Abstract
Purpose
Breast cancer is the most common malignancy in developed countries and the main cause of deaths in women worldwide. Lactoferrin (Lf) is an iron-binding protein constituted for a single polypeptide chain that is folded into two symmetrical lobes that bind Fe2+ or Fe3+. Lf has the ability to reversibly bind Fe3+ and is found free of Fe3+ (Apo-Lf) or associated with Fe3+ (Holo-Lf) with a different three-dimensional conformation. However, the role of bovine Apo-Lf (Apo-BLf) and bovine Holo-Lf (Holo-BLf) in the migration and invasion induced by linoleic acid (LA) and fetal bovine serum (FBS), as well as in the expression of mesenchymal and epithelial proteins in breast cancer cells has not been studied.
Methods and results
Scratch wound assays demonstrated that Holo-BLf and Apo-BLf do not induce migration, however they differentially inhibit the migration induced by FBS and LA in breast cancer cells MDA-MB-231. Western blot, invasion, zymography and immunofluorescence confocal microscopy assays demonstrated that Holo-BLf partly inhibit the invasion, FAK phosphorylation at tyrosine (Tyr)-397 and MMP-9 secretion, whereas it increased the number and size of focal adhesions induced by FBS in MDA-MB-231 cells. Moreover, Holo-BLf induced a slight increase of E-cadherin expression in MCF-7 cells, and inhibited vimentin expression in MCF-7 and MDA-MB-231 breast cancer cells.
Conclusion
Holo-BLf inhibits cellular processes that mediate the invasion process in breast cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common invasive cancer in developed countries and the leading cause of death among women worldwide [1]. The highest incidence of breast cancer occurs in high-income countries. However, an increase of breast cancer has been reported in low- to middle-income countries, which is the consequence of a variety of factors including the age and lifestyle changes, such as dietary factors [2,3,4].
Lactoferrin (Lf) is an iron-binding glycoprotein of 80 kDa constituted for a single polypeptide chain, which is folded into two symmetrical lobes that are connected by a hinge region constituted for α-helix regions. The lobes of Lf bind Fe2+ or Fe3+ ions, however Lf has the ability to reversibly bind Fe3+, and then it is found free of Fe3+ (Apo-Lf) or associated with Fe3+ (Holo-Lf). The binding of Fe2+ or Fe3+ to Lf generates a different three-dimensional conformation of the protein [5, 6]. Lf has a high homology between species, transport iron in blood serum and is produced and secreted by mucosal epithelial cells in a variety of mammalian species including cows, goats, horses, humans and some rodents [5]. Moreover, Lf is present in mucosal secretions and body fluids, such as saliva, tears, vaginal fluids, semen, blood plasma, amniotic fluid and is abundant in milk and colostrum [7, 8].
Lf has antimicrobial activity against a variety of bacteria, fungi, yeast, virus and parasites, and regulates the absorption of iron and modulates the immune system [9,10,11,12]. Moreover, Lf has protective effects in gastrointestinal cancers, such as cancer of colon, stomach, liver and pancreas. Particularly, Lf inhibits proliferation and Akt activation in SGC-7901 stomach cancer cells, whereas bovine Lf (BLf) from milk decreases viability and proliferation and increase apoptosis in breast cancer cells HS578T and T47D [13,14,15]. Human Lf induces arrest at G1 to S transition of cell cycle, inhibition of Cdk2 kinase activity, Rb hypophosphorylation and reduction of Cdk2 and cyclin E protein levels in breast cancer cells MDA-MB-231 [16, 17]. However, the role of Apo-BLf and Holo-BLf in the inhibition of migration and invasion induced by linoleic acid (LA) and fetal bovine serum (FBS) in breast cancer cells has not been studied.
We demonstrate here that Holo-BLf and Apo-BLf differentially inhibit migration induced by FBS and LA in breast cancer cells MDA-MB-231. Holo-BLf partly inhibit invasion, FAK phosphorylation at tyrosine (Tyr)-397 and metalloproteinase (MMP)-9 secretion, and it also increases the number and size of focal adhesions induced by FBS in MDA-MB-231 cells. Moreover, Holo-BLf induces a slight increase of E-cadherin expression in MCF-7 cells, and inhibits vimentin expression in MCF-7 and MDA-MB-231 breast cancer cells.
Materials and methods
Materials
Apo-BLf (97% purity) was from NutriScience Innovations, LCC (Connecticut, USA). LA sodium salt and tetramethylrhodamine (TRITC)-conjugated phalloidin were from Sigma-Aldrich (St. Louis MO, USA). Anti-vimentin antibody (Ab), anti-E-cadherin Ab and anti-focal adhesion kinase (FAK) Ab were from Santa Cruz Biotechnology (Sta. Cruz, CA, USA). Anti-paxillin Ab was from Abcam® (Waltham, MA, USA). Phospho-specific Ab to tyrosine (Tyr)-397 of FAK (anti-FAK-p-Tyr397) was from Invitrogen (Camarillo, CA, USA). Anti-actin Ab was from R&D Systems, Inc (Minneapolis, MN, USA). FBS was from ByProductos (Mexico).
Preparation of Holo-BLf
Holo-BLf was obtained by saturation of Apo-BLf with iron according to a method previously described [18]. Quantification of iron in Holo-BLf was determined by an enzymatic automated method, (MicroTech Laboratories, Mexico), and it was of 93%.
Cell lines and culture
Human breast cancer cells MDA-MB-231 and MCF-7 were acquired from American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) complemented with 5% FBS, 3.7 g/l sodium bicarbonate and antibiotics. Cultures were incubated under a humidified atmosphere with 5% CO2 and 95% air at 37 ºC. MDA-MB-231 cells were starved with DMEM without FBS for 24 h, and MCF-7 cells were starved in DMEM without FBS for 18 h before treatment with BLf, FBS and/or LA.
Cell stimulation
Cultures of MDA-MB-231 and MCF-7 cells were washed twice with phosphate-buffered saline (PBS), equilibrated in DMEM for 30 min and then untreated or treated with Apo-BLf, Holo-BLf, FBS or LA. After stimulation, conditioned media were obtained and cells were solubilized in 500 µl ice-cold RIPA buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, 100 mM NaF, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X100, 1% sodium deoxycholate, 1.5 mM MgCl2, 0.1% SDS and 1 mM PMSF). Protein concentration of lysates was determined by the micro-Bradford protein assay (Bio-Rad, USA).
Western blot (WB)
Proteins were separated by using 10% SDS-PAGE separating gels, and then proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dried milk in PBS pH 7.2/0.1% Tween 20 (wash buffer) for 2 h at room temperature. Primary Abs were incubated with membranes overnight at 4 ºC, and washed three times with wash buffer. Secondary Abs conjugated to horseradish peroxidase were incubated with membranes for 2 h at room temperature and were washed three times with wash buffer. Immunoreactive bands were visualized using WB luminol reagent and an autoradiography film. Autoradiograms were scanned and bands were analyzed by using the ImageJ software v. 1.52e (NIH, USA).
Scratch-wound assay
Cell cultures were treated with 12 µM mitomycin C for 2 h to inhibit proliferation. Cultures were scratched, washed with PBS and supplemented with serum-free DMEM without or with BLf, FBS and LA for 48 h at 37 ºC. Cultures were photographed with an inverted microscope coupled to a camera. Images from at least three fields per experimental condition were obtained and analyzed using the ImageJ software v. 1.52e (NIH, USA).
Invasion assays
Inserts of 24-well plates were covered with 30 µl Matrigel (3 mg/ml) and incubated overnight at 37 ºC. MDA-MB-231 cells (1 × 105) in FBS-free DMEM were plated on Matrigel of each insert (Upper chamber). Lower chamber contained 600 µl DMEM without or with Holo-BLf and/or FBS. Plates were incubated for 48 h at 37 °C under a humidified atmosphere with 5% CO2 and 95% air. After incubation, cells and Matrigel on the upper surface of membranes were removed with cotton swabs, and cells on the lower surface of membranes were washed with PBS and fixed with methanol at room temperature for 5 min. Quantification of invaded cells was obtained by staining of membranes with a solution of crystal violet (0.5%) for 15 min at room temperature and elution of dye with 300 µl acetic acid (10%). Absorbance of samples was measured at 600 nm.
Zymography
Conditioned media were obtained and concentrated with 3,000 NMWL Amicon Ultra Centrifugal filters (Merck Millipore). Equal volumes of non-heated conditioned medium and sampler buffer (2% sucrose ,2.5% SDS, 4 µg/ml phenol red) were mixed and loaded into 8% polyacrylamide gels copolymerized with gelatin (1 mg/ml). Electrophoresis was performed at 72 V for 2 h and gels were rinsed three times with 2.5% Triton X-100 for 30 min, and incubated in assay buffer (50 mM Tris–HCl pH 7.4, 5 mM CaCl2) at 37 °C for 48 h. Gels were fixed and stained with developer solution (0.125% Coomassie Brilliant Blue G-250, 50% acetic acid, 10% methanol). Proteolytic activity was identified as clear bands against the background stain of undigested substrate. Controls of MMP-2 and MMP-9 secretion were obtained by treatment of MDA-MB-231 cells with 400 mg/dl ethanol or 100 ng/ml PDB for 24 h at 37 ºC respectively [19, 20]. Controls were included.
Immunofluorescence confocal microscopy
MDA-MB-231 cells were grown on coverslips, washed with PBS, equilibrated in FBS-free DMEM for 30 min and treated with Holo-BLf and/or FBS for 20 min at 37 °C. Cells on coverslips were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.5% Triton X-100 for 20 min and blocked with gelatin solution (0.5% gelatin, 1 mM CaCl2, 0.5 mM MgCl2) for 20 min at room temperature. Staining was performed by incubation of cells on converslips with anti-paxillin Ab (1:1000) for 12 h at 4 °C followed by FITC-labeled anti-mouse secondary Ab for 2 h at room temperature. Fibrillar actin was stained by incubation of cells with TRITC-conjugated phalloidin for 2 h at room temperature. Cells were mounted on glass slides with Vectashield and analyzed by confocal microscopy (Model TCS SP2; Leica Microsystems, Inc). Analysis of images was performed by using the ImageJ software v. 1.52e (NIH, USA).
Statistical analysis
Data are expressed as mean ± SD of at least three independent experiments. Analysis of data was performed by one-way ANOVA and Dunnett’s multiple comparison test. Statistical probability of P < 0.05 was considered significant.
Results
BLf does not induce migration in MDA-MB-231 breast cancer cells
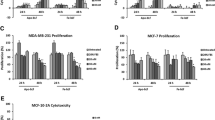
We determined whether Holo-BLf and Apo-BLf induced migration in breast cancer cells MDA-MB-231. Cultures of MDA-MB-231 cells were scratch-wounded and untreated or treated for 48 h with increasing concentrations of Holo-BLf or Apo-BLf. As illustrated in Fig. 1 A and B, treatment with Holo-BLf and Apo-BLf did not induce migration in breast cancer cells MDA-MB-231.
BLf does not induce migration in MDA-MB-231 breast cancer cells. a and b Migration assays of MDA-MB-231 cells treated with increasing concentration of Holo-BLf and Apo-BLf. One positive control of migration was included (FBS). Graphs are the mean ± S.D. of at least three independent experiments, and indicate the fold of migration above unstimulated cells (Control, Ctrl) value
BLf inhibits migration induced by FBS
Since Holo-BLf and Apo-BLf did not induce migration, we studied whether Holo-BLf and Apo-BLf inhibited migration induced by FBS. Cultures of MDA-MB-231 cells were scratch-wounded and treated without or with 5% FBS and increasing concentrations of Holo-BLf or Apo-BLf for 48 h. Results showed that treatment with Holo-BLf and Apo-BLf partly inhibited the migration induced by FBS in MDA-MB-231 cells. Particularly, treatment with 2500 nM Holo-BLf inhibited FBS-induced migration by ∼90%, whereas treatment with 2500 nM Apo-BLf inhibited FBS-induced migration by ∼50% in MDA-MB-231 cells (Fig. 2 A and B).
BLf inhibits migration induced by FBS. a and b Migration assays of MDA-MB-231 cells cotreated with increasing concentrations of Holo-BLf and Apo-BLf and 5% FBS. One positive control of migration was included (FBS). Graphs represent the mean ± S.D. of at least three independent experiments, and indicate the fold of migration above Ctrl value. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
BLf inhibits migration induced by LA
LA induces migration and invasion of MDA-MB-231 cells [21, 22]. We determined whether Holo-BLf and Apo-BLf inhibited migration induced by LA. Cultures of MDA-MB-231 cells were scratch-wounded and treated without or with 90 µM LA and increasing concentrations of Holo-BLf or Apo-BLf for 48 h. Findings demonstrated that treatment with 125 nM and 1250 nM Holo-BLf inhibited LA-induced migration by 90%, whereas treatment with 2500 nM Holo-BLf inhibited LA-induced migration to basal levels in MDA-MB-231 cells. Moreover, treatment with 1250 nM and 2500 nM Apo-BLf inhibited LA-induced migration by 50% in MDA-MB-231 cells (Fig. 3 A and B).
BLf inhibits migration induced by LA. a and b Migration assays of MDA-MB-231 cells cotreated with increasing concentrations of Holo-BLf and Apo-BLf and 90 µM LA. One positive control of cell migration was included (FBS). Graphs represent the mean ± S.D. of at least three independent experiments, and indicate the fold of migration above Ctrl value. **P < 0.01. ****P < 0.0001
BLf inhibits invasion induced by FBS
Since, Holo-BLf inhibited migration to basal levels, we determined whether Holo-BLf inhibited invasion and secretion of MMP-2 and MMP-9 induced by FBS. Invasion assays were performed with MDA-MB-231 cells cotreated with 2500 nM Holo-BLf and 5% FBS for 48 h. Results demonstrated that treatment with Holo-BLf partly inhibited invasion induced by FBS (Fig. 4 A).
BLf inhibits invasion and the expression of mesenchymal proteins. a Invasion assays of MDA-MB-231 cells cotreated with 2500 nM Holo-BLf and 5% FBS. One positive control of invasion was included (FBS). b Analysis of MMP-2 and MMP-9 secretion from MDA-MB-231 cells cotreated with 2500 nM Holo-BLf and 5% FBS. Positive controls of MMP-2 (EtOH) and MMP-9 (PDB) were included. c Analysis of E-cadherin and vimentin expression in lysates from MDA-MB-231 and MCF-7 cells treated with 125 nM and 1250 nM Holo-BLf. Graphs represent the mean ± S.D. of at least three independent experiments, and indicate the fold of invasion or MMP-2 and − 9 secretion above Ctrl value. **P < 0.01, ***P < 0.001, ***P < 0.001
We determined whether Holo-BLf inhibited MMP-2 and MMP-9 secretion induced by FBS. Cultures of MDA-MB-231 cells were cotreated with 2500 nM Holo-BLf and 5% FBS for 48 h, and conditioned media were obtained. Secretion of MMP-2 and MMP-9 was analyzed by gelatin zymography of conditioned media. Findings showed that treatment with Holo-BLf partly inhibited the secretion of active MMP-9 induced by FBS in MDA-MB-231 cells. In contrast, treatment with Holo-BLf did not inhibit secretion of Pro-MMP-9 and MMP-2 induced by FBS in MDA-MB-231 cells (Fig. 4B).
BLf regulates the expression of mesenchymal markers associated with the epithelial to mesenchymal transition (EMT) process
EMT process involves migration, invasion and secretion of MMPs [23]. We studied whether Holo-BLf regulates the expression of the epithelial protein E-cadherin and the mesenchymal protein vimentin in breast cancer cells. MDA-MB-231 and MCF-7 breast cancer cells were treated with 125 nM and 1250 nM Holo-BLf for 24 h and cells were lysed. Cell lysates were analyzed by WB with anti-E-cadherin Ab, anti-vimentin Ab and anti-actin Ab as loading control. Findings showed that treatment with Holo-BLf induced a slight increase of E-cadherin expression in MCF-7 cells, however Holo-BLf did not induce E-cadherin expression in MDA-MB-231 cells. In contrast, treatment with 125 nM Holo-BLf partly inhibit vimentin expression in MCF-7 cells, and 1250 nM Holo-BLf completely inhibited expression of vimentin in MCF-7 and MDA-MB-231 cells (Fig. 4 C).
BLf promotes the formation of focal adhesions
We studied whether Holo-BLf regulates FAK activation induced by FBS. FAK activation was analyzed by determination of its phosphorylation at Tyr-397. MDA-MB-231 cells were treated for 1 h with 1250 nM Holo-BLf and then cells were stimulated with 5% FBS for 60 min and lysed. Cell lysates were analyzed by WB with anti-FAK-p-Tyr397 Ab and anti-actin Ab as loading control. Results demonstrated that Holo-BLf partly inhibited FAK phosphorylation at Tyr-397 induced by FBS in MDA-MB-231 cells (Fig. 5 A).
BLf inhibits FAK activation and induces assembly of focal adhesions. a Analysis of FAK-p-Tyr397, FAK and actin in lysates from MDA-MB-231 cells cotreated with 2500 nM Holo-BLf and 5% FBS b Immunofluorescence analysis of paxillin (green) and F-actin (red) in MDA-MB-231 cells cotreated with 2500 nM Holo-BLf and 5% FBS. Graph represents the mean ± S.D. of at least three independent experiments, and indicates the fold of FAK phosphorylated at Tyr397 (p-FAk) above Ctrl value. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Next, we studied whether Holo-BLf inhibited the formation of focal adhesions induced by FBS. Focal adhesions were analyzed by immunofluorescence analysis of paxillin, because the paxillin protein is concentrated in focal adhesions [24]. MDA-MB-231 cells cultured on coverslips were treated without or with 1250 nM Holo-BLf for 1 h and stimulated with 5% FBS for 20 min. Results showed that FBS induced the assembly of focal adhesions, whereas treatment with Holo-BLf and FBS increased the number and size of focal adhesions in MDA-MB-231 cells (Fig. 5B).
Discussion
Metastasis is a process that implicate the dissemination of cancer cells from primary tumors to distant organs in adenocarcinomas, and it requires the migration and invasion of cancer cells into adjacent tissues and then intravasation into blood and/or lymphatic vessels [25, 26]. Therefore, migration and invasion play a pivotal role in metastasis. BLf (1 µM) enhances migration of WI-38 human fetal fibroblasts, whereas recombinant human Lf (50–200 µg/ml) induces migration of human dermal fibroblasts [27, 28]. In contrast, we demonstrate that Holo-BLf and Apo-BLf do not induce migration in MDA-MB-231 breast cancer cells. Since, human fetal fibroblasts WI-38 and human dermal fibroblasts are not cancerous cells, we propose that BLf induces migration in non-cancer cells but it does not induce migration in breast cancer cells.
An association between diets containing high levels of omega-6 polyunsaturated fatty acids, particularly LA, and an increased risk of developing breast cancer has been suggested [29,30,31,32]. LA is an essential fatty acid and represents the main polyunsaturated fatty acid in most Western diets, and these diets increase the risk for development of chronic diseases [29]. Particularly, LA induces FAK and PLD activation, migration and invasion via a FFAR1-, FFAR4- and PI3K/Akt-dependent pathway in MDA-MB-231 cells [21, 22, 33]. We demonstrate that treatment with 2500 nM Holo-BLf completely inhibit the migration induced by LA, whereas treatment with 2500 nM Holo-BLf inhibit the migration induced by FBS by ∼90% in MDA-MB-231 cells. However, treatment with 2500 nM Apo-BLf inhibit the migration induced by LA and FBS by ∼50% in MDA-MB-231 cells. Since, Apo-BLf and Holo-BLf have a different three-dimensional conformation depending on its binding to Fe3+ [5], our results demonstrate that Holo-BLf conformation has a bigger capacity than Apo-BLf conformation to inhibit migration induced by FBS and LA in MDA-MB-231 breast cancer cells. In contrast, it has been reported that treatment with 10 nM Apo-BLf has a bigger capacity than Holo-BLf to inhibit migration induced by FBS in MDA-MB-231 and MCF-7 breast cancer cells [34]. We propose that Apo-BLf and Holo-BLf and their different concentrations used in the different studies show different capacities of inhibition of specific cell processes in breast cancer cells. Supporting our proposal, Holo-BLf has a bigger capacity than Apo-BLf to inhibit proliferation induced by FBS in MDA-MB-231 cells, whereas Holo-BLf and Apo-BLf inhibit proliferation induced by FBS to similar levels in MCF-7 cells [34]. Moreover, Apo-BLf is more cytotoxic than Holo-BLf in MDA-MB-231 and MCF-7 cells [34].
EMT is a cellular process implicated in tumor progression, because through EMT the epithelial cells acquire mesenchymal properties, and then the ability to execute the steps implicated in invasion and metastasis [23]. EMT process is mediated by the loss of apico-basal polarity, disassembly of adherens junctions, MMPs secretion, expression of mesenchymal proteins including vimentin, N-cadherin, α-smooth muscle actin, myosin isoforms, fibronectin and FSP-1, and the loss of epithelial characteristics, such as downregulation of E-cadherin expression [23, 35]. In this study, we analyzed MCF-7 breast cancer cells because they are non-invasive with a low metastatic potential that still have epithelial characteristics including expression of E-cadherin, estrogen and progesterone receptors. However, MCF-7 cells express some mesenchymal markers, such as vimentin. In contrast, MDA-MB-231 breast cancer cells are invasive with high metastatic potential and have mesenchymal characteristics, such as the expression of vimentin and the absence of E-cadherin, estrogen and progesterone receptors expression [36,37,38]. We demonstrate that Holo-BLf induces downregulation of vimentin expression in MDA-MB-231 and MCF-7 cells. Moreover, Holo-BLf induces a slight increase of E-cadherin expression in MCF-7 cells, however it does not induce E-cadherin expression in MDA-MB-231 cells. We propose that treatment with Holo-BLf promotes the expression of epithelial characteristics in breast cancer cells, such as the expression of E-cadherin and downregulation of vimentin, and then Holo-BLf is able to inhibit the EMT process in breast cancer cells. Supporting our proposal, Holo-BLf induces a higher inhibition of vimentin expression than Apo-BLf in human glioblastoma GL-15 cells, whereas high concentrations of BLf (10 and 100 µg/ml) induce upregulation of E-cadherin expression and downregulation of vimentin expression in malignant oral squamous carcinoma cells HOC3313 [39, 40].
EMT induces the activation of signal transduction pathways that mediate the invasion process through extracellular matrix. Particularly, EMT induces a variety of specific cellular processes, including migration, invasion, focal adhesion assembly and secretion of some MMPs, including MMP-2 and MMP-9 (gelatinases) [35]. We demonstrate that Holo-BLf partly inhibits invasion and MMP-9 secretion induced by FBS in MDA-MB-231 cells. In agreement with our findings, BLf partly inhibits invasion in HOC3313 and SCCVII oral squamous carcinoma cells [39]. Our findings support the proposal that Holo-BLf inhibits the EMT process in breast cancer cells.
Focal adhesions are structures wherein integrin receptors mediate the interaction between the actin cytoskeleton of cells and the extracellular matrix. The composition of focal adhesion is varied and includes scaffolding proteins, adaptor proteins, GTPases, phosphatases and kinases, such as FAK and Src [41, 42]. FAK is a protein tyrosine kinase of 125 kDa, which is activated by a variety of agonists and regulates cell spreading, differentiation, proliferation, migration, invasion survival and angiogenesis [43, 44]. FAK activation is given by its phosphorylation at Tyr-397, which is a binding site for SH2 domains of proteins including Src family kinases. Particularly, formation of FAK/Src complex, mediated by a SH2 domain, participates in the regulation of assembly and disassembly of focal adhesions, and then it mediates migration, invasion, and then the EMT process [43, 45, 46]. We demonstrate that Holo-BLf inhibits phosphorylation of FAK at Tyr-397, and promotes an increase in the number and size of focal adhesions induced by FBS in MDA-MB-231 cells. We propose that Holo-BLf induces inhibition of migration and invasion through inhibition of FAK activation and the inactivation of proteins that participate in the disassembly of focal adhesions. Supporting our proposal, a peptide corresponding to residues 17–25 of BLf induces paxillin tyrosine phosphorylation in intestinal epithelial cells IEC-6 [47]. These findings support our proposal that Holo-BLf inhibits the EMT process in breast cancer cells.
In conclusion, our findings demonstrate Holo-BLf exhibit a higher capacity of inhibition of migration than Apo-BLf in MDA-MB-231 breast cancer cells. In addition, Holo-BLf inhibits migration and invasion in MDA-MB-231 cells, and the expression of epithelial characteristics in MDA-MB-231 and MCF-7 cells. We propose that Holo-BLf inhibit the EMT process in breast cancer cells.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Albuquerque RC, Baltar VT, Marchioni DM (2014) Breast cancer and dietary patterns: a systematic review. Nutr Rev 72:1–17
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F (2019) Breast cancer. Nat Rev Dis Primers 5:66
Chajes V, Romieu I (2014) Nutrition and breast cancer. Maturitas 77:7–11
Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q (2009) Lactoferrin: structure, function and applications. Int J Antimicrob Agents 33:301e301–301e308
Schanbacher FL, Goodman RE, Talhouk RS (1993) Bovine mammary lactoferrin: implications from messenger ribonucleic acid (mRNA) sequence and regulation contrary to other milk proteins. J Dairy Sci 76:3812–3831
van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK (2001) Antiviral activities of lactoferrin. Antiviral Res 52:225–239
Alexander DB, Iigo M, Yamauchi K, Suzui M, Tsuda H (2012) Lactoferrin: an alternative view of its role in human biological fluids. Biochem Cell Biol 90:279–306
Conneely OM (2001) Antiinflammatory activities of lactoferrin. J Am Coll Nutr 20:389S–395S discussion 396S-397S
Hao L, Shan Q, Wei J, Ma F, Sun P (2019) Lactoferrin: Major Physiological Functions and Applications. Curr Protein Pept Sci 20:139–144
Gruden S, Poklar Ulrih N (2021): Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides.Int J Mol Sci 22
Cutone A, Rosa L, Ianiro G, Lepanto MS, Bonaccorsi di Patti MC, Valenti P, Musci G (2020): Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 10
Iigo M, Alexander DB, Long N, Xu J, Fukamachi K, Futakuchi M, Takase M, Tsuda H (2009) Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 91:86–101
Tsuda H, Sekine K, Fujita K, Ligo M (2002) Cancer prevention by bovine lactoferrin and underlying mechanisms–a review of experimental and clinical studies. Biochem Cell Biol 80:131–136
Xu XX, Jiang HR, Li HB, Zhang TN, Zhou Q, Liu N (2010) Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J Dairy Sci 93:2344–2350
Damiens E, El Yazidi I, Mazurier J, Duthille I, Spik G, Boilly-Marer Y (1999) Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J Cell Biochem 74:486–498
Duarte DC, Nicolau A, Teixeira JA, Rodrigues LR (2011) The effect of bovine milk lactoferrin on human breast cancer cell lines. J Dairy Sci 94:66–76
Avalos-Gomez C, Reyes-Lopez M, Ramirez-Rico G, Diaz-Aparicio E, Zenteno E, Gonzalez-Ruiz C, de la Garza M (2020) Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2. Vet Res 51:36
Ke Z, Lin H, Fan Z, Cai TQ, Kaplan RA, Ma C et al (2006) MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int J Cancer 119:8–16
Park MJ, Park IC, Hur JH, Rhee CH, Choe TB, Yi DH, Hong SI, Lee SH (2000) Protein kinase C activation by phorbol ester increases in vitro invasion through regulation of matrix metalloproteinases/tissue inhibitors of metalloproteinases system in D54 human glioblastoma cells. Neurosci Lett 290:201–204
Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP (2017) Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol 34:111
Serna-Marquez N, Villegas-Comonfort S, Galindo-Hernandez O, Navarro-Tito N, Millan A, Salazar EP (2013) Role of LOXs and COX-2 on FAK activation and cell migration induced by linoleic acid in MDA-MB-231 breast cancer cells. Cell Oncol (Dordr) 36:65–77
Thiery JP (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15:740–746
Schaller MD (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459–6472
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Tang L, Cui T, Wu JJ, Liu-Mares W, Huang N, Li J (2010) A rice-derived recombinant human lactoferrin stimulates fibroblast proliferation, migration, and sustains cell survival. Wound Repair Regen 18:123–131
Takayama Y, Mizumachi K (2001) Effects of lactoferrin on collagen gel contractile activity and myosin light chain phosphorylation in human fibroblasts. FEBS Lett 508:111–116
Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 60:502–507
Tsubura A, Uehara N, Kiyozuka Y, Shikata N (2005) Dietary factors modifying breast cancer risk and relation to time of intake. J Mammary Gland Biol Neoplasia 10:87–100
Lee MM, Lin SS (2000) Dietary fat and breast cancer. Annu Rev Nutr 20:221–248
Buja A, Pierbon M, Lago L, Grotto G, Baldo V (2020): Breast Cancer Primary Prevention and Diet: An Umbrella Review. Int J Environ Res Public Health 17
Diaz-Aragon R, Ramirez-Ricardo J, Cortes-Reynosa P, Simoni-Nieves A, Gomez-Quiroz LE, Perez Salazar E (2019) Role of phospholipase D in migration and invasion induced by linoleic acid in breast cancer cells. Mol Cell Biochem 457:119–132
Gibbons JA, Kanwar JR, Kanwar RK (2015) Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 15:425
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Ziegler E, Hansen MT, Haase M, Emons G, Grundker C (2014) Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res Treat 148:269–277
Dai X, Cheng H, Bai Z, Li J (2017) Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer 8:3131–3141
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:179
Chea C, Miyauchi M, Inubushi T, Okamoto K, Haing S, Nguyen PT, Imanaka H, Takata T (2018) Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem Biophys Res Commun 507:142–147
Cutone A, Colella B, Pagliaro A, Rosa L, Lepanto MS, Bonaccorsi di Patti MC, Valenti P, Di Bartolomeo S, Musci G (2020) Native and iron-saturated bovine lactoferrin differently hinder migration in a model of human glioblastoma by reverting epithelial-to-mesenchymal transition-like process and inhibiting interleukin-6/STAT3 axis. Cell Signal 65:109461
Guo W, Giancotti FG (2004) Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5:816–826
Wozniak MA, Modzelewska K, Kwong L, Keely PJ (2004) Focal adhesion regulation of cell behavior. Biochim Biophys Acta 1692:103–119
Parsons JT (2003) Focal adhesion kinase: the first ten years. J Cell Sci 116:1409–1416
Zhao J, Guan JL (2009) Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28:35–49
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6:154–161
Avizienyte E, Frame MC (2005) Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol 17:542–547
Jeong YY, Lee GY, Yoo YC (2021) Bovine Lactoferricin Induces Intestinal Epithelial Cell Activation through Phosphorylation of FAK and Paxillin and Prevents Rotavirus Infection. J Microbiol Biotechnol 31:1175–1182
Acknowledgements
We are grateful to Nora Ruiz for her technical assistance.
Funding
This research was funded by CONACYT (255429), Mexico. Grants from CONACYT supported N R-O and K R-R.
Author information
Authors and Affiliations
Contributions
N R-O, K R-R: design, conduction, data analysis, methodology, validation and manuscript preparation. P C-R, MG, EPS: Conceptualization, Methodology, Writing-Review and editing, Project administration, Funding acquisition, Visualization, Supervision.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez-Ochoa, N., Cortes-Reynosa, P., Rodriguez-Rojas, K. et al. Bovine holo-lactoferrin inhibits migration and invasion in MDA-MB-231 breast cancer cells. Mol Biol Rep 50, 193–201 (2023). https://doi.org/10.1007/s11033-022-07943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07943-8