Abstract
Carotenoids are liposoluble pigments found in plant chromoplasts that are responsible for the yellow, orange, and red colors of carrot taproots. Drought is one of the main stress factors affecting carrot growth. Carotenoids play important roles in drought resistance in higher plants. In the present work, the carotenoid contents in three different-colored carrot cultivars, ‘Kurodagosun’ (orange), ‘Benhongjinshi’ (red), and ‘Qitouhuang’ (yellow), were determined by ultra-high-performance liquid chromatography (UPLC) after 15% polyethylene glycol (PEG) 6000 treatment. Real-time fluorescence quantitative PCR (RT-qPCR) was then used to determine the expression levels of carotenoid synthesis- and degradation-related genes. Increases in β-carotene content in ‘Qitouhuang’ taproots under drought stress were found to be related to the expression levels of DcPSY2 and DcLCYB. Increases in lutein and decreases in α-carotene content in ‘Qitouhuang’ and ‘Kurodagosun’ under PEG treatment may be related to the expression levels of DcCYP97A3, DcCHXE, and DcCHXB1. The expression levels of DcNCED1 and DcNCED2 in the three cultivars significantly increased, thus suggesting that NCED genes could respond to drought stress. Analysis of the growth status and carotenoid contents of carrots under PEG treatment indicated that the orange cultivar ‘Kurodagosun’ has better adaptability to drought stress than the other cultivars and that β-carotene and lutein may be involved in the stress resistance process of carrot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrot (Daucus carota L.) is a biennial root vegetable crop of the Apiaceae featuring taproots that are rich in carotenoids (Luby et al. 2014; Que et al. 2019). Carrots have a variety of colors, including purple, red, orange, yellow, and white (Sun et al. 2009; Ma et al. 2017, 2018; Xu et al. 2019, 2020). Carotenoids are the second most abundant natural pigments in nature and include over 700 types (Ben-Amotz and Fishler 1998). Most carotenoids are 40-carbon isoprenoid polymers that are ubiquitous in animals, higher plants, algae, and fungi (Nisar et al. 2015). In higher plants, carotenoids are essential components for photosynthesis, photoprotection, and antioxidant and plant hormone production, such as abscisic acid (ABA) and strigolactone (SL). In addition, plants rich in carotenoids can attract insects and birds via their bright colors, thus contributing to the spread of pollen and seeds and plant reproduction (Cazzonelli 2011; Walter and Strack 2011).
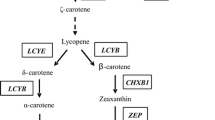
The biosynthetic pathway of carotenoids has been well studied in carrots (Ma et al. 2017; Wang et al. 2020). The precursor of carotenoid biosynthesis is isopentenyl diphosphate (IPP) (Cunningham and Gantt 1998; Nisar et al. 2015). Under the action of IPP isomerase (IPI) and geranylgeranyl diphosphate synthase (GGPS), dimethylallyl diphosphate (DMAPP) and IPP condense into geranylgeranyl diphosphate (GGPP) (Eisenreich et al. 2001). Two molecules of GGPP condense to produce phytoene by phytoene synthase (PSY) (Fraser et al. 2007). Then, under the action of four enzymes, namely, phytoene desaturase (PDS), ξ-carotene desaturase (ZDS), carotenoid isomerase (CRTISO), and ξ-carotene isomerase (Z-ISO), phytoene produces a red all-trans lycopene through a series of dehydrogenation and isomerization steps (Bramley 2002; Isaacson et al. 2002; Park et al. 2002; Nisar et al. 2015). After the production of lycopene, the biosynthetic pathway of carotenoids begins to branch. Lycopene ε-cyclase (LCYE) and lycopene β-cyclase (LCYB) act on both ends of the molecule to form α-carotene and β-carotene, respectively (Cunningham and Gantt 1998). Subsequently, ε-carotene hydroxylase (CHXE), β-carotene hydroxylase (CHXB), and cytochrome P450-type monooxygenase 97A3 (CYP97A3) catalyze the conversion of α-carotene into lutein, while CHXB catalyzes the conversion of β-carotene into zeaxanthin (Tian et al. 2003; Arango et al. 2014). Under the action of zeaxanthin epoxidase (ZEP), zeaxanthin is epoxidated to produce violaxanthin, which is converted to neoxanthin under the influence of neoxanthin synthase (NXS) (Marin et al. 1996; North et al. 2007). Neoxanthin generates xanthoxin under the action of 9-cis-epoxycarotenoid dioxygenase (NCED), followed by phytohormone ABA (Schwartz et al. 2003; Auldridge et al. 2006a). β-Carotene can also be degraded into apocarotenoids and SL by carotenoid cleavage dioxygenase (CCD) (Auldridge et al. 2006b; Koltai and Kapulnik 2011).
Changes in environmental conditions and the expansion of the carrot cultivation area have led to the noticeable influences of environmental factors on the growth and development of carrots (Huang et al. 2015; Wang et al. 2017; Que et al. 2019). Drought is the main abiotic stress factor in carrot production and seriously affects the yield and quality of the crop (Herppich et al. 2001). Studies have shown that drought increases the content of free proline, glycinebetaine, and total phenols in carrot leaves (Razzaq et al. 2017), but decreases storage root diameters (Reid and Gillespie 2017). Ali et al. screened drought-resistant genotypes from different varieties of carrot by analyzing root weights and membrane thermo-stability (Ali et al. 2019). Carotenoids are important antioxidant substances in plants (Treutter 2006); these substances play direct roles in eliminating and reducing the reactive oxygen species (ROS) damage caused by drought and other adverse conditions (Niyogi et al. 1997). Carotenoids are among the important indices used to evaluate drought resistance in various higher plants (Farooq et al. 2009). Parida et al. found that the degree of decrease in carotenoids in cotton under drought is higher in the sensitive genotype than in the moderately tolerant genotype (Parida et al. 2007). Ma et al. and Wang et al. analyzed the carotenoid contents and gene expression levels of several carrots with different colors under different growth stages. The authors found that the accumulation of α-carotene and formation of lutein may be related to the expression level of carotene hydroxylase genes and that the high expression of genes related to lycopene synthesis may lead to the accumulation of lycopene in red carrot (Ma et al. 2017; Wang et al. 2020). However, the mechanism of the carotenoid response to drought stress in carrot taproots remains clear.
In this study, three different-colored carrot cultivars were selected as experimental materials: ‘Kurodagosun’ (orange), ‘Benhongjinshi’ (red), and ‘Qitouhuang’ (yellow). The content changes of four types of carotenoids (lutein, lycopene, α-carotene, and β-carotene) in carrot taproots were determined after treatment with 15% polyethylene glycol (PEG) 6000, and the real-time fluorescence quantitative PCR (RT-qPCR) technology was used to determine the expression levels of carotenoid synthesis- and degradation-related genes. Analysis of the relationship between carotenoid contents and gene expression levels in the carrot taproots was conducted to determine the characteristics of carotenoids in carrot taproots under drought stress. The results of this work provide a potential theoretical basis for research on drought resistance mechanisms and selective cultivation of drought-resistant carrot cultivars.
Materials and methods
Plant materials and growth conditions
Three different-colored carrot cultivars were selected as experimental materials: ‘Kurodagosun’ (orange), ‘Benhongjinshi’ (red), and ‘Qitouhuang’ (yellow). Carrot materials were planted in the artificial climate laboratory of State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University (32° 04′ N, 118° 85′ E). The temperature was set at 25 °C (day) and 18 °C (night). The photoperiod was light of 16 h, and the light intensity was 300 μmol m−2 s−1. After germination on filter paper for 10 days, the carrot seeds were transferred to plastic pots (20 × 20 × 25 cm) mixed with organic substrate, vermiculite, and perlite (1:1:1, v/v). Three plastic pots were used for each cultivar, and eight seedlings were added to each plastic pot. Ninety days after sowing (DAS), the carrot plants were irrigated with 1 L of 15% PEG 6000 (Wang et al. 2011; Li and Li 2014). Samples on day 0 were taken as the control. The taproots treated for 2, 4, and 6 days were frozen in liquid nitrogen and preserved at − 80 °C for subsequent experiments.
Extraction and determination of carotenoids
Carotenoids were extracted according to the method of Chen et al. (2013) with some modifications. Briefly, the taproots were ground into powder in liquid nitrogen. Approximately 50 mg of the vacuum-frozen materials was weighed and extracted thrice in 2 mL of acetone. The combined supernatants were filtered through 0.45-μm filters, and carotenoid contents were measured by Waters ACQUITY UPLC Hclass system (Waters, USA). The injection volume of the extract in the ultra-high-performance liquid chromatography (UPLC) Hedera ODS-2 C18 analytical column (inner diameter 250 mm × 4.6 mm, particle size 5 μm) at 30 °C was 20 μL. The detection wavelength was 450 nm; the mobile phase was methanol/acetonitrile = 10:90, and the flow rate was set as 0.25 mL min−1. Carotenoid contents were calculated from standard curves (lutein, lycopene, α-carotene, and β-carotene) and expressed as dry weight (mg g−1 DW). Three technical replicates were performed for each sample.
Extraction of total RNA and synthesis of cDNA
Total RNA was extracted from the carrot taproots by using a plant total RNA extraction kit (Beijing Tiangen Biochemical Technology Co., Ltd., China), and the concentrations were determined by micro-ultraviolet detector Nano-Drop. The extracted RNA samples were reverse-transcribed into cDNA by HiScript II Q RT SuperMix for qPCR (+gDNA wiper) kit (Nanjing Vanzyme Co., Ltd., China).
RT-qPCR analysis
The expression of carrot carotenoid pathway genes under 15% PEG 6000 treatment was detected by RT-qPCR according to the instructions of Hieff qPCR SYBR Green Master Mix (Shanghai Yeason Biotechnology Co., Ltd.). DcActin1 gene was used as the reference gene (Xu et al. 2014). The fluorescent quantitative detection primers were designed by Primer Premier 5.0 software, and the primer’s specificity was detected by the NCBI website, as shown in Table S1 (Xu et al. 2014; Wang et al. 2015; Iorizzo et al. 2016). The total RT-qPCR reaction system was 20 μL, including 10 μL of SYBR Premix Ex Taq enzyme, 2 μL of cDNA template, 7.2 μL of ddH2O, and 0.4 μL of forward and reverse primers. The reaction procedure was pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 10 s, and annealing at 60 °C for 30 s for a total of 40 cycles, and the melting curves were drawn during the gradual heating of 65 to 95 °C. The relative expression of genes were calculated by 2−ΔΔCt method (Pfaffl 2001), ΔΔCt = (Ct, target gene − Ct, DcActin1)treatment − (Ct, target gene − Ct, DcActin1)control. Three biological repeats were set up for each treatment sample. IBM SPSS Statistic 20 and WPS Excel 2019 were used to analyze the significant differences in the values obtained.
Results
Growth status of three carrot cultivars under PEG treatment
The growth status of three different-colored carrots treated with PEG is shown in Fig. 1. As the number of treatment days increased, the three cultivars showed different degrees of wilting. Wilting in ‘Qitouhuang’ was the most obvious, followed by that in ‘Benhongjinshi’ and then in ‘Kurodagosun’. The plant size of the three cultivars also decreased with increasing number of PEG treatment days.
Carotenoid contents in carrot taproots under PEG treatment
The content of carotenoids in different carrot taproots treated with PEG was determined. According to the UPLC peak spectra obtained (Fig. 2), lutein, lycopene, α-carotene, and β-carotene contents peaked at 2, 8, 14, and 15 min, respectively. As shown in Fig. 3, lycopene was detected only in the red cultivar ‘Benhongjinshi’; the content of this carotenoid decreased and reached minimum levels, at approximately 0.52 times lower than that of the control, 2 days after PEG treatment. As the number of PEG treatment days increased, the contents of α-carotene and lutein showed an up–down–up trend whereas the contents of lycopene showed the opposite trend in ‘Benhongjinshi’. Under drought stress, the contents of α-carotene in ‘Kurodagosun’ and ‘Qitouhuang’ showed a decreasing trend; indeed, the level of this carotenoid in ‘Qitouhuang’ was virtually undetectable. The contents of lutein in the three carrot cultivars showed an increasing trend 2 days after PEG treatment. Changes in β-carotene content among the three carrot cultivars during PEG treatment differed. The orange cultivar ‘Kurodagosun’ was rich in α-carotene and β-carotene, while the yellow cultivar ‘Qitouhuang’ was main rich in lutein.
Expression level of lycopene synthesis genes in carrot taproots under PEG treatment
The expression levels of seven genes (DcIPI, DcGGPS1, DcPSY1, DcPSY2, DcPDS, DcZDS1, and DcCRTISO) related to the biosynthesis of lycopene were determined in three carrot taproots under PEG treatment (Fig. 4). Except for that of DcPDS, the expression levels of other genes in ‘Kurodagosun’ reached minimum levels 4 days after PEG treatment and then increased. In ‘Benhongjinshi’, the expression level of DcGGPS1 increased significantly 2 days after treatment, but the expression levels of all seven genes were lower than those of the control 4 days after treatment. The expression levels of five genes (DcGGPS1, DcPSY1, DcPSY2, DcPDS, and DcCRTISO) in ‘Qitouhuang’ peaked after 6 days of PEG treatment and were 5.67, 6.17, 1.62, 2.89, and 6.50 times higher than those of the control, respectively.
Expression level of lutein and carotene synthesis genes in carrot taproots with PEG treatment
Figure 5 shows the expression levels of five genes (DcLCYE, DcLCYB, DcCHXB1, DcCHXE, and DcCYP97A3) related to the biosynthesis of carotene and lutein under PEG treatment. The expression levels of all five genes in ‘Kurodagosun’ peaked 6 days after treatment. The expression of DcCYP97A3 and DcCHXE in ‘Qitouhuang’ increased significantly 6 days after PEG treatment, which were 4.54 and 6.80 times higher than those of the control.
Expression level of lutein and carotene synthesis-related genes in carrot taproots treated with PEG. Cultivar abbreviations: KRD Kurodagosun, BHJS Benhongjinshi, QTH Qitouhuang. Different lowercase letters indicate significant differences between days of PEG treatment for the same carrot cultivar at P < 0.05
Expression level of carotene degradation–related genes in carrot taproots with PEG treatment
The expression levels of seven genes (DcZEP, DcVDE, DcNXS, DcCCD4, DcCCD8, DcNCED1, and DcNCED2) related to carotene degradation under PEG treatment are shown in Fig. 6. As the number of PEG treatment days increased, the expression levels of DcZEP in the three carrot cultivars showed a consistent increasing trend. The expression levels of DcCCD4, DcCCD8, and DcNCED1 significantly increased in ‘Qitouhuang’ 6 days after PEG treatment, which were 11.29, 4.74, and 6.45 times higher than those of the control. DcNCED2 was highly induced in response to PEG treatment in ‘Benhongjinshi’.
Expression level of carotene degradation-related genes in carrot taproots treated with PEG. Cultivar abbreviations: KRD Kurodagosun, BHJS Benhongjinshi, QTH Qitouhuang. Different lowercase letters indicate significant differences between days of PEG treatment for the same carrot cultivar at P < 0.05
Schematic view of carotenoid metabolism after 6 days of PEG treatment in carrot taproots
The variation trend of each enzyme-encoding gene after 6 days of PEG treatment in the three carrot taproots is summarized in Fig. 7. Compared with the control, the expression levels of DcPSY1, DcLCYB, DcCYP97A3, DcZEP, and DcNCED2 in the three carrot cultivars showed increasing trends 6 days after PEG treatment. The expression levels of DcPSY2, DcCRTISO, DcCHXE, DcVDE, and DcNCED1 were downregulated in ‘Benhongjinshi’; the expression levels of DcNXS and DcCCD8 were upregulated in ‘Qitouhuang’, and these trends were reversed in the two other cultivars. The expression levels of DcIPI, DcZDS1, DcLCYE, and DcCHXB1 were upregulated only in ‘Kurodagosun’.
Schematic view of carotenoid metabolism after 6 days of PEG treatment in carrot taproots. Cultivar abbreviations: KRD Kurodagosun, BHJS Benhongjinshi, QTH Qitouhuang. The variation trends of gene expression levels were represented by ↑, ↓ and −. ↑ represents upregulation, ↓ represents downregulation, − represents no significant change
Discussion
Carotenoids are a type of antioxidant pigment in plants that are of great significance in plant stress resistance; these pigments eliminate ROS and free radicals and maintain the redox balance (Havaux 2013; Hou et al. 2016). Under adversity, plants regulate the accumulation and degradation of carotenoids by regulating genes related to carotenoid metabolism. The accumulation and degradation of carotenoids involves a complex process of gene regulation (Nisar et al. 2015). Drought is an important factor affecting the biosynthesis and metabolism of carotenoids. Drought stress has been reported to affect the content of carotenoids in many crops, including strawberry (Munné-Bosch and Peñuelas 2004), maize (Mohammadkhani and Heidari 2007), soybean (Rys et al. 2015), and so on. Carrot taproots are rich in carotenoids. The red, orange, or yellow color of the carrot taproots is mainly attributed to lycopene, α-carotene, β-carotene, and lutein, respectively (Just et al. 2009). In the present study, three carrot cultivars with different colors were selected as experimental materials, and 15% PEG 6000 was used to simulate drought treatment at 90 DAS. The contents of carotenoid and expression of the related genes in the carrot taproots were determined at 0, 2, 4, and 6 days after treatment.
A previous study identified two PSYs in carrots (Just et al. 2007). DcPSY1 and DcPSY2 mainly play roles in leaves and roots, respectively (Fuentes et al. 2012). Simpson et al. proposed that salt stress and ABA could induce the expression of DcPSY2 by binding the AREB transcription factor (maybe DcAREB3) with the ABRE cis-element in the promoter. The expression of DcPSY2 in carrot taproots increases the production of carotenoids, thereby increasing the level of ABA and protecting the plant from abiotic stress (Simpson et al. 2018). In this work, among the cultivars examined, ‘Qitouhuang’ showed the highest and most significant increases in DcPSY2 expression levels. The contents of lutein and β-carotene in this cultivar also increased. These results suggest that increases in carotenoid contents in ‘Qitouhuang’ may be related to the high expression of the DcPSY2 gene.
Lycopene ε-cyclase (LCYE) and lycopene β-cyclase (LCYB) play important roles in the branching point of the carotenoid biosynthetic pathway and catalyze the cyclization of lycopene (Cunningham and Gantt 1998). Research has shown that downregulation of the LCYB gene causes lycopene accumulation in tomato fruits during ripening (Ronen et al. 2000). Bang et al. found that the mutation of LCYB allele in red watermelon may reduce the activity of LCYB and lead to the accumulation of lycopene (Bang et al. 2007). In this study, lycopene was only accumulated in ‘Benhongjinshi’. The content of lycopene in this cultivar first decreased, subsequently increased, and then finally decreased under PEG treatment. In addition, the expression level of DcLCYB showed a corresponding up–down–up trend. These results are consistent with the conclusions of other researchers (Ronen et al. 2000; Bang et al. 2007). Therefore, lycopene accumulation in carrot may be speculated to be related to the downregulation of DcLCYB gene. Studies have shown that overexpression of NtLCBY1 and inhibition of NtLCYE in tobacco could promote the accumulation of β-carotene and improve the tolerance of the plant to salt and drought (Shi et al. 2015a, 2015b). Kim et al. confirmed that the downregulated expression of LCYE in sweet potato enhances the accumulation of carotenoids and ABA and improves the salt tolerance of the plant (Kim et al. 2012). In the present study, the expression level of DcLCYE in ‘Qitouhuang’ decreased whereas the expression level of DcLCYB and content of β-carotene increased with PEG treatment. Considering these results, the increase in β-carotene content in ‘Qitouhuang’ under drought stress appears to be related to the upregulation of the DcLCYB gene and downregulation of the DcLCYE gene. Under PEG treatment, the contents of lutein and expression levels of DcCYP97A3, DcCHXE, and DcCHXB1 increased in the yellow and orange cultivars, but their α-carotene contents decreased. In plants, the carotene hydroxylase encoded by these genes hydroxylates α-carotene into lutein in some branch of the carotenoid biosynthetic pathway (Quinlan et al. 2012). Drought stress may promote the synthesis of lutein in carrot taproots by affecting the expression of carotene hydroxylase genes.
Shafiq et al. showed that, under drought stress, the contents of carotenoids and malondialdehyde (MDA) in radish roots increase and the weight of radish roots decreases (Shafiq et al. 2015). Increases in carotenoid contents may be related to their role as antioxidants, which have largely been proven to be involved in the plant response to drought stress condition. Riggi et al. and Atkinson et al. found that, compared with sufficiently watered plants, plants under drought stress show reduced lycopene contents and increased β-carotene contents (Riggi et al. 2008; Atkinson et al. 2011). In the present study, lycopene was detected only in ‘Benhongjinshi’ and the content of this carotenoid significantly decreased 2 days after PEG treatment. Moreover, α-carotene contents and the expression levels of DcLCYE and DcLCYB genes significantly increased in this cultivar. Based on these results, we speculate that, under 2 days of PEG treatment, lycopene in the red cultivar ‘Benhongjinshi’ preferentially transforms into the α-branch and produces α-carotene in response to drought stress. Studies have shown that ABA and carotenoids are indicators that could determine whether plants are drought-resistant; under drought stress, the contents of ABA in plants increase and β-carotene is accumulated accordingly (Chaves et al. 2008). Some crops accumulate large amounts of carotenoids in storage roots, such as carrots and potatoes. Perrin et al. demonstrated that the amount of total carotenoids decreases in the xylem of orange genotypes but increases in the xylem and phloem of purple genotypes and in the phloem of red genotypes under restricted-water conditions (Perrin et al. 2017). Results have shown that the response of potato to drought stress is highly cultivar-specific, but the contents of β-carotene consistently increase in all cultivars (Andre et al. 2009). The drought stress of carrot increased with increasing number of PEG treatment days, and, among the cultivars studied, ‘Kurodagosun’ showed the strongest adaptability to drought stress. The results showed that the contents of β-carotene in this cultivar increased 4 days after PEG treatment and decreased 6 days after PEG treatment whereas lutein contents increased significantly 6 days after PEG treatment. The change trends of β-carotene and lutein in the two other cultivars were identical to those in ‘Kurodagosun’. Thus, β-carotene and lutein may be speculated to respond to drought stress and participate in the drought resistance process of carrot.
The enzymes involved in carotenoid cleavage in plants are mainly carotenoid cleavage oxidases (CCDs) (Tan et al. 2003). The CCD family includes carotenoid cleavage dioxygenase (CCD) and 9-cis-epoxycarotenoid dioxygenase (NCED), both of which can catalyze the decomposition of carotenoids and apocarotenoids. NCEDs are related to the drought resistance of plants (Zhang et al. 2009). They can cleave the 11,12 double bonds of 9-cis-epoxy carotenoids and produce xanthoxin, which is the precursor of the plant hormone ABA (Rodrigo et al. 2013). In this study, the expression levels of DcNCED1 and DcNCED2 in the three cultivars significantly increased under PEG treatment, but the degree of increase differed according to the cultivar. These results indicate that NCED plays a role in the drought resistance of carrot. Differences in the expression of this gene under drought stress may be related to differences in the various cultivars, drought degree, and other factors. Although only a small amount of carotenoid accumulation was detected in the cultivar, ‘Qitouhuang’ showed higher expression of DcCCD4 and DcNCED2 than the two other cultivars. These results reveal that these genes may degrade carotenes and lutein into apocarotenoid or other carotenoid-degrading derivatives.
The drought resistance mechanism of carotenoids in plants is relatively complex. The expression and regulation of the related genes are involved not only in transcription but also in post-transcriptional processing and modification, as well as in protein translation, editing, and transport (Cunningham and Gantt 1998; Park et al. 2002). The contents of carotenoids and expression of related genes in three different-colored carrot cultivars under PEG stress were studied in this experiment to understand the relationship between drought resistance and carotenoids. The results provide a reliable basis for the selective cultivation of cultivars with high contents of carotenoids and strong stress resistance. We noted that the contents of the four carotenoids studied in this work and the expression levels of the related genes show variations in response time and intensity of PEG treatment, thereby indicating that other synergistic and antagonistic pathways may be involved in the plants’ response to adversity. The relevant response mechanism requires further study.
Abbreviations
- ABA:
-
Abscisic acid
- DAS:
-
Days after sowing
- DW:
-
Drought weight
- LCYB:
-
Lycopene β-cyclase
- LCYE:
-
Lycopene ε-cyclase
- NCED:
-
9-cis-epoxycarotenoid dioxygenase
- PEG:
-
Polyethylene glycol
- PSY:
-
Phytoene synthase
- ROS:
-
Reactive oxygen species
- RT-qPCR:
-
Real-time fluorescence quantitative
- SL:
-
Strigolactone
- UPLC:
-
Ultra-high-performance liquid chromatography
References
Ali A, Naveed NH, Shah AI, Hussain R, Jamil M, Nijabat A, Manzoor S, Faiz S, Yasin NA, Simon PW (2019) Phylogenetic relationship and screening of diverse germplam of carrot (Daucus carota) for drought resistance. Fresenius Environ Bull 28(11a):8474–8479
Andre CM, Schafleitner R, Guignard C, Oufir M, Aliaga CAA, Nomberto G, Hoffmann L, Hausman JF, Evers D, Larondelle Y (2009) Modification of the health-promoting value of potato tubers field grown under drought stress: emphasis on dietary antioxidant and glycoalkaloid contents in five native andean cultivars (Solanum tuberosum L.). J Agric Food Chem 57(2):599–609
Arango J, Jourdan M, Geoffriau E, Beyer P, Welsch R (2014) Carotene hydroxylase activity determines the levels of both α-carotene and total carotenoids in orange carrots. Plant Cell 26:2223–2233
Atkinson NJ, Dew TP, Orfila C, Urwin PE (2011) Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum L.). J Agric Food Chem 59:9673–9682
Auldridge ME, McCarty DR, Klee HJ (2006a) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9(3):315–321
Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006b) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45(6):982–993
Bang H, Kim S, Leskovar D, King S (2007) Development of a codominant CAPS marker for allelic selection between canary yellow and red watermelon based on SNP in lycopene β-cyclase (LCYB) gene. Mol Breed 20:63–72
Ben-Amotz A, Fishler R (1998) Analysis of carotenoids with emphasis on 9-cis β-carotene in vegetables and fruits commonly consumed in Israel. Food Chem 62:515–520
Bramley PM (2002) Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot 53(377):2107–2113
Cazzonelli CI (2011) Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 38:833–847
Chaves MM, Flexas J, Pinheiro C (2008) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen MD, Zhu HS, Wen QF, Ma HQ, Lin YZ (2013) Determination of carotenoids in strawberry by UPLC. J Fruit Sci 30(4):706–711
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Phys 49:557–583
Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6(2):78–84
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29(1):185–212
Fraser PD, Enfissi EMA, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19:3194–3211
Fuentes P, Pizarro L, Moreno JC, Handford M, Rodriguez-Concepcion M, Stange C (2012) Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant Mol Biol 79:47–59
Havaux M (2013) Carotenoid oxidation products as stress signals in plants. Plant J 79(4):597–606
Herppich WB, Mempel H, Geyer M (2001) Drought- and low temperature-acclimation in carrot (Daucus carota L.) roots. J Appl Bot Food Qual 75(3):138–143
Hou X, Rivers J, León P, Mcquinn RP, Pogson BJ (2016) Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci 21(9):792–803
Huang Y, Li MY, Wang F, Xu ZS, Huang W, Wang GL, Ma J, Xiong AS (2015) Heat shock factors in carrot: genome-wide identification, classification, and expression profiles response to abiotic stress. Mol Biol Rep 42(5):893–905
Iorizzo M, Ellison S, Senalik D, Zeng P, Satapoomin P, Huang JY, Bowman M, Iovene M, Sanseverino W, Cavagnaro P, Yildiz P, Macko-Podgórni A, Moranska E, Grzebelus E, Grzebelus D, Ashrafi H, Zheng ZJ, Cheng SF, Spooner D, Deynze AV, Simon P (2016) A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat Genet 48(6):657–666
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of betacarotene and xanthophylls in plants. Plant Cell 14:333–342
Just BJ, Santos CAF, Fonseca MEN, Boiteux LS, Oloizia BB, Simon PW (2007) Carotenoid biosynthesis structural genes in carrot (Daucus carota): isolation, sequence-characterization, single nucleotide polymorphism (SNP) markers and genome mapping. Theor Appl Genet 114(4):693–704
Just BJ, Santos CAF, Yandell BS, Simon PW (2009) Major QTL for carrot color are positionally associated with carotenoid biosynthetic genes and interact epistatically in a domesticated × wild carrot cross. Theor Appl Genet 119(7):1155–1169
Kim SH, Ahn YO, Ahn MJ, Lee HS, Kwak SS (2012) Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweet potato. Phytochemistry 74:69–78
Koltai H, Kapulnik Y (2011) Strigolactones as mediators of plant growth responses to environmental conditions. Plant Signal Behav 6(1):37–41
Li HY, Li DH (2014) Expression of AtP5CS1 gene enhanced drought tolerance of transgenic Brassica oleracea plants. Plant Physiol J 50(7):1009–1013
Luby CH, Maeda HA, Goldman IL (2014) Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic Res 1:15
Ma J, Xu ZS, Tan GF, Wang F, Xiong AS (2017) Distinct transcription profile of genes involved in carotenoid biosynthesis among six different color carrot (Daucus carota L.) cultivars. Acta Biochim Biophys Sin 49(9):817–826
Ma J, Li JW, Xu ZS, Wang F, Xiong AS (2018) Transcriptome profiling of genes involving in carotenoid biosynthesis and accumulation between leaf and root of carrot (Daucus carota L.). Acta Biochim Biophys Sin 50(5):481–490
Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J 15(10):2331–2342
Mohammadkhani N, Heidari R (2007) Effects of water stress on respiration, photosynthetic pigments and water content in two maize cultivars. Pak J Biol Sci 10(22):4022–4028
Munné-Bosch S, Peñuelas J (2004) Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci 166(4):1105–1110
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82
Niyogi KK, Bjorkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci U S A 94(25):14162–14167
North HM, Almeida AD, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50(5):810–824
Parida AK, Dagaonkar VS, Phalak MS, Umalkar GV, Aurangabadkar LP (2007) Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol Rep 1(1):37–48
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Perrin F, Hartmann L, Dubois-Laurent C, Welsch R, Huet S, Hamama L, Briard M, Peltier D, Gagne S, Geoffriau E (2017) Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 245(4):737–747
Pfaffl MW (2001) New mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Que F, Hou XL, Wang GL, Xu ZS, Tan GF, Li T, Wang YH, Khadr A, Xiong AS (2019) Advances in research on the carrot, animportant root vegetable in the Apiaceaefamily. Hortic Res 6:69
Quinlan RF, Shumskaya M, Bradbury LMT, Beltrán J, Ma CH, Kennelly EJ, Wurtzel ET (2012) Synergistic interactions between carotene ring hydroxylases drive lutein formation in plant carotenoid biosynthesis. Plant Physiol 160:204–214
Razzaq M, Akram NA, Ashraf M, Hira N, Al-Qurainy F (2017) Interactive effect of drought and nitrogen on growth, some key physiological attributes and oxidative defense system in carrot (Daucus carota L.) plants. Sci Hortic 225:373–379
Reid JB, Gillespie RN (2017) Yield and quality responses of carrots (Daucus carota L.) to water deficits. N Z J Crop Hortic Sci 45(4):299–312
Riggi E, Patanè C, Ruberto G (2008) Content of carotenoids a different ripening stages in processing tomato in relation to soil water availability. Aust J Agric Res 59:348–353
Rodrigo MJ, Alquézar B, Alós E, Medina V, Carmona L, Bruno M, Al-Babili S, Zacarías L (2013) A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J Exp Bot 64(14):4461–4478
Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci U S A 97(20):11102–11107
Rys M, Szaleniec M, Skoczowski A, Stawoska I, Janeczko A (2015) FT-Raman spectroscopy as a tool in evaluation the response of plants to drought stress. Open Chem 13:1091–1100
Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JAD (2003) Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochim Biophys Acta 1619(1):9–14
Shafiq S, Akram NA, Ashraf M (2015) Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci Hortic 185:68–75
Shi YM, Liu PP, Xia YZ, Wei P, Li WZ, Zhang W, Chen X, Cao PJ, Xu YL, Jin LF, Li F, Luo ZP, Wei CY, Zhang JF, Xie XD, Qu LB, Yang J, Lin FC, Wang R (2015a) Downregulation of the lycopene ε-cyclase gene confers tolerance to salt and drought stress in Nicotiana tabacum. Acta Physiol Plant 37:210
Shi YM, Guo JG, Zhang W, Jin LF, Liu PP, Chen X, Li F, Wei P, Li ZF, Li WZ, Wei CY, Zheng QX, Chen QS, Zhang JF, Lin FC, Qu LB, Snyder JH, Wang R (2015b) Cloning of the lycopene β-cyclase gene in Nicotiana tabacum and its overexpression confers salt and drought tolerance. Int J Mol Sci 16(12):30438–30457
Simpson K, Fuentes P, Quiroz-Iturra LF, Flores-Ortiz C, Contreras R, Handford M, Stange C (2018) Unraveling the induction of phytoene synthase 2 expression by salt stress and abscisic acid in Daucus carota. J Exp Bot 69(16):4113–4126
Sun T, Simon PW, Tanumihardjo SA (2009) Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J Agric Food Chem 57:4142–4147
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35(1):44–56
Tian L, Magallanes-Lundback M, Musetti V, DellaPenna D (2003) Functional analysis of β- and ε-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15:1320–1332
Treutter D (2006) Significance of flavonoids in plant resistance: a review. Environ Chem Lett 4(3):147–157
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28(4):663–692
Wang JJ, Ye WW, Wang DL, Fan WL, Wang S (2011) Germination characteristics and comprehensive evaluation of drought resistance of 41 accessions of cotton germplasm at seed germination stage under PEG-6000 stress. J Plant Genet Resour 12(6):840–846
Wang GL, Jia XL, Xu ZS, Wang F, Xiong AS (2015) Sequencing, assembly, annotation, and gene expression: novel insights into the hormonal control of carrot root development revealed by a high-throughput transcriptome. Mol Gen Genomics 290(4):1379–1391
Wang GL, Que F, Xu ZS, Wang F, Xiong AS (2017) Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 254:839–848
Wang YH, Li T, Zhang RR, Khadr A, Tian YS, Xu ZS, Xiong AS (2020) Transcript profiling of genes involved in carotenoid biosynthesis among three carrot cultivars with various taproot colors. Protoplasma 257:949–963
Xu ZS, Huang Y, Wang F, Song X, Wang GL, Xiong AS (2014) Transcript pro-filing of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol 14:262
Xu ZS, Yang QQ, Feng K, Xiong AS (2019) Changing carrot color: insertions in DcMYB7 alter the regulation of anthocyanin biosynthesis and modification. Plant Physiol 181(1):195–207
Xu ZS, Yang QQ, Feng K, Yu X, Xiong AS (2020) DcMYB113, a root-specific R2R3-MYB, conditions anthocyanin biosynthesis and modification in carrot. Plant Biotechnol J 18:1585–1597
Zhang M, Leng P, Zhang G, Li X (2009) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166(12):1241–1252
Funding
The research was supported by National Natural Science Foundation of China (31872098), the Open Fund of the State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University (ZW201905), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ASX and RRZ. Performed the experiments: RRZ, YHW, and TL. Analyzed the data: RRZ, YHW, TL, GFT, JPT, XJS, ZSX, and YST. Contributed reagents/materials/analysis tools: ASX. Wrote the paper: RRZ. Revised the paper: ASX and YHW. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 9.69 kb)
Rights and permissions
About this article
Cite this article
Zhang, RR., Wang, YH., Li, T. et al. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 258, 379–390 (2021). https://doi.org/10.1007/s00709-020-01570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01570-5










