype confirm this matter. ABA can upregulate the expression of PAL genes that leads to phenolic compound biosynthesis [36]. During water stress conditions, the two genotypes encountered with membrane damage as MDA results indicated. Different intensities of injuries between tolerant and sensitive plants may be due to their ABA contents in dehydration. Exogenously ABA treatment exhibits a lower membrane injury index in drought-stressed plants compared to untreated ones [37]. The lower increase in H2O2 content in tolerant genotype comared to sensitive one during stressful conditions probably be effect of ABA as an endogenous messenger for alleviation of H2O2 upsurge in tolerant genotype. Previous study reported that ABA treatment reduce generation of H2O2 in chickpea seedlings [38]. Here we observed ABA accumulation in contrast with the dwindling trend of RWC in both tolerant and sensitive plants under stress. It seems that accumulation of ABA can indirectly decreases water content in drought affected-tissues. ABA involved in the reduction of volume and turgor of guard cells through ion efflux causes stomatal closure and controls transpirational rate [39]. In this regard, ABA accumulation led to a lower dropped RWC in the tolerant genotype. Probably, over accumulation of ABA that made guard cells rapidly closed to water loss was triggered osmotic balance in tolerant genotype to effortlessly came over drought tragedy. In an equal stress situation, ABA-mediated responses may be effective to distinguish between two genotypes in terms of tolerance as our results indicated. The increase in ABA content was subsequent of upregulation of ABA biosynthesis genes. A higher transcriptional level maybe is representative of genetically drought tolerance capacity in tolerant plants. For developing superior genotypes of chickpea in breeding programs, understanding the genetic basis and identification of molecular markers for drought tolerance is require [40]. The previous study in Arabidopsis indicated that overexpression of ZEP gene (AtZEP) plays important role in response to osmotic stress [41]. A previous report about the rice aldehyde oxidase gene (OsAO3) participating in ABA biosynthesis is revealed the effect of this gene in drought tolerance. Here, NCED gene showed noteworthy overexpression in tolerant genotype in severe stress compared to non-stress conditions. Previously, an experiment confirmed the potential of overexpression of NCED gene to improve drought tolerance in soybean plants [42]. It is possible that some upstream regulatory mechanisms are involved in drought-induced gene expression reprogramming to create drought tolerance responses in two Desi-type chickpeas, especially in the tolerant plant. For evaluation of the plant’s physiological status, performance, and stability of photosynthesis pigment content was assessed. The synchronization of the intensity of drought stress with decreasing trend of photosynthetic pigments could engender differences between two genotypes. Lower intensity of decrease and more stability in the tolerant genotype can strengthen probability of tolerance in tolerant genotype than the sensitive one. First of all, ABA probably has positive influence to create this competence. It is recognized that ABA has a positive function to alleviates stress injuries in the PSII system [43]. Based on our results, the lower decline in total cholorophyll content may be consequent of the lower H2O2 production in the tolerant genotype compared to sensitive one. The water crisis in plants causes stomatal closure, the decline of CO2 influx, and overproduction of ROS in the principal sites such as plant chloroplast and finally hampers the photosynthetic machinery [44]. In this study, a decreasing trend in chlorophyll content was accompained by severity of dehydration limiting photosynthesis rate. In dehydrated plants, RuBP (Ribulose 1,5-bisphosphate) regeneration depended on efficient electron transport rate causes photochemical limitation of photosynthesis [45]. Also, the sustainability of the chlorophylls maybe related to defensive indices. In agreement with our results, a research in drought-stressed wheat genotypes showed higher chlorophyll and carotenoid contents in tolerant genotypes compared to sensitive ones [46]. Probably, a mutual relationship rationalizes the simultaneous decrease of carotenoid content with lower ABA biosynthesis in sensitive plant than tolerant one in severe stress. Carotenoids as essential molecules are involve in some phytohormone biosynthesis like ABA and strigolactone [47]. Exogenous ABA application is involved in increasing carotenoid content [48]. Also, carotenoid content changed in genotypes probably be effective in cell homeostasis during stress conditions. The increase of carotenoid as a non-enzymatic antioxidant agent and its ability to remove the damage of the ROS and free radicals are signals of drought tolerance used to evaluation of various plants under drought stress [47].
Conclusion
In this paper, we tried to indicate that drought stress induces overexpression of genes triggering ABA-mediated drought responses simultaneously in two genotypes while more increment expression was related to the tolerant genotype. At first thought, it seems that the tolerant genotype compared to the sensitive genotype has a genetically inherent ability to cope with and drop adverse effects of drought stress through over-accumulation of ABA as a drought stress signal molecule. So, tolerant Desi genotype because of this genetic potential is an appropriate candidate to be involved in the breeding programs.
Data availability
Data will be available in case of need.
Abbreviations
- ABA:
-
Abscisic acid
- RWC:
-
Relative water content
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- ZEP :
-
Zeaxanthin epoxidase
- NCED:
-
9-cis-epoxycarotenoid dioxygenase
- AAO:
-
Abscisic aldehyde oxidase
References
Agarwal G, Jhanwar S, Priya P, Singh VK, Saxena MS, Parida SK, Garg R, Tyagi AK, Jain M (2012) Comparative analysis of Kabuli chickpea transcriptome with Desi and wild chickpea provides a rich resource for development of functional markers. PLoS ONE 7(12), e52443
Amini S, Maali Amiri R, Kazemi Shahandashti SS, López Gómez M, Sadeghzadeh B, Sobhani Najafabadi A, Kariman K (2021) Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J Plant Physiol 258:153387
Maphosa L, Richards MF, Norton SL, Nguyen GN (2020) Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): a comprehensive review. CBGG 4(3)
Maqbool MA, Aslam M, Ali H (2017) Breeding for improved drought tolerance in chickpea (Cicer arietinum L). Plant Breed 136(3):300–318
Moussa H, Gamal E SM (2010) Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant 54(2):315–320
González L, González Vilar M (2001) Determination of relative water content. In handbook of plant ecophysiology techniques. Springer 207–212
Sallam A, Alqudah AM, Dawood MF, Baenziger PS, Börner A (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20(13):3137
Saed Moucheshi A, Pakniyat H, Pirasteh Anosheh H, Azooz M (2014) Role of ROS as signaling molecules in plants. In Oxidative damage to plants Elsevier 585–620
Hassan N, Ebeed H, Aljaarany A (2020) Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol Mol Biol 26(2):233–245
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Sustain Agric re Springer 153–188
Zhang JY, Cruz De Carvalho MH, Torres Jerez I, Kang Y, Allen SN, Huhman DV, Tang Y, Murray J, Sumner LW, Udvardi MK (2014) Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ 37(11):2553–2576
Aslam M, Maqbool MA, Cengiz R (2015) Mechanisms of drought resistance in drought stress in Maize (Zea mays L). Springer, pp 19–36
Takahashi F, Kuromori T, Urano K, Yamaguchi Shinozaki K, Shinozaki K (2020) Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front Plant Sci 11:556972
Chiappero J, del Rosario Cappellari L, Alderete LGS, Palermo TB, Banchio E (2019) Plant growth promoting rhizobacteria improve the antioxidant status in Mentha Piperita L. grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod 139:111553
Md MH, Raushanara A, Mariam J, Md EHM, Shafiqur R (2009) DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J Med Plant Res 3(11):875–879
Jaleel CA, Manivannan P, Wahid A, Farooq M, Al Juburi HJ, Somasundaram R, Panneerselvam R (2009) Drought stress in plants: a review on morphological characteristics and pigments composition. IJAB 11(1):100–105
McELROY JS, Kopsell DA (2009) Physiological role of carotenoids and other antioxidants in plants and application to turfgrass stress management. NZJ Crop Hortic Sci 37(4):327–333
Pouresmael M, Khavari Nejad RA, Mozafari J, Najafi F, Moradi F (2013) Efficiency of screening criteria for drought tolerance in chickpea. Arch Agron Soil Sci 59(12):1675–1693
Taleei A, Shaabani J (2017) Yield potential screening of Desi chickpea genotypes in water stress conditions. Int J Biotechnol 9(1):89–102
Naderi S, Fakheri BA, Maali Amiri R, Mahdinezhad N (2020) Tolerance responses in wheat landrace Bolani are related to enhanced metabolic adjustments under drought stress. Plant Physiol Biochem 150:244–253
Harkousse O, Slimani A, Jadrane I, Aitboulahsen M, Mazri MA, Zouahri A, Ouahmane L, Koussa T, Al Feddy MN (2021) Role of local biofertilizer in enhancing the oxidative stress defence systems of date palm seedling (Phoenix dactylifera L.) against abiotic stress. Appl Environ Soil Sci 2021:1–13
Gharibi S, Tabatabaei BES, Saeidi G, Goli SAH (2016) Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl Biochem Biotechnol 178(4):796–809
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58(1):339–366
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127(4):1781–1787
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem 125(1):189–198
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–ciocalteu reagent. Nat Protoc 2(4):875–877
Abe N, Murata T, Hirota A (1998) Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci Biotechnol Biochem 62(4):661–666
Li XJ, Yang MF, Chen H, Qu LQ, Chen F, Shen SH (2010) Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochim et Biophys Acta (BBA)-Proteins Proteom 1804(4):929–940
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In: Portland Press Ltd
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25(4):402–408
Rahimi Y, Taleei A, Ranjbar M (2018) Long-term water deficit modulates antioxidant capacity of peppermint (Mentha Piperita L). Sci Hortic 237:36–43
Wang Y, Ma F, Li M, Liang D, Zou J (2011) Physiological responses of Kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul 64(1):63–74
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V (2014) Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia Mirzayanii. S Afr J Bot 93:92–97
Sarker U, Oba S (2018) Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol 18(1):1–15
Kaur G, Asthir B (2020) Impact of exogenously applied ABA on proline metabolism conferring drought and salinity stress tolerance in wheat genotypes. Cereal Res Commun 48(3):309–315
Khaleghnezhad V, Yousefi AR, Tavakoli A, Farajmand B (2019) Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L). Sci Hortic 250:302–309
Bandurska H (1998) Implication of ABA and proline on cell membrane injury of water deficit stressed barley seedlings. Acta Physiol Plant 20(4):375–381
Nayyar H, Bains T, Kumar S (2005) Chilling stressed chickpea seedlings: effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. EEB 54(3):275–285
Hsu PK, Dubeaux G, Takahashi Y, Schroeder JI (2021) Signaling mechanisms in abscisic acid-mediated stomatal closure. TPJ 105(2):307–321
Varshney RK, Thudi M, Nayak SN, Gaur PM, Kashiwagi J, Krishnamurthy L, Jaganathan D, Koppolu J, Bohra A, Tripathi S (2014) Genetic dissection of drought tolerance in chickpea (Cicer arietinum L). Theor Appl Genet 127(2):445–462
Park HY, Seok HY, Park BK, Kim SH, Goh CH, Lee Bh, Lee CH, Moon YH (2008) Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. BBRC 375(1):80–85
Molinari MDC, Fuganti Pagliarini R, Marin SRR, Ferreira LC, Barbosa DdA, Marcolino Gomes J, Oliveira MCNd M, Kanamori N, Takasaki H (2020) Overexpression of AtNCED3 gene improved drought tolerance in soybean in greenhouse and field conditions. Genet Mol Biol 43
Teng K, Li J, Liu L, Han Y, Du Y, Zhang J, Sun H, Zhao Q (2014) Exogenous ABA induces drought tolerance in upland rice: the role of chloroplast and ABA biosynthesis-related gene expression on photosystem II during PEG stress. Acta Physiol Plant 36(8):2219–2227
Garcia Caparros P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT (2021) Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev 87:421–466
Beckett M, Loreto F, Velikova V, Brunetti C, Di Ferdinando M, Tattini M, Calfapietra C, Farrant JM (2012) Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta Humilis during dehydration and rehydration. Plant Cell Environ 35(12):2061–2074
Sairam R, Saxena D (2000) Oxidative stress and antioxidants in wheat genotypes: possible mechanism of water stress tolerance. J Agron Crop Sci 184(1):55–61
Zhang RR, Wang YH, Li T, Tan GF, Tao JP, Su XJ, Xu ZS, Tian YS, Xiong AS (2021) Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 258(2):379–390
Kou X, Yang S, Chai L, Wu C, Zhou J, Liu Y, Xue Z (2021) Abscisic acid and fruit ripening: multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci Hortic 281:109999
Acknowledgements
We do acknowledge the University of Tehran for providing a grant to carry out this research work.
Funding
University of Tehran.
Author information
Authors and Affiliations
Contributions
Professor Taleei contributed for this research work scientifically and used from his research grant.
Corresponding author
Ethics declarations
Competing interests
There is no competing interest.
Ethical approval
N/A.
Consent to participate
N/A.
Consent to publish
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasbi, E.A., Taleei, A. & Amiri, R.M. Effect of drought stress on the expression pattern of genes involved in ABA biosynthesis in Desi-type chickpea (Cicer arietinum L.). Mol Biol Rep 51, 469 (2024). https://doi.org/10.1007/s11033-024-09402-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09402-y