Abstract
Background
Nerium oleander L. is ethnopharmacologically used for diabetes. Our aim was to investigate the ameliorative effects of ethanolic Nerium flower extract (NFE) in STZ-induced diabetic rats.
Methods
Seven random groups including control group, NFE group (50 mg/kg), diabetic group, glibenclamide group and NFE treated groups (25 mg/kg, 75 mg/kg, and 225 mg/kg) were composed of forty-nine rats. Blood glucose level, glycated hemoglobin (HbA1c), insulin level, liver damage parameters and lipid profile parameters were investigated. Antioxidant defense system enzyme activities and reduced glutathione (GSH) and malondialdehyde (MDA) contents and immunotoxic and neurotoxic parameters were determined in liver tissue. Additionally, the ameliorative effects of NFE were histopathologically examined in liver. mRNA levels of SLC2A2 gene encoding glucose transporter 2 protein were measured by quantitative real time PCR.
Results
NFE caused decrease in glucose level and HbA1c and increase in insulin and C-peptide levels. Additionally, NFE improved liver damage biomarkers and lipid profile parameters in serum. Moreover, lipid peroxidation was prevented and antioxidant enzyme activities in liver were regulated by NFE treatment. Furthermore, anti-immunotoxic and anti-neurotoxic effects of NFE were determined in liver tissue of diabetic rats. Histopathogically, significant liver damages were observed in the diabetic rats. Histopathological changes were decreased partially in the 225 mg/kg NFE treated group. SLC2A2 gene expression in liver of diabetic rats significantly reduced compared to healthy rats and NFE treatment (25 mg/kg) caused increase in gene expression.
Conclusion
Flower extract of Nerium plant may have an antidiabetic potential due to its high phytochemical content.
Graphical Abstract

Highlights
-
Nerium flower extract (NFE) may have antidiabetic and antioxidant potential.
-
Nerium flower extract (NFE) may have antidiabetic and antioxidant potential.
-
NFE treatment resulted with decrease in lipid peroxidation and liver biomarkers.
-
SLC2A2 gene expression level decreased in liver of diabetic rats.
-
Histopathological improvements were observed in NFE treated diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is an endocrinological disorder that develops due to genetic origin or endocrine disrupting chemicals, stress, sedentary lifestyle and diet type. In other words, DM is a disorder characterized by high circulating glucose levels accompanied by carbohydrate, protein and fat metabolism disorders as a result of the absence or low insulin secretion from pancreatic β-cells. DM prevalence and treatment costs are increasing year by year. The global prevalence of diabetes in adults will rise to 10.5% (536.6 million people) in 2021 and 12.2% (783.2 million) by 2045, with global diabetes-related healthcare spending of US$ 966 billion in 2021 and it is estimated to reach US$ 1.054 billion by 2045 [1]. Although recent developments in pharmaceutical industry, people are interested in alternative approaches to struggle with diabetes. People suffering from diabetes pay attention medicinal plants, their extracts and isolated active compounds to improve their daily life. Therefore, the investigation of healing effects of the medicinal plants has importance, recently. The World Health Organization (WHO) has listed a total of 21,000 herbs used for medicinal purposes worldwide, more than 400 of which can be used to treat diabetes [2].
Glucose in the circulation is taken into the cell by glucose transporters (GLUT) on cell membranes as a result of the effect of insulin. Each GLUT is known to be specific or more functional in certain tissues. GLUT family has five types of GLUT called as GLUT1, GLUT2, GLUT3, GLUT4 and GLUT5. These transmembrane proteins are approximately the same size, consisting of 500 amino acids and 12 helix structures that cross the membrane. GLUT2, encoded by the Solute Carrier Family 2 Member 2 (SLC2A2) gene, is a transmembrane glycoprotein that mediates glucose transport in liver, pancreatic beta cells, small intestine, duodenum and renal system [3]. GLUT2 is required for the secretion of glucose-stimulated insulin hormone in beta cells. Mutations in the human SLC2A2 gene that impair its function are directly related to Fanconi-Bickel syndrome, which is one of the types of glycogen storage disease and is characterized by hepatomegaly and kidney disease [4].
Nerium oleander L. (N. oleander) belongs to Apocynaceae family is an ornamental tree distributed in subtropical regions of the world [5]. Although it is a poisonous plant, it has lots of pharmacologically active substances and gut microbiota is important factor for pharmaco-toxicological properties of N. oleander [6]. Glycosides (kaneroside, neriumoside, odoroside-H, neridiginoside, nerizoside and neritaloside), triterpenes (betulinic acid, ursolic acid, oleanderol, oleandric acid, oleanerolide, epoxydammarane 3β, 25-diol, cis and trans karenin and oleanolic acid) and phenolics (3‐O‐Caffeoylquinic acid (3‐CQA) and 5‐O‐Caffeoylquinic acid (5‐CQA)) have been previously reported as bioactive compounds [7,8,9,10,11,12]. Antimicrobial activity, cardioprotective, antioxidant, hepatoprotective, anticancer, anti-HIV and antidiabetic effects of N.oleander have been reported [13,14,15,16,17,18]. Additionally, it was reported that people who live in the Malda region of India use N. oleander leaves in their diabetic formulations [19].
There are many studies on the in vitro and in vivo antidiabetic effects of the N. oleander plant. It has been stated that N. oleander flower ethanolic, hexane and water extracts may have an antidiabetic effect due to its inhibitory effect on α-amylase, α-glucosidase, cholinesterase and tyrosinase as in vitro [18]. Nerium flower extract had the high phenolic content and antioxidant potential according to leaf extract [20]. However, in vivo effects of Nerium flower remain unclear. In the present study, we aimed to investigate the healing effects of ethanolic Nerium flower extract (NFE) in STZ induced diabetic rat model with biochemical, histological and molecular approaches.
Materials and methods
Chemicals and reagents
All chemicals and reagents used in this study were purchased as technical grade from Sigma Chemical Co. (St. Louis, MO, USA). The kits for the antioxidant enzymes analysis were purchased from Randox/Ransod Laboratories Ltd (Crumlin, UK). The kits for the molecular analysis were purchased from Thermo Fisher Scientific (Germany).
Plant material and extract preparation
Flowers of N. oleander L. were collected by Assoc. Dr. by Abdulahad DOĞAN in the province of Mardin, Turkey (37°54′48.1"N 41°78′78.7"E) in July 2016. Assoc. Prof. Dr. Süleyman Mesut PINAR from Van Yüzüncü Yıl University, Department of Biology, Department of Botany identified the plant. One voucher specimen (Herbarium number: 164,212) was deposited at the Herbarium (VANF) of Yüzüncü Yıl University. The ethanol extract was prepared from air-dried flowers according to Dogan et al. [21]. Briefly, dry flowers were cut in small pieces and extracted in 80% ethanol. Ethanol was evaporated using a rotavapor after filtration. Subsequently, water was evaporated from frozen extract by lyophilization process to obtain lyophilized ethanolic flower extract. Lyophilized extract was used in this study.
Animals
For this study 49 adult male Wistar albino weighting approximately 250 g each were divided as 7 groups (n = 7). Animals, which were purchased from the Van Yüzüncü Yıl University Experimental Animal Unit, were acclimatized to experimental laboratory conditions for 7 days before the experiments were done and housed in groups of seven in plastic material cages with a stainless steel lit kept at 25 ± 1 ◦C in an illumination-controlled room (photoperiod: 12 h light and 12 h darkness). Animals were fed standard feed and allowed to have water ad libitum. Animals were handled according to the guidelines of the Institutional Committee of Care and Use of Laboratory Animals of the Van Yüzüncü Yıl University Experimental Animal Unit, that approved the experiments under the file 27552122-604.01.02-E.41,738.
Toxicity test
Eighteen rats were grouped in the six different groups (n = 3) for toxicity test. OECD guideline (Test 423: acute oral toxicity method; 2002) was followed. NFE was dissolved in the distilled water. NFE (20, 100, 250, 500, 1000 and 2000 mg/kg) was given via gavage. Rats were observed for any sign of morbidity and mortality at 0.5, 2, 4, 8, 24, 48, 72, 96 and 120 h. Any sign of morbidity and mortality was not observed for 20, 100 and 250 mg/kg applications. On the other hand, 500, 1000 and 2000 mg/kg of NFE application caused mortality after 8 h. Treatment doses were selected as 25, 75 and 225 mg/kg after toxicity test.
Experimental design
To induce diabetes in rats, a 50 mg/kg dose of STZ (in citrate buffer at pH 4.5) was administered intraperitoneally (i.p.) to 35 animals selected at random [21]. Fasting blood glucose of rats was measured from tail after 72 h STZ injection by Accu-Check Go (Roche) strip. Rats with 200 mg/dL fasting blood glucose were accepted as diabetic. The study was conducted for 21 days. Rats were fed with standard pellet food and water ad libitum and they were grouped as follow:
C: Non diabetic control. Normal saline solution via gavage per day for 21 days.
N-50: Non diabetic rats treated with NFE (50 mg/kg/day) via gavage for 21 days.
D: Diabetic control. Normal saline solution via gavage per day for 21 days.
DG-3: Diabetic rats treated with glibenclamide (an oral antidiabetic drug) (3 mg/kg/day) via gavage for 21 days.
DN-25: Diabetic rats treated with NFE (25 mg/kg/day) via gavage for 21 days.
DN-75: Diabetic rats treated with NFE (75 mg/kg/day) via gavage for 21 days.
DN-225: Diabetic rats treated with NFE (225 mg/kg/day) via gavage for 21 days.
Tissue homogenization
At the end of the treatment, after 21 days, all the animals were weighed, anesthetized with ketamine (50 mg/kg) i.p. and blood samples were taken using a cardiac puncture. Samples were put immediately into disposable biochemical tubes. Subsequently, the samples were centrifuged (NF 1200 R, Turkey) at 3000×g for 15 min at 4 °C in order to obtain serum samples for the measurement of the biochemical parameters. The liver tissue was dissected and stored at − 78 °C until analysis in freeze (Thermo Scientific™ Forma™ 88,000 Series, Germany). The tissues were homogenized according to Dogan et al. [22].
Analysis of serum biochemical parameters and glycated hemoglobin
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), cholesterol (CHOL) triglyceride (TRIG), low density lipoprotein cholesterol (LDL_c), high density lipoprotein cholesterol (HDL_c) and glucose (GLU) were measured in serum by biochemistry auto analyzer (ARCHIT 16,200, Abbott Park, IL 600 USA) using Abbott biochemistry kits. Insulin (Rat INS Elisa Kit) and C-peptide (Rat C-P Elisa Kit) were measured in serum. Additionally, glycosylated hemoglobin (HbA1c) was measured in blood using Integra 400-Roche kit (DPC; Diagnostic Products Corporation, USA). Homeostatic model assessment for insulin resistance (HOMA-IR)[23] and quantitative insulin sensitivity check index (QUICKI) [24] were evaluated.
Determination of lipid peroxidation, antioxidant, immune toxic and neurotoxic parameters in liver
Malondialdehyde (MDA) content being a major product of lipid peroxidation was determined according to Jain et al. [25]. Reduced glutathione (GSH), glutathione S transferase (GST), glutathione reductase (GR), catalase (CAT), glutathione peroxidase (GPX) and superoxide dismutase (SOD) were measured using a spectrophotometer [22].
Histopathology
Necropsies of all group rats were performed at the end of the study. Tissue samples taken from liver were fixed in 10% neutral formalin solution for 48 h. All samples were washed in tap water for 12 h and paraffin-embedded. Then, 5-µm thick sections using a microtome (RM 2135; Leica, Nussloch, Germany) were cut from paraffin-embedded blocks after series of alcohol and xylene treatments, deparaffinized in xylene, and stained with Hematoxylin and Eosin [26] after being passed through alcohol series (100%, 96%, 80%, and 70%, respectively). Microphotographs of the specimens were obtained by a digital imaging system (Olympus DP12BSW, Tokyo, Japan) that was assembled in the light microscope (Olympus BX50-F4, Tokyo, Japan). Histopathological changes were evaluated semiquantitively according to degree of the lesion such as (-): none; (+): slight; (++): moderate; (+++): severe according to method [26].
Gene expression analysis of SLC2A2 gene
Liver tissue (100 mg) was homogenized with 1 mL of Trizol solution (Catalog number: 15,596,026) in an Eppendorf tube using a homogenizer and chloroform (200 µL) was added into Eppendorf tube. After centrifugation, aqueous phase (upper) was taken into a new Eppendorf tube and 500 µL of isopropanol was added into this tube to collect RNA. Tubes were centrifuged and pellet was collected. 1 mL of 75% of ethanol was added into tube and centrifuged. After supernatant throw away, pellet was washed with 1 mL of cold absolute ethanol and centrifuged. Tubes were air dried in a laminar flow cabinet. 100 µL of nuclease free water was used to solve pellet. RNA samples were treated with DNase I (Catalog number: 18068-015) to prevent DNA contamination.
RNA concentration was determined Multiskan Go (Thermo Scientific, USA) nanodrop at 260 nm. Additionally, RNA samples were read at 280 nm for protein contamination. 5 µL of RNA samples were run on agarose gel (1%) prepared with 1X TBE buffer.
cDNA libraries were constructed with RevertAid First Strand cDNA Synthesis Kit (Catalog number: K1622) according to supplier protocol. TaqMan gene expression assay (Rn00563565_m1) for rat SLC2A2 gene and TaqMan gene expression assay (Rn01775763_g1) for GAPDH gene were used to determine relative gene expression.
Statistical analysis
Data was used from at least seven independent sample for in vivo assay. Also, data was collected from at least three independent samples for gene expression assay. Minitab 13 Package Program One-Way ANOVA test was used for statistical analysis. Also, data was presented as average and standard deviation. Significantly difference between groups was accepted as P < 0.05.
Results
Serum biochemical parameters and glycated hemoglobin
GLU and HbA1c in all of the diabetic groups were significantly higher than C and N-50 groups. On the other hand, treatment with 225 mg/kg flower extract caused statistically decrease in GLU compared to other diabetic groups (Table 1). Additionally, NFE treatment showed dose dependent decrease in HbA1c level according to D and DG-3 groups. C-Peptide level of DN-75 and DN-225 groups was significantly higher than D group. Insulin level of D group was significantly lower than C and N-50 groups. Additionally, treatment of NFE extract caused increase in insulin level in DN-75 and DN-225 groups. According to Table 1, the lowest HOMA-IR values were calculated in C and N-50 groups. On the other hand, diabetic rats had the significantly higher HOMA-IR compared to C and N-50 groups. However, 225 mg/kg NFE treatment caused significant decrease in HOMA-IR compared to other diabetic groups. Similarly, 225 mg/kg NFE treatment significantly improved QUICKI value according to diabetic groups. Significant decrease in AST and ALT levels in DN-225 group was determined according to other diabetic groups, while their levels was importantly higher than C and N-50 groups. NFE caused significantly decrease in LDH level in diabetic rats according to D and DG-3 groups. NFE treatment resulted with decrease in ALP level compared to only diabetic rats, especially in DN-225 group. According to Table 1, TRIG level in DN-225 was significantly lower than C, N-50, D and DG-3 groups. The significant decrease in CHOL in N-50, D, DN-25, DN-75 and DN-225 groups was determined according to C and DG-3. The highest levels of HDL-c and LDL_c was measured in DG-3 group.
Lipid peroxidation, antioxidant, neurotoxic and immunotoxic parameters in liver
MDA content of D group was significantly higher than all other groups. Additionally, NFE extract treatment caused significant decrease in MDA content (Table 2). GSH content of D group was significantly lower than all other groups. On the other hand, GST activity in D group was importantly higher than all other groups. D group had significantly more GR activity than C and N-50 groups. However, GR activity in treatment groups was similar to C, N-50 and D groups. On the other hand, NFE treated groups, C and N-50 groups had importantly more CAT activity than D group. According to Table 2, STZ induction to crate diabetic rats caused significantly decrease in SOD activity according to C and N-50 groups. NFE treatment of diabetic rats resulted with increase in SOD activity similar to C and N-50 groups.
Liver AChE activities of D and DG-3 were significantly higher than C and N-50 groups (Fig. 1A). NFE treatment caused the decrease in liver AChE activity. Liver BChE activities of D, DG-3 and DN-50 was importantly higher than C, DG-3, DN-25 and DN-75 groups. Additionally, BChE activity of DN-225 was markedly lower than D, DG-3 and DN-25 groups. Moreover, NFE treatment caused dose dependent decrease in BChE activity (Fig. 1B).
The effects of NFE on neurotoxic and immunotoxic parameters. Different lowercase letters on the columns indicates significantly difference between groups (P ≤ 0.05). C: Non diabetic control; N-50: Non diabetic rats treated with NFE (50 mg/kg/day); D: Diabetic control; DG-3: Diabetic rats treated with glibenclamide (3 mg/kg/day); DN-25: Diabetic rats treated with NFE (25 mg/kg/day); DN-75: Diabetic rats treated with NFE (75 mg/kg/day) and DN-225: Diabetic rats treated with NFE (225 mg/kg/day)
According to liver ADA activity results, the highest activity was measured in D group. Liver ADA activity in treatment groups was significantly lower than D group (Fig. 1C). While the lowest liver MPO activity was determined in C group, the highest activity was in DG-3 group (Fig. 1CD). DN-225 application caused significantly decrease in MPO activity according to D group.
Histopathological findings in liver
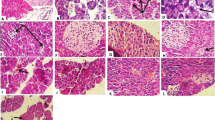
The normal histological view of the liver was seen in the C (Fig. 2A) and N-50 (Fig. 2B) groups. Degeneration in hepatocytes and coagulative necrosis as a single component were observed in liver sections of D group rats. Vacuoles in varying size were determined in the cytoplasm of degenerated hepatocytes. There was marked dilation of sinusoids. Additionally, severe congestion of veins and sinusoids and slight intrahepatic bile ducts proliferation were detected. The normal arrangement of hepatocyte cords was observed to be impaired (Fig. 2C). These findings were found to be significantly reduced in the liver of rats in DG-3 treatment group. Rare coagulative necrosis in hepatocytes and mild congestion in sinusoids were detected in this group (Fig. 2D). Groups DN-25 (Fig. 2E) and DN-75 (Fig. 2F) had similar lesions to group D, but lesions were lighter in these groups than in group D. Pathological changes were considerably reduced in group DN-225. Sinusoidal dilatation and congestion were negligible. Degeneration and necrosis were not observed. Nearly normal histology of liver was found in this group (Fig. 2G). The histopathological findings for liver are presented in Table 3.
Photomicrographs of H&E stained histological sections of the liver. A and B: Normal histological appearance of C and N groups. C: Appearance of necrosis in the hepatocytes (thick arrows), congestion of sinusoids (dashed arrows) and veins (stars), vacuolization (tiny arrows) and bile duct proliferation (arrow heads) in the D group. D: Appearance of necrosis (thick arrows) in some hepatocytes and congestion of sinusoids (dashed arrows) in the DG-3 group. E: Appearance of from slight to moderate necrosis in the hepatocytes (thick arrows), congestion of sinusoids (dashed arrows), vacuolization (tiny arrows) and bile duct proliferation (arrow heads) in the DN-25 group. F: Appearance of slight necrosis in the hepatocytes (thick arrows), congestion of sinusoids (dashed arrows) and veins (stars), vacuolization (tiny arrows) and bile duct proliferation (arrow heads) in the DN-75 group. G: Almost normal histological appearance of liver except very rare of necrosis in the hepatocytes (thick arrow), minimal congestion of sinusoids (dashed arrows) in the DN-225 group. C: Non diabetic control; N-50: Non diabetic rats treated with NFE (50 mg/kg/day); D: Diabetic control; DG-3: Diabetic rats treated with glibenclamide (3 mg/kg/day); DN-25: Diabetic rats treated with NFE (25 mg/kg/day); DN-75: Diabetic rats treated with NFE (75 mg/kg/day) and DN-225: Diabetic rats treated with NFE (225 mg/kg/day)
Gene expression analysis of SLC2A2 gene in liver
Decrease in SLC2A2 gene expression in liver tissue of D group was determined according to C group (P < 0.01) (Fig. 3A). On the other hand, there was increase in SLC2A2 gene expression in DN-25 group compared to D group (P < 0.05) (Fig. 3B).
Gene expression level of SLC2A2 gene. (A) Relative gene expression of SLC2A2 gene compared to C (** indicates p < 0.01). C: Non diabetic control; N-50: Non diabetic rats treated with NFE (50 mg/kg/day) and D: Diabetic control. (B) Relative gene expression of SLC2A2 gene compared to D (** indicates p < 0.01). C: Non diabetic control; N-50: Non diabetic rats treated with NFE (50 mg/kg/day); D: Diabetic control; DG-3: Diabetic rats treated with glibenclamide (3 mg/kg/day); DN-25: Diabetic rats treated with NFE (25 mg/kg/day); DN-75: Diabetic rats treated with NFE (75 mg/kg/day) and DN-225: Diabetic rats treated with NFE (225 mg/kg/day)
Discussion
Diabetes mellitus is a disease that its prevalence is increasing globally year by year. Although there are some medical drugs to treat DM and its complication or to improve comfortable of the people who are suffering from DM, the use of ethnomedicinal plants having antidiabetic properties is an increasing trend in the world. Leaves of N. oleander has been used as traditionally in diabetes [27] even though its toxic effects. Additionally, the antidiabetic potential of N. oleander has been reported both of in vitro and in vivo studies [28]. Moreover, antioxidant, enzyme inhibitory, cardioprotective, hepatoprotective, antibacterial, anti-inflammatory and neuroprotective activities of N. oleander flower has been reported up to now [15, 18, 29,30,31]. In this study, we aimed to explore healing effects of NFE in liver of the STZ induced diabetic rats in the light of the biochemical parameters, histopathological changes and gene expression analysis.
DM is characterized by high blood glucose and glycated hemoglobin. According to our findings, the highest NFE concentration caused decrease in blood glucose level. Additionally, NFE treatment was resulted with dose dependent decrease in HbA1c (Table 1). Similar to our results, decrease in blood glucose level was reported in diabetic mice model treated with 200 mg/kg of N. oleander leaf extract [28]. Different fractions of N. oleander flower extract showed in vitro α-amylase and α-glycosidase inhibition activity because of its high phenolic and flavonoid content [18]. Additionally, in the same study, oleanolic acid isolated from N. oleander flower extract had the high α-glycosidase inhibition activity. The lower levels or absence of insulin secretion is one of the important symptoms in diabetics. C-peptide is a key factor in insulin secretion by connecting proinsulin polypeptides. We found that DN-75 and DN-225 treatments caused increase in C-peptide level according to D group (Table 1). Additionally, insulin level in DG-3 group was higher than D group. Nerium leaf extract and glibenclamide caused the increase in insulin level in diabetic mice induced by alloxan [28] and their findings in accordance with our results. HOMA-IR and QUICKI are indexes used for evaluation of insulin resistance and sensitivity. HOMA-IR value should be lower than 2.5 and QUICKI value should be higher than 0.339 [23, 24]. According to HOMA-IR and QUICKI results, 50 mg/kg STZ induction to create rat model was resulted with insulin resistance and sensitivity in accordance with previous studies [32, 33]. We investigated that HOMA-IR and QUICKI values were reduced to reference values by 225 mg/kg NFE treatment of diabetic rats. Similar to our results, 200 mg/kg treatment of hydro methanolic root and stem extract of N. oleander had ameliorative effects on insulin resistance in diabetic mice [34].
DM is not only negatively affected the glucose and insulin levels, but also liver biomarkers such as AST, ALT, LDH and ALP and lipid profile parameters such as TRIG, CHOL, HDL_c and LDL_c are dramatically affected by DM. Oxidative stress induced by free radicals in the cell is one of the important factors in DM development. Free radicals attack cell components as carbohydrate, protein, DNA and membranes. Liver biomarkers leakage from damaged cell membrane to circulation system. Their levels in the circulation present important information in the evaluation of clinical findings. In this study, AST, ALT, LDH, ALP, TRIG, CHOL, HDL_c and LDL_c levels in the circulation system were evaluated (Table 1). We found fluctuated results for lipid profile parameters. LDL_c and HDL_c of N-50 group were significantly lower than C group. Additionally, NFE treatment caused significantly decrease in HDL_c levels of DN-25 and DN-225 compared to other diabetic groups. Thus, fluctuations in lipid profile parameters may be related with extract independent from diabetes. According to our findings, NFE treatment caused improve in liver biomarkers in STZ induced diabetic rats, especially 225 mg/kg treatment. N. oleander flower extract has hepatoprotective effect by against carbon tetrachloride-induced liver toxicity in rats [15]. Additionally, Nerium leaf extract contributed to decrease in AST, ALT and ALP levels in alloxan induced mouse model [28]. Moreover, many plant extracts caused improve in liver enzyme biomarker levels in diabetic rat models [21, 35, 36].
Cells activate their endogenous defense systems (CAT, GPx, SOD, GST and GSH) to prevent or to decrease oxidative stress damage induced by free radicals. Additionally, medicinal plants are used to as exogenous antioxidant sources against oxidative stress. Both of endogenous and exogenous antioxidant sources suppress generation of free radicals and minimize their dangerous effects. Lipid peroxidation occurs when free radicals attack to unsaturated fatty acids in membranes and MDA is produced as a major compound as a result of this process. We found the highest MDA level in D group. NFE treatment prevented lipid peroxidation by decreasing MDA levels in liver of STZ-induced rats (Table 2). Additionally, we found that NFE treatment positively affected GSH content, a non-enzymatic antioxidant, and CAT enzyme activity. Moreover, we determined that GST, GR and SOD activities of NFE treatment groups were reduced to C group. Furthermore, 225 mg/kg NFE treatment caused histopathological improvements in accordance with our biochemical parameters (Fig. 2; Table 3). The use of Nerium leaf extract in diabetic mice caused the increase in CAT enzyme activity and the decrease in MDA content [28]. In another study, stem and root extracts of Nerium showed ameliorative effects on mouse liver injury induced by a haloalkane according to histopathologic findings, antioxidant enzyme activities, liver biomarker levels and lipid peroxidation [37].
AChE and BChE enzymes were proposed as useful parameters to predict prognosis of diabetes and Alzheimer’s disease [38]. They stated that higher levels of AChE and BChE were reported in serum and in tissues of diabetic people compared to healthy ones. Our findings presented that AChE and BChE enzyme activities in liver tissue of diabetic rats were higher than non-diabetic rats. NFE treatment caused reduction of these enzyme activities (Fig. 2A and B). Neuroprotective role of methanolic extract of Nerium flowers was reported in non-classical Alzheimer’s disease rat model [30].
ADA catalyzes irreversibly adenosine to inosine to regulate its intra cellular and extra cellular concentrations. Adenosine metabolism might have been affected by DM [39] and ADA activity in serum of diabetic patients was higher than healthy ones [40]. According to our findings, while STZ caused increase in ADA activity, NFE and glibenclamide treatments resulted with decrease in ADA activity in STZ induced rat liver tissue (Fig. 2D). Similar to our results, the decrease in ADA activity was reported in diabetic rat liver tissue treated with zinc compared to diabetic rats [41]. MPO level in D group was higher than C group as expected (Fig. 2C). Additionally, NFE treatment caused decrease in MPO activity in liver tissue according to only diabetic rats. Higher MPO activity in diabetic rats was reported that results were compatible with our findings [39].
TaqMan probe is a real time quantitative PCR method providing more accurate and faster results and avoiding contamination [42]. SLC2A2 TaqMan probes were used to determine its expression in this study. GLUT2 expression is required for the physiological control of glucose-sensitive genes and its inactivation in the liver leads to disruptions in the secretion of insulin hormone [43]. Significant decrease in mRNA level of SLC2A2 gene because of relative insulin deficiency induced by STZ in D group was determined according to C group (Fig. 3A) and our results were in accordance with Abdel-Rahman et al. [44]. Decreases in GLUT2 expression are directly related to degenerated insulin secretion in diabetic mice and rats [45, 46]. On the other hand, 25 mg/kg NFE treatment caused increase in SLC2A2 expression in liver compared to only diabetic rats (Fig. 3B). Ficus deltoidea extract caused increase in SLC2A2 gene expression in rat hepatocytes [44]. mRNA levels of glucose uptake and insulin signaling related genes were regulated by polyphenol extract of green tea in rats fed with high fructose diet [47].
Nerium plant possess many bioactive compounds which are pharmacologically important. Triterpenes, flavonoids, cardenolides, pregnanes and steroids are bioactive compounds presence in different organs of Nerium plant [14, 18, 48]. Additionally, leaves of N. oleander had more phytochemicals than root and stem according to data obtained from gas chromatography analysis of hexane and methanol subfractions [48]. Moreover, the presence of ferulic acid, vanillic acid, 4-hydyoxybenzoic acid, syringic acid and myricetin in Nerium plant was reported and they have antioxidant and antidiabetic effects [34] Kaempferol, kaempferol-3-O-β-D-glucoside and chlorogenic acid were determined as active components of Nerium flower sub-extract [49]. The ameliorative effects of kaempferol in skeletal muscle and liver were reported in STZ induced diabetic rats [50]. Additionally, kaempferol was defined as a potential natural antidiabetic agent and its mechanism was revealed in the treatment of diabetes [51]. Oleanolic acid and β-sitosterol were isolated from Nerium flowers and their in vitro inhibitory effects of α-amylase, α-glucosidase, cholinesterase and tyrosinase activities were stated [18]. Kaempferol and ursolic acid were stated as the major components of Nerium plant [52]. Ursolic acid showed insulin receptor activity and proposed as having anti-diabetic potential [53].
Conclusion
NFE treatment caused decrease in glucose and HbA1c levels and HOMA-IR index in STZ induced diabetic rats. On the other hand, NFE treatment resulted with increase in QUICKI index. Increase in insulin and C-peptide levels were determined in serum of NFE treated diabetic rats. Additionally, NFE treatment improved liver damage biomarkers and prevented lipid peroxidation. Moreover, neurotoxic and immunotoxic effects of NFE were investigated in liver of diabetic rats. Furthermore, histopathologic findings in liver supported our results. While SLC2A2 mRNA level in liver was reduced in STZ induced diabetic rats, 25 mg/kg NFE administration caused increase in this gene expression. As a conclusion, flower extract of Nerium plant might have an antidiabetic potential because of its high phytochemical content.
Data Availability
All data generated or analyzed during this study is included in this article.
Ethical Animals were handled according to the guidelines of the Institutional Committee of Care and Use of Laboratory Animals of the Van Yüzüncü Yıl University Experimental Animal Unit, that approved the experiments under the file 27552122-604.01.02-E.41,738.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WHH, Zhang P, Bommer C, Kuo SH, Boyko EJJ, Magliano DJ et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183.
Kumar S, Mittal A, Babu D, Mittal A (2021) Herbal Medicines for Diabetes Management and its secondary complications. Curr Diabetes Rev 17(4):437–456
Gould GW, Thomas HM, Jess TJ, Bell GI et al (1991) Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 30(21):5139–5145
Sharari S, Abou-Alloul M, Hussain K, Khan FA et al (2020) Fanconi-Bickel syndrome: a review of the Mechanisms that lead to Dysglycaemia. Int J Mol Sci 21(17):6286
Dolgova AA, Ignateva NS et al (1963) [Morphologo-Anatomical features of Oleander Leaves]. Aptechn Delo 12:36–41
Dey P (2020) The pharmaco-toxicological conundrum of oleander: potential role of gut microbiome. Pharmacotherapy 129:110422
Siddiqui S, Hafeez F, Begum S, Siddiqui BS et al (1986) Isolation and structure of two cardiac glycosides from the leaves of Nerium oleander. Phytochemistry 26(1):237–241
Siddiqui S, Hafeez F, Begum S, Siddiqui BS et al (1988) Oleanderol, a New Pentacyclic Triterpene from the Leaves of Nerium-Oleander. J Nat Prod 51(2):229–233
Siddiqui BS, Begum S, Siddiqui S, Lichter W et al (1995) Two cytotoxic pentacyclic triterpenoids from Nerium oleander. Phytochemistry 39(1):171–174
Begum S, Siddiqui BS, Sultana R, Zia A, Suria A et al (1999) Bio-active cardenolides from the leaves of Nerium oleander. Phytochemistry 50(3):435–438
Fu L, Zhang S, Li N, Wang J, Zhao M, Sakai J, Hasegawa T, Mitsui T, Kataoka T, Oka S, Kiuchi M, Hirose K, Ando M et al (2005) Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J Nat Prod 68(2):198–206
Wong SK, Lim YY, Ling SK, Chan EWC et al (2014) Caffeoylquinic acids in leaves of selected Apocynaceae species: their isolation and content. Pharmacognosy Res 6(1):67–72
El Sawi NM, Geweely NS, Qusti S, Mohamed M, Kamel A et al (2010) Cytotoxicity and antimicrobial activity of Nerium oleander extracts. J App Anim Res 37(1):25–31
Siddiqui BS, Khatoon N, Begum S, Farooq AD, Qamar K, Bhatti HA, Ali SK et al (2012) Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry 77:238–244
Singhal KG, Das Gupta G et al (2012) Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac J Trop Med 5(9):677–685
Singh S, Shenoy S, Nehete PN, Yang PY, Nehete B, Fontenot D, Yang GJ, Newman RA, Sastry KJ et al (2013) Nerium oleander derived cardiac glycoside oleandrin is a novel inhibitor of HIV infectivity. Fitoterapia 84:32–39
Ayaz M, Baba F, Akgun N, Bas AL, Uney K, Dik B et al (2015) Protective effect of distillated Nerium oleander on heart of type 2 diabetic rats. Bratisl Lek Listy 116(7):451–456
Balkan IA, Dogan HT, Zengin G, Colak N, Ayaz FA, Goren AC, Kirmizibekmez H, Yesilada E et al (2018) Enzyme inhibitory and antioxidant activities of Nerium oleander L. flower extracts and activity guided isolation of the active components. Ind Crop Prod 112:24–31
Saha MR, Sarker DD, Kar P, Gupta PS, Sen A et al (2014) Indigenous knowledge of plants in local healthcare management practices by tribal people of Malda district, India. J Intercult Ethnopharmacol 3(4):179–185
Mohadjerani M (2012) Antioxidant activity and total phenolic content of Nerium oleander L. grown in North of Iran. Iran J Pharm Res 11(4):1121–1126
Dogan A, Celik I, Kaya MS et al (2015) Antidiabetic properties of lyophilized extract of acorn (Quercus brantii Lindl.) On experimentally STZ-induced diabetic rats. J Ethnopharmacol 176:243–251
Dogan A, Dalar A, Sadullahoglu C, Battal A, Uzun Y, Celik I, Demirel K et al (2018) Investigation of the protective effects of horse mushroom (Agaricus arvensis Schaeff.) Against carbon tetrachloride-induced oxidative stress in rats. Mol Biol Rep 45(5):787–797
Matthews DR, Hosker J, Rudenski A, Naylor B, Treacher D, Turner RJD et al (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocr Metab 85(7):2402–2410
Jain SK, Mcvie R, Duett J, Herbst JJ et al (1989) Erythrocyte-membrane lipid-peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38(12):1539–1543
Uyar A, Yener Z, Dogan A et al (2016) Protective effects of Urtica dioica seed extract in aflatoxicosis: histopathological and biochemical findings. Br Poult Sci 57(2):235–245
Hamza N, Berke B, Umar A, Cheze C, Gin H, Moore N et al (2019) A review of algerian medicinal plants used in the treatment of diabetes. J Ethnopharmacol 238:111841
Dey P, Saha MR, Chowdhuri SR, Sen A, Sarkar MP, Haldar B, Chaudhuri TK et al (2015) Assessment of anti-diabetic activity of an ethnopharmacological plant Nerium oleander through alloxan induced diabetes in mice. J Ethnopharmacol 161:128–137
Gayathri V, Ananthi S, Chandronitha C, Ramakrishnan G, Lakshmisundaram R, Vasanthi HR et al (2011) Cardioprotective effect of Nerium oleander flower against isoproterenol-induced myocardial oxidative stress in experimental rats. J Cardiovasc Pharmacol Ther 16(1):96–104
Singhal KG, Gupta GD et al (2012) Neuroprotective Appraisal of Methanolic Extract of Flowers of Nerium oleander in a non classical rat model of Alzheimer Disease. Nat Prod J 2(3):235–245
Shafiq Y, Naqvi SBS, Rizwani GH, Asghar MA, Bushra R, Ghayas S, Rehman AA, Asghar MA et al (2021) A mechanistic study on the inhibition of bacterial growth and inflammation by Nerium oleander extract with comprehensive in vivo safety profile. BMC Complement Med Ther 21(1):1–19
Okoduwa SIR, Umar IA, James DB, Inuwa HM et al (2017) Appropriate insulin level in selecting fortified diet-fed, streptozotocin-treated rat model of type 2 diabetes for anti-diabetic studies. PLoS ONE 12(1):e0170971
Wickramasinghe ASD, Attanayake AP, Kalansuriya P et al (2022) Biochemical characterization of high fat diet fed and low dose streptozotocin induced diabetic Wistar rat model. J Pharmacol Toxicol Methods 113:107144
Dey P, Saha MR, Roy Choudhuri S, Sarkar I, Halder B, Poddar-Sarkar M, Sen A, Chaudhuri TK et al (2019) Oleander Stem and Root Standardized Extracts Mitigate Acute Hyperglycaemia by Limiting Systemic Oxidative Stress Response in Diabetic Mice. Adv Pharmacol Sci 2019: 7865359
Dogan A, Celik I et al (2016) Healing effects of sumac (Rhus coriaria) in streptozotocin-induced diabetic rats. Pharm Biol 54(10):2092–2102
Agirman E, Celik I, Dogan A et al (2022) Consumption of the syrian mesquite plant (Prosopis farcta) fruit and seed lyophilized extracts may have both protective and toxic effects in STZ-induced diabetic rats. Arch Physiol Biochem 128(4):887–896
Dey P, Dutta S, Biswas-Raha A, Sarkar MP, Chaudhuri TK et al (2016) Haloalkane induced hepatic insult in murine model: amelioration by Oleander through antioxidant and anti-inflammatory activities, an in vitro and in vivo study. BMC Complement Altern Med 16(1):1–15
Rao AA, Sridhar GR, Das UN et al (2007) Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med Hypotheses 69(6):1272–1276
Uluoglu C, Cimen B, Ozbey G, Karasu C, Durakoglugil DB, Gunes A, Turkozkan N, Zengil H et al (2008) The effect of experimental diabetes on the circadian pattern of adenosine deaminase and myeloperoxidase activities in rat liver. Gen Physiol Biophys 27(1):25–31
Kurtul N, Pence S, Akarsu E, Kocoglu H, Aksoy Y, Aksoy H et al (2004) Adenosine deaminase activity in the serum of type 2 diabetic patients. Acta Medica (Hradec Kralove) 47(1):33–35
Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R et al (2021) Protective role of zinc in liver damage in experimental diabetes demonstrated via different biochemical parameters. J Biochem Mol Toxicol 35(1):e22617
Heid CA, Stevens J, Livak KJ, Williams PMJGr et al (1996) Real time quantitative PCR. Genome Res 6(10):986–994
Thorens BJD (2015) GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58(2):221–232
Abdel-Rahman RF, Ezzat SM, Ogaly HA, Abd-Elsalam RM, Hessin AF, Fekry MI, Mansour DF, Mohamed S et al (2020) Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: a new insight into its antidiabetic mechanism. J Nutr Sci 9(2):1–18
Orci L, Unger RH, Ravazzola M, Ogawa A, Komiya I, Baetens D, Lodish H, Thorens B et al (1990) Reduced beta-cell glucose transporter in new onset diabetic BB rats. J Clin Investig 86(5):1615–1622
Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S et al (1990) Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci 87(17):6492–6496
Cao H, Hininger-Favier I, Kelly MA, Benaraba R, Dawson HD, Coves S, Roussel AM, Anderson RA et al (2007) Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem 55(15):6372–6378
Dey P, Chaudhuri TKJAoBS et al (2016) Comparative phytochemical profiling and effects of Nerium oleander extracts on the activities of murine peritoneal macrophages. 68:515–5313
Atay Balkan İ, Gören AC, Kırmızıbekmez H, Yeşilada E et al (2018) Evaluation of the in vitro anti-inflammatory activity of Nerium oleander L. flower extracts and activity-guided isolation of the active constituents. Rec Nat Prod 12(2):128–141
Alkhalidy H, Moore W, Wang Y, Luo J, McMillan RP, Zhen W, Zhou K, Liu D et al (2018) The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 23(9):2338
Yang Y, Chen Z, Zhao X, Xie H, Du L, Gao H, Xie C et al (2022) Mechanisms of Kaempferol in the treatment of diabetes: a comprehensive and latest review. Front Endocrinol 13:990299
Ibrahim A, Khalifa SI, Khafagi I, Youssef DT, Khan S, Mesbah M, Khan I et al (2008) Microbial metabolism of biologically active secondary metabolites from Nerium oleander L. Chem Pharm Bull 56(9):1253–1258
Jung SH, Ha YJ, Shim EK, Choi SY, Jin JL, Yun-Choi HS, Lee JR et al (2007) Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J 403(2):243–250
Acknowledgements
We want to thank to Assoc. Prof. Dr. Süleyman Mesut PINAR because of identification of plant material.
Funding
This work was supported by Van Yüzüncü Yıl University Scientific Research Coordination Unit with grant numbers (TSA-2017-5927) and (TSA-2016-5097).
Author information
Authors and Affiliations
Contributions
Abdulhamit Battal: Development of methodology, analysis, data interpretation, manuscript writing and editing; Abdulahad Dogan: Development of methodology, analysis, data interpretation, manuscript writing and editing; Ahmet Uyar and Ömer Faruk Keleş: Histological studies; Abdulbaki Demir: Analysis data; Ismail Celik: Design of experiment and manuscript review; Mehmet Cengiz Baloglu: Analysis data and manuscript review; Ali Aslan: Data analysis.
Corresponding author
Ethics declarations
Competing Interests
Authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battal, A., Dogan, A., Uyar, A. et al. Exploring of the ameliorative effects of Nerium (Nerium oleander L.) ethanolic flower extract in streptozotocin induced diabetic rats via biochemical, histological and molecular aspects. Mol Biol Rep 50, 4193–4205 (2023). https://doi.org/10.1007/s11033-023-08332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08332-5