Abstract
Introduction
: Diabetes Mellitus is a major health problem characterized by hyperglycemia and disturbances in metabolism and implicated in causing oxidative stress. Treatment includes administration of oral hypoglycaemic agents with lifestyle modifications, these offer glycemic control, however, present limitations about availability, affordability and side effects. Traditional anti-diabetic plants are becoming popular in management of diabetes mellitus. This study was carried out to determine the efficacy of Leptadenaia hastata in treatment of diabetes.
Materials and methods
Diabetes mellitus was induced in using a single injection of streptozotocin (50 mg kg− 1 i.p.). The rats were divided into four groups of 5 rats each. Groups 3–6 received olive oil, 100 mg kg− 1 extract, 200 mg.kg− 1 extract and insulin (6IU kg− 1), respectively. 10 non-diabetic rats were grouped into two group receiving olive oil and 200 mg kg− 1 extract for 28 days. All groups were sacrificed by injecting with ketamine hydrochloride, blood was collected by cardiac puncture and centrifuged. The serum was analyzed for biochemical parameters. The liver was removed and homogenized with the supernatant of the resultant homogenate collected and used for analysis of oxidative stress enzymes.
Results
The extract significantly decreased serum AST (p < 0.05), ALP (p < 0.001), ALT (p < 0.05), TG (p < 0.01), TC (p < 0.001), creatinine (p < 0.001). It had no effect on SOD and CAT levels but it significantly increased (p < 0.001) GSH levels and reduced (p < 0.05) MDA level.
Conclusions
The n-hexane extract of Leptadenia hastata significantly decreased the levels of hepatic and renal serum biomarkers proving that it was beneficial in ameliorating diabetic related complications. The extract significantly increased GSH levels and reduced MDA level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is defined as a heterogeneous group of metabolic disorders which are characterized physiologically and functionally by deficiencies in insulin or insulin activity and clinically by hyperglycemia/ elevated blood glucose levels or impaired glucose tolerance and other manifestable disorders [1][2][3]. Hyperglycemia which is observed in diabetes is due to deficiency of insulin secretion or resistance of the body cells to the action of insulin, often also associated with carbohydrate, protein and lipid metabolism. Diabetes mellitus is classified as type I DM and type II DM.
Type I DM is occurs when the body’s immune system attacks the insulin-producing beta cells and it is believed to be caused by genes and/or family history, age and environmental factors like viruses which trigger the disease. Type II diabetes is caused by resistance of muscle and fat cells to insulin thereby preventing cellular glucose intake.
DM is ranked seventh among the leading causes of death and is considered third when its fatal complications are taken into account [4]. It has been estimated that about 171 million people worldwide suffer from diabetes [5] and this number is on an increase daily. In 2019, 463 million people worldwide were estimated to be living with diabetes and the number of people is expected to increase to 578 million by 2030 [6]. There are several predisposing causes and risk factors for diabetes and these include weight, inactivity, pregnancy (gestational diabetes), family history and age. Untreated and uncontrolled diabetes can lead to many chronic complications including blindness, heart failure, and renal failure. In order to prevent this alarming health problem, the research into developing new hypo-glycemic and potentially anti-diabetic agents of treatment is of great interest in order to ameliorate the growing effects of this disease.
Serum biochemical tests are frequently used in the diagnosis of diseases of the heart, liver, kidney and cardiovascular system. They are also widely used in monitoring the body’s response to exogenous toxic exposure. These insults are commonly manifested by changes in enzyme levels and other cell components. Generally, liver cell damage is characterized by a rise in serum enzymes like alanine aminotransferase (ALT), aspartate aminotransferase (AST), Alkaline Phosphatase (ALP) [7].
An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules [8]. Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. This can produce free radicals, which begin chain reactions that damage cells. Antioxidants terminate these chain reactions by removing free radical intermediates, and thus inhibit other oxidation reactions by being oxidized themselves [8]. Free radicals are chemical species that contain unpaired or free electrons; they can be obtained from normal metabolism or external source such as exposure to an organic chemical in the environment [5]. Normally there is a balance between oxidants and antioxidants in a cell, these levels are controlled by the body’s anti-oxidative defense system. Nevertheless, failure of the body’s anti-oxidative defense system to appropriately function or overproduction of reactive oxygen species may lead to increased production of free radicals therefore creating an imbalance in the cell [9]. Oxidation reactions are both beneficial and harmful to life, if not balanced they may lead to oxidative stress resulting in tissue damage. Oxidative stress refers to the existence of products called free radicals and reactive oxygen species (ROS), which are formed under normal physiological conditions but become harmful when not being removed by the endogenous systems present in the body. Oxidative stress is caused by an increase in the levels of free radicals in the body due to an imbalance between oxidants and antioxidants; it is the cause of many disease conditions including DM [9].
ROS are known to produce adverse modifications to cellular components, such as lipids, proteins, and DNA. It has been implicated in cardiovascular disease [10], neural disorders [11], Alzheimer’s disease [12] mild cognitive impairment [13], Parkinson’s disease [14], alcohol induced liver disease [15], ulcerative colitis [16], ageing [17], atherosclerosis and diabetes [7]. Oxygen derived free radicals such as superoxide anions, hydroxyl radicals and hydrogen peroxide are cytotoxic and give rise to tissue injuries [18][19].
The human body can to some extent fight the effects of free radicals (oxidants) on its own, there are four sources of antioxidants in a biological system these include enzymatic antioxidants, large molecules, small molecules and some hormones [20].
Antioxidants protect cells from being damaged by slowing down or preventing oxidation of biomolecules, they impede the initiation of oxidation chain reactions and therefore turn ROS into stable and harmless molecules [21]. The body’s effective defense mechanism in preventing and counteracting the free radical-induced damage is accomplished by a set of endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX). These enzymes constitute a defense against ROS [22].
Superoxide dismutase is an antioxidant enzyme that catalyses the dismutation of the highly reactive superoxide anion to O2 and to the less reactive species hydrogen peroxide (H2O2). Peroxide can be destroyed by CAT or GPX reactions. Catalase is a primary component of the antioxidant system that defends against oxidative stress which is ubiquitously associated with pathologic conditions, including cancer, diabetes, cataracts, atherosclerosis, ischemic /reperfusion injury, arthritis, neurodegenerative disease, nutritional deficiencies and aging. Catalase decomposes H2O2 which is a major reactive oxygen species in the presence of iron or other metal ions oxidizes cellular biochemical to cause cytotoxicity [23].
Glutathione reduced (GSH) is a super oxide radical scavenger that protects thiol protein groups required for maintaining the integrity of cell against oxidation. GSH serves as the most important intracellular radical scavenger and is a substrate for the antioxidant enzyme GPx [24]. Decreased glutathione levels in diabetes have been considered to be an indicator of increased oxidative stress. GSH also functions as free radical scavenger in the repair of radical based biological damage. A decrease in glutathione levels is usually observed in the liver during diabetes [25].
Lipid peroxidation is a free radical mediated chain reaction that can inactivate cellular components and are purportedly associated with various chronic disorders [26]. Lipid peroxidation evaluated by the thiobarbituric acid reactive substances method assays for malondialdehyde, the last product of lipid breakdown caused by oxidative stress. Malondialdehyde (MDA) is a useful index of lipid peroxidation being a major breakdown product of lipid peroxides. The engagement and overwhelming of antioxidant enzymes by free radicals result in the depletion of the antioxidant defenses and induction of lipid peroxidation evident in elevation of MDA level [27]. Oxidative stress induced by hyperglycemia in DM accelerates a state in which ROS increases and anti-oxidant status of enzymes like GSH, CAT and SOD reduces [27].
Under diabetic conditions, chronic hyperglycemia and chronic augmentation of reactive oxygen species deteriorates cell function and increases insulin resistance which leads to aggravation of type II diabetes. Considerable studies suggest that hyperglycemia substantially leads to the generation of reactive oxygen species (ROS), triggering oxidative stress as well as numerous cellular and molecular modifications affecting normal physiological functions in the body [28]. It has been proven that beside its osmotic effect on cells, hyperglycemia activates diacylglyceride (DAG) formation together with an activation of protein kinase C (PKC) and NADPH-oxidase which leads to the production of ROS and oxidative stress in diabetes [29].
Leptadenia hastata (Pers.) Decne is an edible, non-domesticated vegetable found in the wild throughout Africa. The crushed leaves have been also used as dressing for fresh cuts, wounds and ulcers. The plant has been reported to have antibacterial, anti-inflammatory and trypanocidal properties [30]. A decoction made from the leaves are also ingested to manage diabetes mellitus. Studies conducted by [30][31][32]evaluated the anti-diabetic, hypoglycaemic and hypolipidaemic effects of water, methanol and n-hexane extracts of Leptadenia hastata in alloxan and streptozotocin induced diabetic rat models demonstrating the hypoglycaemic and hypolipidaemic effects of Leptadenia hastata extracts as well its potential in the management of diabetes mellitus [30].
Phytochemical screening of n-hexane extract of Leptadenia hastata leaf indicated the presence of steroids, triterpenoids and cardiac glycosides. The elemental analysis revealed that Na, SO4 and PO4 had the highest levels in the extract. Pb, Hg and Cd were not detected which showed that the plant extract was not toxic. Other elements (NO4, Mn, Fe, K, Ni, Si, Cn, Zn, Ca, Mg, NH4, Cr and F) were present in varying concentrations. Triterpenes which is a phytochemical found in the n-hexane extract of Leptadenia hastata is reported to play an important role as a plant antioxidant [33].
Despite the presence of many known anti-diabetic medicines in the pharmaceutical market, the search for new anti-diabetic and hypoglycemic agents recovered from natural plants is still attractive and preferable for individuals in rural areas that have no access to orthodox medication and because these herbs contain substances which may have an alternative and innocuous effect on diabetes mellitus [34]. The present study was undertaken with the expectation of finding an available and affordable medication that would also enhance the treatment/management of diabetes and its complications.
Materials and methods
Chemicals Used
Streptozotocin (Bristol Scientific Company, Missouri, United States), soluble insulin injection (Novo Nordsick, Denmark), ketamine hydrochloride (Rotexmedica, Trittau, Germany) were purchased online, Sodium citrate granules (BDH chemicals, Poole, England) and citric acid granules (BDH chemicals, Poole, England) were dissolved in distilled water to make citrate buffer in which streptozotocin powder was dissolved. Sodium dihydrogen orthophosphate and sodium hydroxide (BDH chemicals, Poole, England) was also dissolved in distilled water to give phosphate buffer. Randox kits protocol (United Kingdom) were purchased to determine the biochemical parameters that were required.
Collection, Identification and Storage of Plant Material
Leptadenia hastata leaves was collected from a private garden in the University of Maiduguri staff quarters, Borno State, the plant was authenticated by a plant taxonomist from the Department of Biological Sciences, Faculty of Science, University of Maiduguri. The leaves were collected, cleaned and shade-dried for a period of two weeks and then ground to fine powder. The powder was sifted to obtain the fine powder, which was then packaged, labeled and stored for use.
Extraction of Plant Material
Maceration technique as described by [33] was used for extraction in the current study. The leaf powder weighing 500 g was dissolved in 3 L of n-hexane in a 5 L stoppered bottle. The maceration process involved soaking the leaf powder and allowing it to stand at room temperature for a period of 3 days at the minimum with intermittent agitation. The process softened and broke down the plant’s cell wall to release the soluble phytochemicals present. After 3 days, the mixture was filtered using filter paper. The resultant n-hexane filtrate was concentrated to dryness in-vacuo using an evaporator and the resulting powder was kept in an air–tight container and refrigerated to preserve.
Experimental Animals
The experimental study was carried out according to the protocol of the European Committee Guidelines for the use of experimental animals. The study was performed using Wistar albino rats of both sexes. A total of 30 albino rats weighing 135–190 g were used. The rats were obtained from the National Veterinary Research Institute (NVRI) Vom, Plateau State, Nigeria. They were kept in the Animal house of the Department of Human Anatomy, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Maiduguri, Borno State The rats were maintained under controlled conditions of humidity (50–60%), temperature of 220 C±30 C, 12 h light and 12 h dark as well as adequate ventilation. They were fed with pelletized ECWA (Jos) feed and water ad libitum.
Animal Grouping
A total of thirty rats (20 diabetic and 10 normal) of both sexes were randomly divided into 6 groups of 5 animals each and treated as indicated below. This treatment commenced after diabetes was confirmed and continued for a period of 28 days.
Group 1 - Non-diabetic control group were administered olive oil as vehicle.
Group 2 - Non-diabetic group that was administered 200 mg kg− 1 of n-hexane extract of Leptadenia hastata.
Group 3 – Induced with 50 mg kg− 1 of Streptozotocin and received olive oil as vehicle.
Group 4 - Induced with 50 mg kg− 1 of Streptozotocin and treated with 100 mg kg− 1 of n-hexane extract of Leptadenia hastata.
Group 5 - Induced with 50 mg kg− 1 of Streptozotocin and treated with 200 mg kg− 1 of n-hexane extract of Leptadenia hastata.
Group 6 – Induced with 50 mg kg− 1 of Streptozotocin and treated with insulin (6IU/kg).
Experimental Induction of Diabetes in Rats
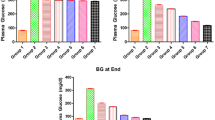
Hyperglycemia was induced in overnight fasted Albino Wistar rats by a single intra-peritoneal injection of 50 mg kg − 1 streptozotocin (Bristol-Sigma, Bristol Scientific Company, Missouri, United States of America) dissolved in 0.1 M ice-cold sodium citrate buffer, (pH = 4.5), immediately before use in a volume of 1ml/kg body weight as described by [35]. Hyperglycemia was confirmed by the elevated plasma glucose levels determined in tail blood sample using a glucometer (Roche, Germany). Rats whose fasting blood glucose levels exceeded 250 mg dl− 1 (13mmol dl− 1) after one week were considered as diabetic and used for the study.
Experimental Protocol
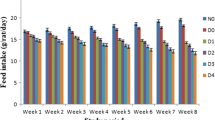
The extract was administered orally for a period of 28 days at a dosage of 100 mg kg− 1 and 200 mg kg− 1. Two dosages of the extract were chosen to determine at what dosage the extract was most effective. Insulin is usually administered to diabetic patients to manage diabetic complications by increasing glucose uptake hence the use of insulin in the present study. Body weight and food/water consumption were noted and at the end of this period, the serum was analyzed for biochemical assays (serum glucose, total cholesterol, triglycerides, high density lipoprotein (HDL), aspartate amino transferase (AST), alanine amino transferase (ALT) levels, Alkaline Phosphatase (AP), Total Protein (TP), Urea, Creatinine and Albumin from rats in all groups (I – VI).
The liver was quickly removed and placed on ice to prevent autolysis and putrefaction. A portion of the liver was homogenized in 5mls chilled phosphate buffer (pH 7.2) using a homogenizer. The homogenate obtained was centrifuged at 15,000 rpm for 10 min, supernatant collected and stored on ice and used for analysis of oxidative stress enzymes (CAT, MDA, GSH and SOD). The method used was as described by [36]. The administration of extract was carried out in the period of 28 days. During the study period, standard feed and water was provided ad libitum. Changes in body weight as well as physical appearance of the rats was noted and recorded every week. On the 28th day, all rats were sacrificed first by inducing sleep with ketamine hydrochloride (Rotexmedica, Trittau, Germany). Blood was collected from the heart chamber by cardiac puncture and centrifuged to separate the blood cells from the serum which was analyzed for biochemical parameters [37].
Statistical Analysis
Data was statistically analyzed using GraphPad InStat software (version 3.75) by using One-way Analysis of Variance (ANOVA) and expressed as mean ± SEM and percentage followed by Bonferroni Multiple Comparisons Test. p < 0.05 was considered to be statistically significant.
Results
Effect of n-hexane leaf Extract of Leptadenia hastata on Biochemical Parameters
The aspartate aminotransferase (AST) content in serum was significantly (P < 0.05) higher in group III diabetic untreated rats (DC) when compared to the non-diabetic groups. Treatment with the extract showed significant (P < 0.05) decrease in the level of AST content when compared to the diabetic untreated group. Treatment of diabetic rats with the extract (groups IV and V) significantly reduced (P < 0.05) ALT activity when compared to the diabetic untreated rats (group III). The value was comparable to the diabetic rats treated with insulin. The result of the AP activity indicated that untreated diabetic rats (Group III) had significantly higher activity, while the extract treated rats (Group IV and V) recorded an activity which was similar to the non-diabetic groups (I and II) [Table 1].
There was no significant (P > 0.05) difference level in TP except for the insulin treated (Group VI), which it was significantly lower (P < 0.05) than all other groups. Analysis of TG level showed that diabetic untreated rats (group III) had the significantly (P < 0.05) highest TG values than other groups. There was a significantly (P < 0.05) higher serum levels of TC in the untreated diabetic rats (group III) than in the non-diabetic rats (groups I and II). However, the values in diabetic rats treated with the extract (groups IV and V) were similar to those of the non-diabetic rats (groups I and II). There was no significant (P > 0.05) difference in high density lipoprotein (HDL) levels in the serum of rats in all groups. There was no significant (P > 0.05) difference in low density lipoprotein (LDL) levels in the serum of rats in all groups (Table 2).
There was no significant (P > 0.05) difference in albumin and urea content in the serum of rats in all groups. Serum creatinine was significantly (P < 0.001) higher in diabetic untreated rats (group III) than non-diabetic rats (groups I and II) [P < 0.05]. However, diabetic rats treated with the extract (groups IV and V) and insulin (group VI) have creatinine levels similar to the non-diabetic rats (P > 0.05) [Table 3].
Antioxidant Parameters from Liver Homogenate
The catalase activity from the liver homogenate was not significantly different in all the groups when compared to the diabetic and non-diabetic groups (Table 4) GSH activity was significantly higher in groups II, IV and VI than in group I (P < 0.05). However, the diabetic untreated (group III) and group V showed similar GSH activity with group I [P > 0.05]. MDA activity was significantly higher in group III than group I (P < 0.05). In addition, the activity was similar in groups IV – VI with that of group I (P > 0.05) [Table 4].
The superoxide dismutase content from the liver homogenate was similar in all the diabetic groups that received either the extract or insulin and the non-diabetic groups (I and II) [P < 0.05] (Table 4).
Discussion
In the present study, experimental DM increased activities of plasma enzymes (ALT, AST, and ALP) in the diabetic groups. This might be primarily due to the leakage of these enzymes, and more specifically ALT, from the liver cytosol into bloodstream [38] demonstrating a hepatocellular damage [39].
In the groups that were administered n-hexane extract of Leptadenia hastata, there was a significant decrease in AST, ALT and ALP levels compared to the diabetic untreated group. This was an indication that the extract may have an effect on reducing hepatic damage in the rats in these groups. Triterpenoids which are rich in this extract has been reported to be hepatoprotective [40][33]. The decrease of ALT and AST levels in rats treated with the extract is consistent with the studies of Hussein et al. (2006), where diabetic rats which were administered the extract exhibited reduced serum AST, ALP and ALT levels. The decrease of the transaminase activity as a result of administration the treatment has resulted in improved liver function [41][42][43].
DM is usually associated with several abnormalities of fats and lipoproteins [44] which were noticed in STZ-induced diabetic animals. There was an observed elevation in plasma concentrations of TG and TC in the present study in the diabetic control group which may be regarded as a result of a profound decrease in catabolism of TG-rich lipoproteins secondary to reduced activity of lipoprotein lipase [45]. The reduction in cholesterol and triglycerides in diabetic rats in the present study may be attributed to increased clearance and decreased production of the major transporters of endogenously synthesized total cholesterol and triglycerides [46]. A similar effect was reported by [46] [47].
STZ-diabetic animals also have enhanced plasma levels of creatinine. This could be attributed to reduced clearance of these substances, reflecting a decline in the glomerular filtration rate (GFR) [48] and/or an increased net tubular absorption. However, during this metabolic disorder, creatinine, the degradation derivative of creatine and phosphocreatine (that are viewed as energy storage compounds in skeletal muscles) could also be a result of an extensive muscles breakdown associated with an increased catabolism of liver and plasma proteins [49] [42]. Treatment with 100 mg.kg− 1 and 200 mg.kg− 1 significantly (P < 0.05) diminished the levels of creatinine compared to the mean values of the diabetic control group. This may be interpreted as an improved renal function associated with decreased protein degradation secondary to reduced glucose concentration and subsequent glycation as also indicated in a study performed by [43][3]. It could also be attributed to a decrease in protein catabolism through increasing glucose uptake in accordance with studies by [50]. Streptozotocin-induced diabetes is accompanied by increased generation of reactive oxygen species (ROS) and also significantly increases lipid peroxides and decreased antioxidant enzyme activities in the plasma of rats which in turn induced lipid peroxidation [51] [52]. In the study, the reduction in MDA activity in diabetic rats treated with the extract is the indication that the extract was effective in decreasing the products of lipid peroxidation and consequently decreasing MDA levels.
Triterpenes is reported to be found in phytochemical analysis of Leptadenia hastata[53] The lupine-type triterpene, bacosine, obtained from the herb of Bacopa monnieri (Scrophulariaceae) also displayed antioxidant properties by significantly decreasing the level of malonylaldehyde and increasing the level of glutathione (GSH) but unlike the present study, the activity of SOD and CAT in the liver of diabetic rats were also increased [54] and [55] showed significant decrease in the MDA concentration for extract treated groups compared with the diabetic control group. This is in line with the present study and indicates that Leptadenia hastata has an antioxidant property in preventing lipid peroxidation seen in diabetes. [56] showed that serum levels GPx were increased after supplementation with zinc. In the present study, the presence of zinc in the extract may have helped to improve the antioxidant status in diabetes. This could probably result from the supportive role played by zinc on the body’s antioxidant defense systems. Such roles include; acting as a co-factor for superoxide dismutase (Isoforms 1 and 3), regulating glutathione metabolism and metallothionein expression, competing with iron and copper in the cell membrane as well as inhibiting the nicotinamide adenine dinucleotide phosphatase-oxidase (NADPH-oxidase) enzyme [57].
Conclusions
The use of affordable and available alternative medication in the treatment of diabetes and its associated complications including oxidative stress is the objective of the current work. Leptadenia hastata has been locally used to reduce serum glucose levels, however, little is known about its effect on oxidative stress biomarkers and liver function enzymes. In the present study, the extract was found to significantly decrease hepatic and renal serum biomarkers proving that it was beneficial in ameliorating diabetic related complications. However, the extract had no effect on SOD and CAT levels of liver homogenate but significantly increased GSH levels and reduced MDA level. The extract is therefore showed promise in improving oxidative stress in diabetic rats.
References
Niamat R, Khan MA, Khan KY, Ahmed-Mazari P, Ali B, Mustafa M, Zafar M. A Review on Zizyphus as Antidiabetic. J Appl Pharm Sci. 2012;02(03):177–9.
Neto MCL, de Vasconcelosa CFB, Thijana VN, Caldasa GFR, Araújob AV, Costa-Silvac JH, Amorima ELC, Ferreirab F, de Oliveirad AFM, Wanderleya AG. Evaluation of anti-hyperglycaemic activity of Calotropis procera leaves extract on streptozotocin-induced diabetes in Wistar rats. Brazillian J Pharmacognosy. 2013;23:913–9.
Réggami Y, Berredjem H, Cheloufi H, Berredjem M, Bouzerna N. Anti-hyperglycemic and antidiabetic effects of Ethyl (S)-2-(1-cyclohexylsulfamide carbamoyloxy) propanoate in streptozotocin-induced diabetic Wistar rats. Eur J Pharmacol. 2016;779:122–30.
Trivedi NA, Majumder B, Bhatt JB, Hermavathi KG. Effects of Shilajit on blood glucose and lipid profile in alloxan-induced diabetic rats. Indian J Pharmacol. 2004;36:373–6.
Lai ZR, Ho YL, Huang SC, Huang TH, Lai SC, Tsai JC, Wang CY, Huang GJ, Chang YS. (2011) Antioxidant, anti-inflammatory and anti-proliferative activities of Kalanchoe gracilis (L.) DC stem. The American Journal of Chinese Medicine, 39:1275–1290.
International Diabetes Foundation (IDF) IDF Diabetes Atlas. 2019, Ninth Edition, 2019. http://www.idf.org retrieved 12th August, 2020, 10.28am.
Rajeshkumar D. (2010), “Evaluation of Antioxidant Property and Toxicological Assessment of Polyalthia Longifolia var. Pendula Leaf”, thesis PhD, Saurashtra University.
Kawsar MH, Raihana R, Sultana T, Sohel D, Sohaily SI. In-Vitro and In-vivo Models for Antioxidant Activity Evaluation: A Review. J SUB. 2014;5(1):21–31.
Sagnia B, Fedeli D, Casetti R, Montesano C, Falcioni G, Colizzi V. Antioxidant and anti-inflammatory activities of extracts from Cassia Alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLOS ONE 2014;9:1-10
Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–42.
Sas K, Robotka H, Toldi J, Vecsei L. Mitochondrial, metabolic disturbances, oxidative stress and kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–39.
Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochimca et Biophysica Acta. 2000;1502:139–44.
Guidi I, Galimberti D, Lonati S, Novembrino C, Bamonti F, Tiriticco M, Fenoglio C, Venturelli E, Baron P, Bresolin N. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2006;27:262–9.
Bolton JL, Trush MA, Penning TM, Dryhurst G. Monks T.J. Role of quinones in toxicology. Chem Reearch Toxicol. 2000;13:135–60.
Arteel GE. Oxidants and Antioxidants in Alcohol-induced Liver Disease. Gastroenterology. 2003;124:778–90.
Ramakrishna BS, Varghese R, Jayakumar S, Mathan M, Balasubramanian KA. Circulating antioxidants in ulcerative colitis and their relationship to disease severity and activity. J Gastroenterol Hepatol. 1997;12:490–4.
Hyun DH, Hernandez JO, Mattson MP, de Cabo R. The plasma membrane redox system in aging. Aging Resesearch Reviews. 2006;5:209–20.
Jainu M, Devi CS. In vitro and in vivo evaluation of free radical scavenging potential of Cissus quadrangularis. Afr J Biomedical Res. 2005;8:95–9.
Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role of inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29.
Prior RL, Wu X, Schaich R. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–302.
Nahak G, Suar M, Sahu RK. Antioxidant potential and nutritional values of vegetables: A review. Research Journal of Medicinal Plants.2014; 8(2):50-81.
Venukumar MR, Latha MS. Antioxidant activity of Curculigo orchioides in carbon tetrachloride induced hepatopathy in rats. Indian J Clin Biochem. 2002;17:80–7.
Vendemiale G, Grattagliano I, Altomare E. An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res. 1999;29:49–55.
Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori induced inflammation and oxidative stress. Free Radical Biology and Medical Journal. 2002;33:323–36.
Ghosh T, Maity TK, Sengupta P, Dash DK, Bose A. Antidiabetic and In Vivo Antioxidant Activity of Ethanolic Extract of Bacopa monnieri Linn. Aerial Parts: A Possible Mechanism of Action. Iran J Pharm Res. 2008;7(1):61–8.
Halliwell B, Wasil M, Grootveld M. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorus acid by ascorbic acid. Implications for antioxidant protection in the inflamed rheumatoid joint. FEBS Lett. 1987;213:15–7.
Akindele AJ, Ezenwanebe KO, Anunobi CC, Adeyemi OO. Hepatoprotective and in vivo Antioxidant effects of Byrsocarpus coccineus Schum. and Thonn.(Connaraceae). J Ethnopharmacol. 2010;129:46–52.
Pasupuleti VR, Arigela CS, Gan SW, Salam SJN, Krishnan KT, Rahman NA, Jeffree MS. A Review on Oxidative Stress, Diabetic Complications and the Role of Honey Polyphenols. Oxidative Medicine and Cellular Longevity. 2020;2020:8878172,1–16.
Volpe CMO, Vilr-Delfino PH, dos Anjos PMF, et al., Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications Cell Death Diseases 2018. 9;119.
Bello A, Aliero AA, Saidu Y, Muhammad. Phytochemical Screening, Polyphenolic Content and Alpha-Glucosidase Inhibitory Potential of Leptadenia hastata (Pers.) Decne. Nigeria Bayero Journal of Pure and Applied Sciences. 2011;19(2):181–6.
Sanda KA, Sandabe UK, Auwal MS, Bulama I, Bashir TM, Sanda FA, Mairiga A. Hypoglycemic and Antidiabetic Profile of the Aqueous Root Extracts of Leptadenia hastata in Albino Rats. Pakistan J Biol Sci. 2013;16(4):190–4.
Attah MOO, Jacks TW, Garba SH, Balogun JB. Hypoglycemic and Anti-Diabetic Profile of N-Hexane Extract of Leptadenia Hastata Leaves on Streptozotocin-Induced Diabetes in Albino Rats. Sumerianz J Med Healthc. 2019;2(4):42–6.
Attah MOO, Jacks TW, Garba SH, Mshelia HE. Physico-Chemical and Phytochemical Screening of N-Hexane Extract of Leptadenia Hastata Leaves: A Proposed Herbal Remedy in the Treatment of Diabetes Mellitus. Int J Res - Granthaalayah. 2019;7(2):45–57. “.” ; ).
Jung BJ, Ji Su K, Chang WC, Hae KL, Tae-Kyun O, Sei CK. (2008). Comparison between ethanolic and aqueous extract from Chines Juniper berries for hypoglycaemic effects in alloxan induced diabetic rats. Journal of Ethnopharmacology. 2008,115: 110–115.
Etuk EU. Animals Models For Studying Diabetes Mellitus. Agric Biology J North Am. 2010;1(2):130–4.
Omodanisi EI, Aboua YG, Chegou NN, Oguntibeju OO, Hepatoprotective. antihyperlipidemic, and anti-inflammatory activity of Moringa oleifera in diabetic-induced damage in male wistar rats. Pharmacol Res. 2017;9:182–7.
Oyebadejo S, Bassey E, Oyewunmi A, Archibong V. and Usoro E. Histopathological study of the liver of Alloxan induced diabetic rats and macerated Allium sativum (garlic) Ameliorative Effect. Asian J Biomedical Pharm Sci. 2014;4(34):72–7.
Navarro MC, Montilla MP, Martín A, Jiménez J, Utrilla MP. Free Radical Scavenger and Anti-hepatotoxic Activity of Rosmarinus tomentosus. Planta Med. 1993;59:312–4.
El-Demerdash FM, Yousef MI, El-Naga N. I.A. Biochemical Study on the Hypoglycemic Effects of Onion and Garlic in Alloxan-induced Diabetic Rats. Food and Chemical Toxicology. 2005;43:57–63.
Nazaruk J, Borzym-Kluczyk M. The Role of Triterpenes in the Management of Diabetes Mellitus and its complications. Phytochemical Reviews. 2015<bi>;</bi>;14:675–90.
Hussein HM, El-Sayed EM, Astaa AS, Antihyperglycemic. Antihyperlipidemic and Antioxidant Effects of Ziziphus spina Christi and Zizipus jujube in Alloxan Diabetic Rats. Int J Pharmacol. 2006;2(5):563–70.
Rivadeneyra-Domíngueza E, Becerra-Contrerasa Y, Vázquez-Lunaa A, Díaz-Sobaca R, Rodríguez-Landaa JF. Alterations of blood chemistry, hepatic and renal function, and blood cytometry in acrylamide-treated rats. Toxicol Rep. 2018;5:1124–8.
Andjelkovic M, Djordjevic AB, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int J Environ Res Public Health. 2019;16:274.
Dunn FL. Hyperlipidemia in Diabetes Mellitus. Diabetes Metabolism Review. 1990;6:47–61.
Laakso M. Lipid Disorders in Type 2 diabetes. Endocrinolology and Nutrition. 2009;56:43–5.
Pepato MT, Folgado VB, Kettethut IC, Branetti TL. Lack of antidiabetic effect of a Eugenia jambolana leaf decoction on rat streptozotocin diabetes. Brazillian J Med Biol Res. 2001;34(3):389–95.
Chen H, Feng R, Guo Y, Sun L, Jiang J. Hypoglycemic effects of aqueous extract of Rhizoma polygonati odorati in mice and rats. J Ethnopharmacol. 2001;74(3):225–9.
Raju J, Kocmarek A, Roberts J, Taylor M, Patry D, Chomyshyn E, Caldwell D, Cooke G, Mehta R. Lack of adverse health effects following 30-weeks of dietary exposure to acrylamide at low doses in male F344 rats. Toxicol Rep. 2016;3:673–8.
Belhadj-Benziane A, Dilmi-Bouras A, Mezaini A, Belhadri A, Benali M. Effect of oral exposure to acrylamide on biochemical and hematologic parameters in Wistar rats, Drugs and Chemical Toxicology (2019) 42(2):157-166
Umesh CS, Yadav K, Moorthy K, Najma Z. Combined treatment of sodium orthovanadate and Mormodica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes an alloxan diabetic rats. Mol Cell Biochem. 2005;268:111–20.
Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 2006;40(2):168–76. “,” .
Kroncke KD, Fehsel K, Sommer A, Rodriguez ML, Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-N-nitroso-urea streptozotozin contributes to islet cell DNA damage. Biol Chem. 2006;376(30):179–85. “,” .
Nikiema JB, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J, Graef CD, Heenen M. Effects of Anti-inflammatory Terpenes Isolated from Leptadenia hastata Latex on Keratinocyte Proliferation. Phototherapy Resour. 2001;15(1):131–4.
Ghosh PK, Gaba A. Phyto-extracts in wound healing. J Pharm Pharm Sci. 2013;16:760–820.
Eze DE, Yusuf T, Abubakar A, Suleiman OS, Rabiu MK, Mohammed A. Lycopene Ameliorates Diabetic-Induced Changes in Erytrocytes Osmotic Fragility and lipid peroxidation in Wistar Rats. J Diabetes Mellitus. 2017;7(3):71–85.
Ranasinghe P, Pigera S, Galappatthy P, Katulanda P, Constantine GR. Zinc and diabetes mellitus: understanding molecular mechanisms and clinical implications. Daru J Pharm Sci. 2015;23(1):44.
Teru GAD, Ahmed MK, Denis IA, Sani M. Ameliorative Effects of Combined Administration of Lycopene and/or Zinc on Biomarkers of Oxidative Stress in Alloxan- Induced Diabetic Wistar Rat. Open Sci J Pharm Pharmacol. 2018;6(3):21–5.
Acknowledgements
The authors would like to acknowledge Prof M. O. Attah, E.O. Attah, Dr. S.T. Balogun, Dr. J.V. Zirahei and Dr. J. Balogun. The authors also appreciate the support from the staff of the histology laboratory, University of Maiduguri and the Pharmacognosy Laboratory, Department of Pharmacy, University of Maiduguri.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there were no conflicts of interest concerning this publication.
Financial Support and Sponsorship
The present research work was privately funded.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martha Orendu Oche, A., Tamunotonye Watson, J., Sani Hyedima, G. et al. Leptadenia hastata Leaf Extract ameliorates oxidative stress and serum biochemical parameters in Streptozotocin-Induced diabetes in Wistar rats. J Diabetes Metab Disord 21, 1273–1281 (2022). https://doi.org/10.1007/s40200-022-01017-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01017-z