Abstract
Medulloblastoma is the most common malignant brain tumor of childhood accounting for about 60% of all pediatric embryonal tumors. Despite improvements in the overall survival rate, this tumor still lacks an efficient, reliable, and less toxic therapeutic approach. Characterization of the molecular mechanisms involved in medulloblastoma initiation and progression is a crucial step for the development of effective therapies. Signal transducer and activator of transcription 3 is a convergence point for several signaling cascades that are implicated in medulloblastoma tumorigenesis. Accumulated evidence has revealed the pivotal role of signal transducer and activator of transcription 3 in medulloblastoma pathogenesis such as proliferation, survival, angiogenesis, and immunosuppression as well as maintenance, drug resistance, and recurrence. In this review, we focus on the role of signal transducer and activator of transcription 3 in medulloblastoma tumorigenesis and discuss the recent advances of signal transducer and activator of transcription 3 inhibition as a promising developed strategy for medulloblastoma therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is the most common malignant brain tumor of childhood affecting mainly the cerebellum and accounting for about 20% of all pediatric central nervous system (CNS) neoplasms [1]. It mainly arises from the primitive neuroectoderm in the cerebellar vermis of the brain affecting mostly infants and children in their first years of life [2]. Males are more frequently diagnosed with MB than females with a male to female ratio of 1.7: 1 [3]. The incidence rate for children younger than 4 years is 0.55 per 100,000 population and 0.16 per 100,000 population for adolescents aged between 15–19 years [3].
MB can be either classified based on histological criteria or molecular features. Based on histological and cellular characteristics, MB is divided into four subtypes: classic MB, desmoplastic-nodular MB, MB with extensive nodularity, and large cell-anaplastic MB [4]. Recently, MB classification was updated according to different molecular phenotypes: wingless-activated MB (MBWNT−activated), sonic hedgehog-activated and TP53-wildtype MB (MBSHH−activated, TP53−wildtype), SHH-activated and TP53-mutant MB (MBSHH−activated, TP53−mutant), and non-WNT/non-SHH MB (MBnon−WNT/non−SHH). The later includes two subtypes: MBnon−WNT/non−SHH, Group 3 and MBnon−WNT/non−SHH, Group 4 [5]. They exhibit highly distinct cytogenetic profiles, transcriptional signatures, mutational spectra, and most importantly, clinical phenotypes such as prognostic outcomes [6].
Currently, the standard regimens used for MB patients include surgical resection followed by adjuvant chemotherapy [7]. Multimodal treatments have improved overall survival (OS) to a 5 year of approximately 80% and 10 year of about 70% of MB patients [8]. However, recurrence, metastasis, and severe long-term side effects related to treatment toxicity remain the major clinical limitations [7]. In this regard, different treatment approaches have been used in MB such as immunotherapy, gene-targeted therapy, and drug repurposing [8, 9]. Nevertheless, a better understanding of the molecular mechanisms in the normal brain and also in MB that drive the tumor’s phenotype will allow identifying a novel therapeutic target and development of safer and more effective treatment to interrupt such aberrant signaling. In this review, we focus our discussion on the role of Signal transducer and activator of transcription (STAT3) activation and signaling in MB pathogenesis highlighting the importance of STAT3 inhibitors as a promising therapeutic approach for targeting MB.
Overview of STAT3 pathway
STAT family
STAT family is a group of cytoplasmic transcription factors that were initially recognized as interferon mediators of several biological processes. In mammals, STAT family comprises seven members, STAT1, -2, -3, -4, -5a/b, and -6 [10]. STATs are activated by extracellular signaling proteins (cytokines, growth factors, and hormones) mainly via binding to specific cell surface receptor-associated Janus kinases (JAK) promoting a wide range of physiological events such as differentiation, proliferation, angiogenesis, apoptosis, and cellular immunity [11]. All STAT proteins share a common N-terminal domain (ND), a coiled-coil domain (CCD), DNA binding domain (DBD), linker domain (LD), Src-homology-2 domain (SH2), and the C-terminal transactivation domain (CTD) which contains two phosphorylation sites, Tyr705 and Ser727 involved in gene transcriptional activity [12].
STAT3 activation and signaling
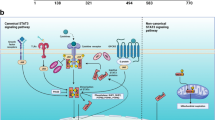
Among all the STATs, STAT3 is the most clinically relevant one due to its pivotal roles in a variety of biological events such as hematopoiesis, neurogenesis, embryogenesis, immune responses, and oncogenesis [12]. STAT3 gene is almost expressed in all body cell types and can be proteolytically cleaved into two isoforms, STAT3α and STAT3β [13]. STAT3 is induced by numerous cytokines and growth factors activating a transduction cascade which results in the amplification of target gene(s) [14]. Biological responses of STAT3 occur at two molecular levels: canonical STAT3 pathway and non-canonical STAT3 pathway.
The most prevalent canonical STAT3 pathway is the IL-6/STAT3 signaling pathway. Upon binding of IL-6 to glycoprotein 130 (GP130) receptor [15], JAK (JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2)) is phosphorylated [16] inducing STAT3 phosphorylation at tyr705 residue in the STAT3 CTD. STAT3 can be also phosphorylated at another crucial residue Ser727 which is located at the CTD [17]. Phosphorylated STAT3 recruits another phosphorylated STAT3 to form a dimer, and translocate to the nucleus [11], where they bind to specific interferon-gamma (IFN-γ)-activated sequence (GAS) sites [18] inducing the expression of target genes including pro-invasive factors such as metallopeptidases and heat shock proteins [19] as well as angiogenic factors such as vascular endothelial growth factor (VEGF) [20]. On the other hand, the non-canonical STAT3 signaling pathway has been implicated in STAT3 activity independently of tyr705 phosphorylation [21]. Evidence of this pathway indicated that an un-phosphorylated STAT3 monomer or dimer can translocate to the nucleus and regulate gene expression via binding to GAS [22].
STAT3 regulation
Although phosphorylation plays a major role in STAT3 activation, post-translational modifications including acetylation, methylation, ubiquitination, and dephosphorylation are also essential for STAT3 signaling regulation [23, 24]. Acetylation is required for STAT3 dimerization and activation. This reversible mechanism is carried out by acetyltransferase p300 at Lys-685 residue of STAT3 [24]. STAT3 acetylation by CREB binding protein (CBP)-histone acetyltransferase enhances its translocation to the mitochondria where acetylated STAT3 participates in pyruvate metabolism by association with pyruvate dehydrogenase complex E1 (PDC-E1) resulting in ATP production. However, STAT3 role in pyruvate metabolism is terminated upon its deacetylation by sirtuin 5 (SIRT5) [25].
Methylation is another fundamental post-translational modification essential for STAT3 regulation. STAT3 can be di-methylated on Lys140 residue by histone methyltransferase (SET9) and demethylated by lysine-specific demethylase (LSD1). This process negatively regulates the expression of STAT3 target genes, knowing that methylation precedes STAT3 Tyr705 and Ser727 phosphorylation [26]. On the other hand, di-methylation of STAT3 on Lys49 by enhancer of zeste homolog 2 (EZH2) activates the transcription of IL-6-dependent genes [27].
STAT3 activity is also regulated by ubiquitin proteasomal degradation. In neonatal hippocampal neurons, calcineurin stimulation results in the degradation of STAT3 via ubiquitin proteasomal pathway through TATA element modulatory factor/androgen receptor coactivator 160 (TMF/ARA 160) [28]. Furthermore, tumor necrosis factor receptor-associated factor (TRAF6) is implicated in STAT3 negative regulation by mediating its ubiquitination and degradation [29].
An important mechanism for regulating the activity of STAT3 is achieved by a wide variety of phosphatases. Protein tyrosine phosphatase receptor T (PTPRT) dephosphorylates phosphorylated STAT3 at Tyr705 residue directly after STAT3 activation by JAK, while phosphorylated STAT3 is migrating to the nucleus, or after its translocation to the nucleus [23]. In addition, non-receptor tyrosine phosphatases Meg2 and SHP2 dephosphorylate STAT3 [30] in cytoplasm and nucleus [30, 31]. STAT3 activity is also influenced by serine/threonine phosphatases by which protein phosphatase 2A (PP2A) is involved in STAT3 dephosphorylation at Ser727 residue interrupting its normal function in transducing signals to the nucleus [32].
Furthermore, other molecules involved in STAT3 regulation are the suppressor of cytokine signaling-3 (SOCS3) and the protein inhibitor of activated STAT3 (PIAS3). SOCS3 acts as a negative feedback loop by binding to the GP130 receptor thus inhibiting JAK from activating STAT3 [33], whereas PIAS3 binds directly to STAT3 hindering its interaction with DNA and preventing the expression of target genes [34].
Role of STAT3 in normal brain physiology
STAT3 in neurodevelopment
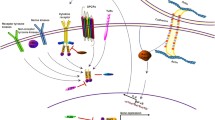
STAT3 is expressed in the cerebral cortex, striatum, and hippocampus during early embryonic life, and continued to be detected till 3 months prenatally [35, 36]. Later, the activation of STAT3 in the brain is limited where it can be found in the subventricular zone of the forebrain and the subgranular zone in the hippocampus which comprises cells that constantly undergo division [37]. Takeda et al. have indicated that STAT3-deficient mice died between E6.5 and E7.5, preceding gastrulation [38]. Furthermore, the production of neural precursor cells (NPCs) and the neural stem cell markers decline significantly in STAT3 dominant-negative cells indicating the critical role of STAT3 in early neural development [39]. Newly formed neurons secrete gliogenic cytokines that activate the JAK/STAT pathway in the undifferentiated neuronal stem cells (NSCs) destined to become astrocytes where Notch signaling triggers the demethylation of astrocytic genes which are then activated when STAT3 and the co-activator p300/CBP bind to their promoters [40]. STAT3 downregulation enhanced hippocampal neuronal maturation through the crosstalk with MAPK signaling. However, STAT3 overexpression induced hippocampal neurospheres characterized by stem cell properties and further attenuated the terminal differentiation of hippocampal neurons [41] (Fig. 1A and B).
Role of STAT3 in neurodevelopment. A STAT3 fluctuations during the perinatal period in the brain of rodents. Prenatally, STAT3 was detected at embryonic day (E14), whereas postnatally, it was identified at postnatal day (P3) and continued to increase till P21 where the amounts of STAT3 declined. B STAT3 role in astrogenesis during neurodevelopment. Newly formed neurons release gliogenic cytokines driving the differentiation of neuronal stem cells into astrocytes by activating JAK/STAT pathway where STAT3 and p300/CBP bind to the promoters of astrocytic genes such as GFAP. C STAT3 role in synaptic plasticity and cognition through the induction of NMDAR-long term depression in the hippocampal synapse
STAT3 in synaptic plasticity
JAK/STAT3 signaling is critical in inducing N-methyl-D-aspartate receptor (NMDAR) dependent-long term depression (NMDAR-LTD) in the hippocampus [42]. NMDAR-LTD has been implicated in spatial reversal learning by eliminating previous memory traces to permit new traces to form when needed [43]. Interestingly, STAT3 anchored to the postsynaptic site was translocated to the nucleus in response to certain synaptic stimuli altering gene expression [44]. During the neonatal period, the activity of STAT3 was blocked by cytokine leptin resulting in the enhancement of glutamatergic synapses through the formation and maturation of dendritic spines in the hippocampal neurons [45]. Thus, STAT3 is implicated in synaptic plasticity and cognition [46] (Fig. 1C).
STAT3 in neuroprotection
STAT3 is characterized by neuroprotection properties in response to various brain stress conditions including cerebral ischemia [47]. Several molecules such as estradiol, secretoneurin, GF-1, and IL-6 activated STAT3 which is, in turn, translocated to the nucleus and induced the transcription of neuroprotective genes such as Bcl-2 [48]. Murase et al. demonstrated that activation of STAT3 regulated the transcription of p53, resulting in the activation of a survival signaling pathway in neurons upon the elevation of neuronal activity [49]. On the other hand, STAT3 pathway has been reported to be implicated in several brain pathophysiologies such as aging, Alzheimer’s disease, Parkinson's disease, and brain tumors including glioma, neuroblastoma, and MB [50, 51].
STAT3 and MB tumorigenesis
STAT3 in MB growth and survival
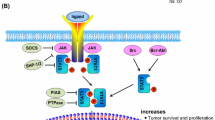
STAT3 is a key cell survival protein involved in growth and anti-apoptotic mechanisms of several tumors including MB [52]. Phosphorylated STAT3 has been found in rapidly proliferated MB cells and implicated in MB cell growth and migration [53], and also attenuated apoptosis [54]. Notably, the constitutive activation of STAT3 via IL-6 and leukemia inhibitory factor (LIF) has induced the expression of a subset of genes such as c-Myc, survivin, Bcl-2, Bcl-xl, and cyclin D1 mediating MB cell viability and proliferation [55, 56]. Furthermore, STAT3 besides other anti-apoptotic molecules such as epidermal growth factor receptor (EGFR) and matrix metalloproteinase-9 (MMP-9) was shown to protect MB cells against apoptosis [57] (Fig. 2). Interestingly, White et al. have indicated the sex-specific role of STAT3 in the initiation and development of SHH MB, by which targeting STAT3 in SHH MB mouse model would protect the male mice from MB initiation with no effect on female mice [58].
STAT3 signaling pathway in MB. The constitutive hyper-activation of STAT3 due to elevated IL-6 level resulted in STAT3 dimerization and translocation to the nucleus inducing the expression of its target genes such as cyclin D1, survivin, c-Myc, VEGF, CD133, and Bcl-2 that mediate MB proliferation and survival, angiogenesis, in addition to maintenance and resistance. Moreover, STAT3 aberrant activation resulted in MB immunosuppression via driving myeloid derived suppressor cell (MDSC) which in turn regulated dendritic cells and regulatory T-cells (Treg)
STAT3 in MB Angiogenesis
Angiogenesis—which is an essential process for MB in order to maintain its pathogenesis and survival -was shown to be dependent on STAT3 signaling [59]. STAT3 has been reported to induce neovascularization via binding to VEGF promoter inducing its expression and upregulation in many cell lines including MB [60]. Craveiro et al. revealed that constitutive activation of STAT3 enhanced the phosphorylation of VEGF2/3, the key receptors in angiogenesis [61] (Fig. 2).
STAT3 in MB immunosuppression
Although several studies have revealed the role of innate and adaptive immune components as extrinsic tumor suppressors, tumor cells including MB usually develop several mechanisms to evade the immune responses [2]. STAT3, one of the most common factors in tumors, acts as a negative regulator of inflammatory responses in immune cells [62, 63]. Interestingly, activated STAT3 was shown to drive myeloid-derived suppressor cells (MDSCs) expansion that is involved in the immunosuppressive process [64]. Abad et al. have demonstrated the involvement of STAT3 in MDSCs to sustain this population in MB [63]. The constitutive activation of STAT3 in MDSCs promoted their survival and inhibited apoptosis in the MB mice model [63]. In addition, STAT3-intact MDSCs were able to regulate other cell types including dendritic cells (DCs) and regulatory T-cells (Tregs) within MB microenvironment to reinforce immunosuppression and promote MB growth [63]. Moreover, overexpression of B7-H3, an immunosuppressive immune checkpoint, was associated with increased activity of STAT3 which may indicate a more malignant and aggressive phenotype of MB [65] (Fig. 2).
STAT3 in MB maintenance and resistance
The cancer stem cell (CSC) hypothesis suggests that CSCs originate from normal stem cells after multiple mutations, and are engaged in tumor formation, growth, and relapse [66, 67]. In MB, it was reported that tumor-associated astrocytes secreted chemokine C–C ligand 2 (CCL2) on MB stem cells (MBSCs) maintaining their stem-like properties by activating the Notch pathway via JAK2/STAT3 signaling [68]. Stemness and maintenance of MB cells showed dependency on STAT3 by which STAT3 was associated with the expression of stemness markers such as c-Myc and CD133, increased proliferation, and decreased cell death [69]. STAT3 was also activated in CD133+ MBCs promoting metastasis, tumorigenesis, and recurrence by the regulation of c-MYC expression [70]. Moreover, it was demonstrated that the self-renewal CD133+ MBSCs are triggered by STAT3 pathway supporting tumor survival [71] (Fig. 2).
Furthermore, it was reported that increased STAT3 activity has been associated with MB radio- and chemotherapy drug resistance [71, 72]. Sreenivasan et al. showed an elevated level of activated STAT3 in MB cell lines. Chemoresistant group 3 MB cell lines displayed an increased IL-6/STAT3 activity which is in turn involved in developing drug resistance. However, drug sensitivity was restored in MB cells lacking STAT3 or IL-6 receptors [72]. Therefore, due to the aberrant role of STAT3 signaling in MB tumorigenesis, STAT3 has been shown to be a potential therapeutic target for developing anti-tumor drugs.
STAT3 inhibitors
Recently, STAT3 has been suggested as a promising molecular target for tumor therapy including MB. Various STAT3 inhibitors have been developed and well-characterized for their efficacy in targeting in vitro and in vivo MB models. In the following section, targeting STAT3 for the prevention and treatment of MB will be discussed.
Multi-kinase inhibitors
Pazopanib
Multi-kinase inhibitors (MKi) target tumor angiogenesis by blocking VEGF receptor (VEGFR) signaling [73]. Pazopanib, one of the MKi members, has the ability to penetrate the blood–brain barrier (BBB) and inhibit oncogenic kinases in neoplastic cells [73]. In MB cell lines, Pazopanib inhibited proliferation, arrested cell growth, promoted apoptosis, and reduced MB cell migration through blocking the phosphorylation of STAT3 by targeting upstream kinases and suppressing STAT3 synthesis after long-term exposure. Furthermore, Pazopanib hindered tumor growth and increased the survival of the MB mice model [73] (Table 1; Part 1).
Sorafenib
Sorafenib, an oral MKi, has the ability to inhibit angiogenesis and promote apoptosis in tumors via targeting the Raf-MEK-MAPK pathway [74]. Similar to Pazopanib, Sorafenib reduced cell viability, cell growth, proliferation, and angiogenesis, and induced apoptosis in MB cell lines by reducing STAT3 phosphorylation. Moreover, Sorafenib reduced tumor growth and improve survival in vivo [73, 75] (Table 1; Part 1).
Vandetanib
Vandetanib is characterized by its antiangiogenic capability by regulating angiogenesis genes which further impact the survival of tumor cells. It also reduced tumor growth by inhibiting target receptors VEGFR 1/2, EGFR, and c-RET [76]. Vandetanib showed antitumor activity against MB where it reduced cell viability and proliferation, enhanced apoptosis, and inhibited cell migration in MB cell lines by decreasing STAT3 phosphorylation and expression [61] (Table 1; Part 1).
Axitinib
Axitinib, an FDA-approved drug for advanced renal cell carcinoma, is an angiogenic inhibitor that targets VEGFR 1/2, platelet-derived growth factor receptor (PDGFR α/β), and c‐kit. In MB, Axitinib displays an antitumor activity by decreasing the phosphorylation of protein kinase B (AKT or PKB) and STAT3 [77]. When combined with GDC‐0941 (PI3K inhibitor), Axitinib displays augmented cytotoxicity and anti-neoplastic efficacy in MB cell lines. Compared to other MKi, Axitinib possesses a higher affinity to STAT3 and AKT, thus it is suggested as a promising therapeutic drug to facilitate MB treatment [77] (Table 1; Part 1).
Receptor tyrosine kinase inhibitor: sunitinib
Sunitinib, a receptor tyrosine kinase (RTK) inhibitor, suppresses tumor growth directly by targeting STAT3 and RTKs or indirectly by inhibiting angiogenesis, representing a promising treatment option for MB patients [52, 78]. Sunitinib exhibits anti-cancerous activity in MB via arresting cell cycle and inducing apoptosis which were associated with the inhibition of STAT3 phosphorylation [52]. Unfortunately, Sunitinib cannot cross the BBB, thereby it can be delivered locally to the cerebrospinal fluid by convection-enhanced delivery in order to contribute to a more effective antitumor effect [52] (Table 1; Part 2).
Peptide inhibitors: S3-NTDi
S3-NTDi is a penetrating-tagged cell-permeable peptide inhibitor that selectively inhibits the N-terminal domain of STAT3 [79]. Treating MB cell lines with S3-NTDi inhibited phosphorylated STAT3 as well as the expression of its target genes including Bcl-2, c-Myc, and cyclin D1. Notably, S3-NTDi reduced the efficiency of STAT3 to bind to its target gene promoters. Moreover, it was shown that S3-NTDi attenuated STAT3 signaling pathway via increasing the expression of protein inhibitor of activated STAT3 (PIAS3) mediated by the downregulation of miRNA-21 [79]. Furthermore, MB cells treated with S3-NTDi were subjected to apoptosis and cell cycle arrest, in addition to suppressed cell migration. Interestingly, S3-NTDi enhanced the apoptotic effect of the chemotherapeutic drug cisplatin in MB cells. Thus, S3-NTDi and cisplatin worked in a synergistic way to sensitize MB cells to chemotherapy [79] (Table 1; Part 3).
Small molecule inhibitors/non-peptide inhibitors
LLL12 and LLL12B
Non-peptide inhibitors selectively inhibit the function of STAT3 SH2 domain regardless of the STAT3 phosphorylation state. They also inhibit STAT3 activation, dimerization, and nuclear translocation as well as the induction of apoptosis in STAT3-dependent cancer cells [80]. LLL12 is a non-peptide small molecule that inhibits STAT3 phosphorylation. It inhibited proliferation and induced apoptosis in MB cell lines [55]. LLL12 inhibits the phosphorylation of STAT3 but not ERK1/2, AKT, and STAT1, indicating that it is specific for STAT3. It also blocks the ability of STAT3 to bind to DNA and suppresses the transcription of STAT3 target genes such as cyclin D1, survivin, Bcl-2, and Bcl-l. Due to its efficacy and ability to cross the BBB, LLL12 should be further investigated as a treatment option in MB [55] (Table 1; Part 4).
LLL12B is a variant of LLL12 that displays greater bioavailability than its original compound. Chen et al. have demonstrated that LLL12B blocked IL-6-mediated STAT3 phosphorylation inducing growth arrest and apoptosis [81]. LLL12B possessed a better anti-neoplastic effect when combined with cisplatin compared to monotherapy in vitro and in vivo [81]. When combined with irradiation therapy, it decreased the expression of STAT3 downstream targets and exhibited enhanced tumorsphere suppression, cell migration, and invasion [54]. Thus, LLL12B offers a novel treatment option that can be used in combination with cisplatin chemotherapy or radiotherapy (Table 1; Part 4).
LY5 and LLY17
LY5, another small molecule inhibitor, suppressed the constitutive activation of STAT3, inhibited its nuclear translocation, and downregulated the expression of downstream targets such as survivin, cyclin D1, Bcl-xl, and micro-RNA-21. LY5 treatment in combination with cisplatin and radiotherapy showed a potent effect in the reduction of cell viability [82]. Similarly, LLY17, another small-molecule inhibitor of STAT3, blocked IL-6-induced phosphorylation of STAT3 and enhanced the anti-tumor effect of irradiation [54]. Therefore, the inhibition of STAT3 by LY5 and LLY17 represents a possible strategy for MB treatment (Table 1; Part 4).
Bazedoxifene
Bazedoxifene is an FDA-approved drug for postmenopausal osteoporosis prevention that is re-purposed for anti-cancer treatment. It suppresses tumor growth and promotes apoptosis via targeting IL-6/IL-6R/GP130 complex [56]. Furthermore, a recent study has shown that bazedoxifene inhibited GP130 signaling in order to overcome the chemoresistance in Group3 MB [83]. Bazedoxifene was also shown to inhibit STAT3 phosphorylation and decrease IL-6-mediated glycolysis and colony formation in MB in vitro models. It also resulted in reduced cell viability and proliferation due to the inhibition of the IL-6/GP130/STAT3 axis [56], thus representing a possible therapeutic drug (Table 1; Part 4).
Polyphenolic compounds
Resveratrol
Resveratrol is a natural polyphenolic compound used for targeting tumors due to its anti-neoplastic properties [84] and biochemical characteristics such as high permeability across BBB and low toxic effect [85]. In MB cell lines, resveratrol has inhibited the activation of STAT3 signaling, suppressed the expression of STAT3-related genes, and also induced neuron-oriented differentiation [31, 86] through Fas-independent pathway [87]. This may further drive MB to growth arrest and apoptosis as well as enhance radiosensitivity in resveratrol-treated MB [88] (Table 1; Part 5).
Curcumin
Curcumin, a natural compound product, is served as an effective agent for pediatric brain tumors due to high BBB permeability [89]. It attenuates many signaling pathways in MB such as Wnt/β-catenin, NF-ĸB, SHH, and STAT3 which are crucial for MB pathogenesis [90,91,92]. Curcumin reduced the level of phosphorylated STAT3, induced a dose-dependent decrease in the growth of MB cells, reduced the population of stem-like cells, arrested cell cycle, and induced apoptosis [90] (Table 1; Part 5).
Non-steroid anti-inflammatory drug (NSAID): celecoxib
Celecoxib is a cyclooxygenase-2 (COX-2) inhibitor and non-steroid anti-inflammatory drug that shows an anti-tumor effect in various human cancers [93, 94]. Celecoxib mediates its anti-tumor effects either directly through suppressing COX-2 activity or in a COX-2 independent manner [94]. In MB, celecoxib inhibited STAT3 activation and suppressed MB stem cell-like properties. It also enhanced the sensitivity to ionizing radiotherapy, thus improving the survival of the animal model [95] (Table 1; Part 6).
Antipsychotic drug: pimozide
Pimozide is an FDA-approved antipsychotic drug used clinically in treating psychological disorders and targeting human cancers through the inhibition of cell proliferation, angiogenesis, and metastatic activity [96]. Pimozide is considered a potential STAT3 inhibitor in targeting the tumor [97]. It was shown that pimozide targeted MB cells by inhibiting STAT3 pathway and its downstream pro-apoptotic markers such c-Myc, Mcl-1, and Bcl-2 thus inducing autophagy-mediated apoptosis in the MB cell line [98] (Table 1; part 7).
JAK inhibitors
AG490
STAT3 can be inhibited indirectly by selective inhibition of the upstream JAK, consequently preventing STAT3 phosphorylation which has been implicated in tumor growth [99]. It was revealed that selective inhibition of STAT3 phosphorylation by AG490 was accompanied by suppression of MB growth, downregulation of survivin, cyclin-D1, Cox-2, and c-Myc, and enhancement of differentiation-like changes in MB cells [86] suggesting the importance of STAT3 signaling in MB survival and maintenance (Table 1; Part 8).
Ruxolitinib and tofacitinib
Ruxolitinib and Tofacitinib are selective inhibitors targeting JAK1/2 [100] and JAK3 exerting a potent anti-tumor effect in several human malignancies [101]. Noteworthy, ruxolitinib and tofacitinib have a safety profile and can be effectively delivered across the BBB [102]. In MB, they reduced JAK/STAT3 phosphorylation a well reduced MB tumorigenesis by decreasing cell viability, colony formation, and migration, and also enhanced apoptosis [103]. They could be an attractive drug for targeting MB (Table 1; Part 8).
Cucurbitacin I
Cucurbitacin I (JSI-124) is also a JAK2/STAT3 inhibitor with an effective anti-neoplastic activity [104]. Suppression of STAT3 phosphorylation by cucurbitacin I has downregulated STAT3 target genes leading to inhibition of cell growth and significant cell death [105]. Moreover, the inhibition of STAT3 signaling pathway notably suppressed CSC-like characteristics and stemness gene expression and induced cell differentiation in MB-derived CD133 (±) cells [71]. Interestingly, the inhibition of JAK2/STAT3 sensitized MB cells to chemotherapy and irradiation resulting in synergistic cell death [71, 105] (Table 1; Part 8).
Src inhibitors
KX2-391
STAT3 signaling can be also activated by non-receptor tyrosine kinases (Src kinases) consequently mediating cancer tumorigenesis and drug resistance [106]. Activated Src were significantly detected in SHH-MB cell lines compared to normal brain cells making them a potential target for SHH-MB [103]. KX2-391 is a non-ATP-competitive inhibitor of Src kinase and tubulin polymerization. It blocked MB cell colony formation and migration through the inhibition of Src/STAT3 [103] (Table 1; Part 9).
Dasatinib
Dasatinib, another FDA-approved Src inhibitor, is used clinically in myeloid leukemia patients and some solid malignancies including MB [103]. It is worthy to note that this drug can be administered orally and easily crosses BBB, hence confirming its potent efficacy in treating pediatric brain tumors [103]. Dasatinib showed a synergistic effect when combined with cisplatin which could significantly inhibit STAT3 phosphorylation and reduce colony formation and migration ability [103]. These results suggest that Src inhibitors could be a novel candidate for MB treatment (Table 1; Part 9).
Other drugs
PG-S3-002, PG-S3-009 and PG-S3-010
PG-S3-002 is a STAT3 inhibitor targeting the SH2 domain preventing its dimerization. It has a selective activity toward CD133+ MBSCs. Garg et al. have shown a decreased level of phosphorylated STAT3 protein and reduced tumor size in group 3 MB xenograft model after treatment with PG-S3-002. However, treating normal neuronal stem cells with PG-S3-002 led to significant neurotoxicity [70]. Other inhibitors of STAT3, PG-S3-009, and PG-S3-010, are highly selective for recurrent MB. They have been shown to decrease tumor burden in vivo demonstrating effectiveness in targeting recurrent MB. Nevertheless, PG-S3-009-treated mice showed no survival benefits with a neurotoxicity side effect [70] (Table 1; Part 10).
NSC74859 and WP1066
NSC 74,859, also known as S3I-201, is a chemical probe STAT3 inhibitor. It prevents STAT3 dimerization and STAT3-DNA interaction thus suppressing its transcriptional activities [107]. WP1066, an AG490 analog, inhibits JAK2 and STAT3 phosphorylation and participates in JAK2 degradation, thereby, affecting STAT3 signaling pathway [108]. Nazio et al. have revealed through that NSC 74,859 and WP1066 decreased STAT3 phosphorylated level and the expression of its target genes including c-Myc and cyclin-D2. Interestingly, the combination of these two drugs with an autophagy inhibitor induced MB cell death and reduced cellular proliferation. WP1066 in combination with an autophagy inhibitor showed an antitumor activity via attenuating MB cell growth and inhibiting metastasis [69] (Table 1; Part 10). Furthermore, a phase I clinical trial is currently evaluating the safety, feasibility and tolerability of WP1066 in brain tumors including MB (ClinicalTrials.gov; NCT4334863). It is worth mentioning that a phase I clinical trial has also investigated the therapeutic effect of WP1066 in patients with recurrent malignant glioma (ClinicalTrials.gov; NCT01904123) where WP1066 showed systemic immune suppression of p-STAT3 with the absence of significant toxicity [109].
LB100
Inhibition of STAT3 activity by increasing Ser727 phosphorylation and decreasing Tyr705 phosphorylation was previously observed upon deactivation of serine/threonine phosphatase 2A (PP2A) [110]. LB100 is a PP2A inhibitor that was tested in preclinical experiments and showed safety and efficacy in targeting solid tumors. Moreover, it exhibited anti-neoplastic properties and was evaluated in a phase I clinical trial (ClinicalTrials.gov; NCT01837667) [111]. Ho et al. reported that LB100 inhibited STAT3 phosphorylation and its downstream targets as well as reduced MB tumorigenicity such as attenuating cell growth and migration and overcoming drug resistance in MB cells [112] (Table 1; Part 10). These results provide strong evidence for evaluating LB100 as an adjunct to chemotherapeutic drugs in MB clinical trials.
Other ways for targeting STAT3 in MB
RNA Interference
RNA interference (RNAi) is a biological process of targeting the silencing of gene expression [113]. It is one of the used approaches for cancer treatment, brain disorders, and viral infection [114]. Kotipatruni et al. showed that downregulation of tumor invasion molecules including urokinase plasminogen activator receptor (uPAR) and MMP-9 via small hairpin RNAs (shRNA) disrupted EGFR-mediated activation of STAT3, thus preventing the recruitment of STAT3 at the promoter of Bcl-2 and survivin genes consequently inhibiting gene expression and further inducing apoptosis in MB cell lines [57].
Fusion Protein GMME1
Fusion protein technology represents one of the used therapeutic approaches in protein-drug development in order to increase drugs' stability, activity, and bioavailability for targeting diseases [115]. It comprises different domains encoded by distinct genes [116]. GMME1 is a fusion protein that consists of granulocyte–macrophage colony-stimulating factor (GMCSF) and truncated C–C motif chemokine ligand 2 (CCL2). It specifically blocked C–C motif chemokine receptor 2 (CCR2)-associated STAT3 phosphorylation and upregulated BCL2-associated X protein (BAX) leading to cell death indicating its potential therapeutic utility [117].
SPARC overexpression
Secreted Protein Acidic and Rich in Cysteine (SPARC), a calcium-binding glycoprotein, is a multifunctional component of extracellular matrix (ECM) that modulates various processes such as angiogenesis, counter adhesion, ECM remodeling, cellular migration, and proliferation [53, 118]. The infection of MB cells with SPARC full-length cDNA adenovirus (Ad-DsRed-SP) resulted in a decline in Notch1 expression as well as a reduction in STAT3 phosphorylation in MB tumor specimens. This enhanced neuron-like morphology and expression of neuronal markers in MB cell lines [53]. Another study demonstrated that SPARC expression suppressed MB cell proliferation and arrested cell cycle at G2/M phase and also decreased STAT3 phosphorylation at Tyr705 residue [119]. These findings indicate that targeting STAT3 signaling via SPARC could be valuable for rational cancer therapy.
Conclusion and future perspectives
The aggressive heterogeneous nature of MB requires more understanding of the underlying abnormal signaling pathways in order to prevent further development of MB, avoid long-term toxicity of the current treatment regimens and prolong patients’ OS. Considering that STAT3 is a well-known oncogene factor in different cancers, and its involvement in the tumorigenesis MB, several studies have attempted to decipher the molecular mechanisms for constitutive activation of STAT3 that are implicated in the malignancy and growth of MB, thereby facilitating the development of more comprehensive and efficient therapeutic strategies. Targeting STAT3 is a promising approach in MB treatment that has shown to have anti-neoplastic effects in preclinical studies. Indeed, several drugs directed toward STAT3 pathway are considered a major part of MB therapies making STAT3 a focal topic in cancer research. Future research and clinical interventions are indeed needed for better delineating the detailed aberrant molecular mechanisms of STAT3 and support further exploration of STAT3 inhibitors in MB therapies.
Data availability
Not applicable.
Code availability
Not applicable.
References
Khatua S et al (2018) Childhood medulloblastoma: current therapies, emerging molecular landscape and newer therapeutic insights. Curr Neuropharmacol 16(7):1045–1058
Audi ZF et al (2021) Immunosuppression in medulloblastoma: insights into cancer immunity and immunotherapy. Curr Treat Options Oncol 22(9):83
Ostrom QT et al (2018) CBTRUS statistical report primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20(suppl_4):vi1-iv86
Komori T (2017) The 2016 WHO classification of tumours of the central nervous System: the major points of revision. Neurol Med Chir (Tokyo) 57(7):301–311
Northcott PA et al (2012) Medulloblastomics: the end of the beginning. Nat Rev Cancer 12(12):818–834
Kaur K et al (2016) Integrating molecular subclassification of medulloblastomas into routine clinical practice: a simplified approach. Brain Pathol 26(3):334–343
Northcott PA et al (2019) Medulloblastoma. Nat Rev Dis Primers 5(1):11
Hammoud H et al (2020) Drug repurposing in medulloblastoma: challenges and recommendations. Curr Treat Options Oncol 22(1):6
Dirven L et al (2020) Neurocognitive functioning and health-related quality of life in adult medulloblastoma patients: long-term outcomes of the NOA-07 study. J Neurooncol 148(1):117–130
Wang Y, Levy DE (2012) Comparative evolutionary genomics of the STAT family of transcription factors. Jakstat 1(1):23–33
Chakraborty D et al (2017) Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 8(1):1130
Gu Y, Mohammad IS, Liu Z (2020) Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol Lett 19(4):2585–2594
Piperi C, Papavassiliou KA, Papavassiliou AG (2019) Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells 8(11):1398
Alonzi T et al (2001) Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation in the liver. Mol Cell Biol 21(5):1621–1632
Benito C et al (2017) STAT3 Controls the long-term survival and phenotype of repair schwann cells during nerve regeneration. J Neurosci 37(16):4255–4269
Huang C et al (2008) JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J Neuroimmunol 204(1–2):118–125
Wen Z, Darnell JE Jr (1997) Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res 25(11):2062–2067
Tkach M et al (2013) p42/p44 MAPK-mediated Stat3Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr Relat Cancer 20(2):197–212
Wu M et al (2019) Negative regulators of STAT3 signaling pathway in cancers. Cancer Manag Res 11:4957–4969
Dong Y et al (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31(12):2097–2104
Floss DM et al (2013) Identification of canonical tyrosine-dependent and non-canonical tyrosine-independent STAT3 activation sites in the intracellular domain of the interleukin 23 receptor. J Biol Chem 288(27):19386–19400
Timofeeva OA et al (2012) Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem 287(17):14192–14200
Zhang X et al (2007) Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci U S A 104(10):4060–4064
Rozovski U et al (2018) STAT3 is constitutively acetylated on lysine 685 residues in chronic lymphocytic leukemia cells. Oncotarget 9(72):33710–33718
Xu YS et al (2016) STAT3 Undergoes acetylation-dependent mitochondrial translocation to regulate pyruvate metabolism. Sci Rep 6:39517
Yang J et al (2010) Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci U S A 107(50):21499–21504
Dasgupta M et al (2015) STAT3-driven transcription depends upon the dimethylation of K49 by EZH2. Proc Natl Acad Sci U S A 112(13):3985–3990
Murase S (2013) Signal transducer and activator of transcription 3 (STAT3) degradation by proteasome controls a developmental switch in neurotrophin dependence. J Biol Chem 288(28):20151–20161
Wei J et al (2012) The ubiquitin ligase TRAF6 negatively regulates the JAK-STAT signaling pathway by binding to STAT3 and mediating its ubiquitination. PLoS ONE 7(11):e49567
Su F et al (2012) Protein tyrosine phosphatase Meg2 dephosphorylates signal transducer and activator of transcription 3 and suppresses tumor growth in breast cancer. Breast Cancer Res 14(2):R38
Li C et al (2016) SHP2, SOCS3 and PIAS3 expression patterns in medulloblastomas: relevance to STAT3 activation and resveratrol-suppressed STAT3 signaling. Nutrients. https://doi.org/10.3390/nu9010003
Woetmann A et al (1999) Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA 96(19):10620–10625
Nicholson SE et al (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA 97(12):6493–6498
Chung CD et al (1997) Specific inhibition of Stat3 signal transduction by PIAS3. Science 278(5344):1803–1805
De-Fraja C et al (1998) Members of the JAK/STAT proteins are expressed and regulated during development in the mammalian forebrain. J Neurosci Res 54(3):320–330
Zhong Z, Wen Z, Darnell JE Jr (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264(5155):95–98
Dziennis S, Alkayed NJ (2008) Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci 19(4–5):341–361
Takeda K et al (1997) Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94(8):3801–3804
Foshay KM, Gallicano GI (2008) Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev 17(2):269–278
Kanski R et al (2014) A star is born: new insights into the mechanism of astrogenesis. Cell Mol Life Sci 71(3):433–447
Ma X et al (2017) Stat3 controls maturation and terminal differentiation in mouse hippocampal neurons. J Mol Neurosci 61(1):88–95
Nicolas CS et al (2012) The Jak/STAT pathway is involved in synaptic plasticity. Neuron 73(2):374–390
McGregor G, Irving AJ, Harvey J (2017) Canonical JAK-STAT signaling is pivotal for long-term depression at adult hippocampal temporoammonic-CA1 synapses. Faseb j 31(8):3449–3466
Murata S et al (2000) Occurrence of a transcription factor, signal transducer and activators of transcription 3 (Stat3), in the postsynaptic density of the rat brain. Brain Res Mol Brain Res 78(1–2):80–90
Sahin GS et al (2020) Leptin stimulates synaptogenesis in hippocampal neurons via KLF4 and SOCS3 inhibition of STAT3 signaling. Mol Cell Neurosci 106:103500
Wan HL et al (2021) STAT3 ameliorates cognitive deficits via regulation of NMDAR expression in an Alzheimer’s disease animal model. Theranostics 11(11):5511–5524
Jung JE et al (2009) Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 29(21):7003–7014
Dziennis S et al (2007) Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci 27(27):7268–7274
Murase S et al (2012) Loss of signal transducer and activator of transcription 3 (STAT3) signaling during elevated activity causes vulnerability in hippocampal neurons. J Neurosci 32(44):15511–15520
Wan J et al (2010) Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer’s disease. J neurosci 30(20):6873–6881
Bian Z et al (2021) Noncoding RNAs involved in the STAT3 pathway in glioma. Cancer Cell Int 21(1):445
Yang F et al (2010) Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res 8(1):35–45
Bhoopathi P et al (2011) SPARC stimulates neuronal differentiation of medulloblastoma cells via the Notch1/STAT3 pathway. Cancer Res 71(14):4908–4919
Pan L et al (2021) STAT3 inhibitor in combination with irradiation significantly inhibits cell viability, cell migration, invasion and tumorsphere growth of human medulloblastoma cells. Cancer Biol Ther 22(7–9):430–439
Ball S et al (2011) The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells. PLoS ONE 6(4):e18820
Chen X et al (2018) Blocking interleukin-6 signaling inhibits cell viability/proliferation, glycolysis, and colony forming activity of human medulloblastoma cells. Int J Oncol 52(2):571–578
Kotipatruni RR et al (2012) Apoptosis induced by knockdown of uPAR and MMP-9 is mediated by inactivation of EGFR/STAT3 signaling in medulloblastoma. PLoS ONE 7(9):e44798
White CL et al (2019) A Sexually Dimorphic Role for STAT3 in Sonic Hedgehog medulloblastoma. Cancers 11(11):1702
Gao P et al (2017) The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 8(40):69139–69161
Niu G et al (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21(13):2000–2008
Craveiro RB et al (2017) The anti-neoplastic activity of vandetanib against high-risk medulloblastoma variants is profoundly enhanced by additional PI3K inhibition. Oncotarget 8(29):46915–46927
Wang T et al (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10(1):48–54
Abad C et al (2014) Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J Leukoc Biol 95(2):357–367
Kumar V et al (2016) CD45 Phosphatase Inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity 44(2):303–315
Purvis IJ et al (2020) B7H3 in medulloblastoma-derived exosomes A novel tumorigenic role. Int J Mol Sci. https://doi.org/10.3390/ijms21197050
Molina-Peña R, Tudon-Martinez JC, Aquines-Gutiérrez O (2020) A mathematical model of average dynamics in a stem cell hierarchy suggests the combinatorial targeting of cancer stem cells and progenitor cells as a potential strategy against tumor growth. Cancers (Basel). https://doi.org/10.3390/cancers12092590
Bahmad HF, Poppiti RJ (2020) Medulloblastoma cancer stem cells: molecular signatures and therapeutic targets. J Clin Pathol 73(5):243–249
Liu H et al (2020) Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro Oncol 22(5):625–638
Nazio F et al (2021) Targeting cancer stem cells in medulloblastoma by inhibiting AMBRA1 dual function in autophagy and STAT3 signalling. Acta Neuropathol 142(3):537–564
Garg N et al (2017) CD133 (+) brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence. Oncogene 36(5):606–617
Chang CJ et al (2012) Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells. Childs Nerv Syst 28(3):363–373
Sreenivasan L et al (2020) Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death Dis 11(12):1035
Craveiro RB et al (2014) In comparative analysis of multi-kinase inhibitors for targeted medulloblastoma therapy pazopanib exhibits promising in vitro and in vivo efficacy. Oncotarget 5(16):7149–7161
Liu L et al (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66(24):11851–11858
Yang F et al (2008) Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther 7(11):3519–3526
Brave SR et al (2011) Vandetanib inhibits both VEGFR-2 and EGFR signalling at clinically relevant drug levels in preclinical models of human cancer. Int J Oncol 39(1):271–278
Ehrhardt M et al (2018) The FDA approved PI3K inhibitor GDC-0941 enhances in vitro the anti-neoplastic efficacy of Axitinib against c-myc-amplified high-risk medulloblastoma. J Cell Mol Med 22(4):2153–2161
Mendel DB et al (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9(1):327–337
Ray S et al (2018) Suppression of STAT3 NH(2) -terminal domain chemosensitizes medulloblastoma cells by activation of protein inhibitor of activated STAT3 via de-repression by microRNA-21. Mol Carcinog 57(4):536–548
Schust J et al (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 13(11):1235–1242
Chen X et al (2021) LLL12B, a small molecule STAT3 inhibitor, induces growth arrest, apoptosis, and enhances cisplatin-mediated cytotoxicity in medulloblastoma cells. Sci Rep 11(1):6517
Xiao H et al (2015) A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J Biol Chem 290(6):3418–3429
Sreenivasan L et al (2022) Targeting the gp130/STAT3 Axis Attenuates tumor microenvironment mediated chemoresistance in group 3 medulloblastoma cells. Cells 11(3):381
Jiao Y et al (2015) Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients 7(6):4383–4402
Mokni M et al (2007) Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem Res 32(6):981–987
Yu LJ et al (2008) Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia 10(7):736–744
Wang Q et al (2003) Resveratrol promotes differentiation and induces Fas-independent apoptosis of human medulloblastoma cells. Neurosci Lett 351(2):83–86
Lu K-H et al (2009) Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv Syst 25(5):543–550
Lee SJ et al (2011) Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 11(1):144
Lim KJ et al (2011) A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther 11(5):464–473
Elamin MH et al (2010) Curcumin inhibits the sonic hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog 49(3):302–314
He M et al (2014) Curcumin suppresses cell proliferation through inhibition of the Wnt/β-catenin signaling pathway in medulloblastoma. Oncol Rep 32(1):173–180
Xu XT et al (2017) Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP. Am J Transl Res 9(3):1088–1100
Liu DB et al (2012) Celecoxib induces apoptosis and cell-cycle arrest in nasopharyngeal carcinoma cell lines via inhibition of STAT3 phosphorylation. Acta Pharmacol Sin 33(5):682–690
Yang MY et al (2014) Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells. Int J Mol Sci 15(6):11013–11029
el Dakir H et al (2018) The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget 9(79):34889–34910
Zhou W et al (2016) The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation. Int J Oncol 48(1):322–328
Ranjan A, Kaushik I, Srivastava SK (2020) Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells. https://doi.org/10.3390/cells9092141
Rahaman SO et al (2002) Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene 21(55):8404–8413
Han ES et al (2018) Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer. Oncotarget 9(36):24304–24319
Ando S et al (2016) Tofacitinib induces G1 cell-cycle arrest and inhibits tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Oncotarget 7(47):76793–76805
Haile WB et al (2016) The janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis 92(Pt B):137–143
Wei J et al (2019) Targeting upstream kinases of STAT3 in human MEDULLOBLASTOMA cells. Curr Cancer Drug Targets 19(7):571–582
Blaskovich MA et al (2003) Discovery of JSI-124 (cucurbitacin I), a selective janus KINASE/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63(6):1270–1279
Lo HW et al (2008) Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res 14(19):6042–6054
Garcia R et al (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20(20):2499–2513
Siddiquee K et al (2007) Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104(18):7391–7396
Ferrajoli A et al (2007) WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res 67(23):11291–11299
Groot J et al (2022) A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma. CNS Oncol. https://doi.org/10.2217/cns-2022-0005
Mandal T et al (2014) Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2—protein phosphatase 2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell Signal 26(8):1725–1734
D’Arcy BM et al (2019) The antitumor drug LB-100 Is a catalytic inhibitor of protein phosphatase 2A (PPP2CA) and 5 (PPP5C) coordinating with the active-site catalytic metals in PPP5C. Mol Cancer Ther 18(3):556–566
Ho WS et al (2016) PP2A inhibition with LB100 enhances cisplatin cytotoxicity and overcomes cisplatin resistance in medulloblastoma cells. Oncotarget 7(11):12447–12463
Jagadeesan S, Hakkim A (2018) RNAi Screening: automated high-throughput liquid RNAI screening in caenorhabditis elegans. Curr Protoc Mol Biol 124(1):e65
Di Silvio D et al (2019) Self-assembly of poly(allylamine)/siRNA nanoparticles, their intracellular fate and siRNA delivery. J Colloid Interface Sci 557:757–766
Kulakova A et al (2020) Albumin-neprilysin fusion protein: understanding stability using small angle X-ray scattering and molecular dynamic simulations. Sci Rep 10(1):10089
Marsh JA et al (2013) Protein complexes are under evolutionary selection to assemble via ordered pathways. Cell 153(2):461–470
Rafei M et al (2011) A MCP1 fusokine with CCR2-specific tumoricidal activity. Mol Cancer 10:121
Chlenski A et al (2006) SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer 118(2):310–316
Chetty C et al (2012) SPARC expression induces cell cycle arrest via STAT3 signaling pathway in medulloblastoma cells. Biochem Biophys Res Commun 417(2):874–879
Funding
Not funded.
Author information
Authors and Affiliations
Contributions
AZ and SN conceived the concept and idea of the present review. AZ and SN worked on the study design strategy and selected the topics to be discussed. AZ, ZA, FS, ZS, RN and HB did literature searches and screened titles and abstracts for relevance. AZ, ZA, FS and ZS abstracted the data from the eligible full text articles, analyzed and interpreted the data and drafted the manuscript. HH, RN and HB revised the final draft of the manuscript. SN critically revised the manuscript with input from the entire team. All authors have read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaiter, A., Audi, Z.F., Shawraba, F. et al. STAT3 in medulloblastoma: a key transcriptional regulator and potential therapeutic target. Mol Biol Rep 49, 10635–10652 (2022). https://doi.org/10.1007/s11033-022-07694-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07694-6