Abstract
Salt stress is one of the leading threats to crop growth and productivity across the globe. Salt stress induces serious alterations in plant physiological, metabolic, biochemical functioning and it also disturbs antioxidant activities, cellular membranes, photosynthetic performance, nutrient uptake and plant water uptake and resulting in a significant reduction in growth and production. The application of osmoprotectants is considered as an important strategy to induce salt tolerance in plants. Trehalose (Tre) has emerged an excellent osmolyte to induce salinity tolerance and it got considerable attention in recent times. Under salinity stress, Tre helps to maintain the membrane integrity, and improves plant water relations, nutrient uptake and reduces the electrolyte leakage and lipid per-oxidation. Tre also improves gas exchange characteristics, protects the photosynthetic apparatus from salinity induced oxidative damages and brings ultra-structure changes in the plant body to induce salinity tolerance. Moreover, Tre also improves antioxidant activities and expression of stress responsive proteins and genes and confers salt tolerance in plants. Additionally, Tre is also involved in signaling association with signaling molecules and phytohormones and resultantly improved the plant performance under salt stress. Thus, it is interesting to understand the role of Tre in mediating the salinity tolerance in plants. Therefore, in this review we have summarized the different physiological and molecular roles of Tre to induce salt tolerance in plants. Moreover, we have also provided the information on Tre cross-talk with various osmolytes and hormones, and its role in stress responsive genes and antioxidant activities. Lastly, we also shed light on research gaps that need to be addressed in future studies. Therefore, this review will help the scientists to learn more about the Tre in changing climate conditions and it will also provide new insights to insights that could be used to develop salinity tolerance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
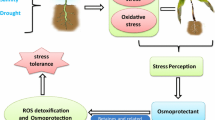
Soil salinization is a major problem across the globe which is negatively affecting crop production and putting global food security at great risk [1]. It has been reported that more than 20% of arable lands are salt affected which causes huge yield losses every year [2, 3]. Soil salinity (SS) adversely affects all phases of plant development, including germination, seedling, vegetative and mature stages [4]. However, the effects of SS largely depend on the severity and duration of stress, plant species and stage of plant growth [5, 6]. Seed germination is the first phase of plant life that is negatively affected by SS. The higher concentration of salts in growing medium reduced the germination by decreasing water uptake, disrupting enzymatic activities and imposing salt induced oxidative damage [7,8,9,10,11]. Salinity stress also disturbs hormones crosstalk, antioxidant activities, ionic homeostasis and induced production of reactive oxygen species (ROS) that reduce the growth and biomass production and cause damage to cellular membranes, proteins and DNA [7, 12,13,14,15,16]. Further, SS also inhibits soil microbial and enzymatic activities which negatively affect soil fertility and overall crop production [18].
Salinity stress is considered to be complex abiotic stress which involves ionic, osmotic and oxidative stresses [19,20,21]. Osmotic stress occurs shortly after exposure of salts and it reduces the plant’s ability to uptake nutrients and water [3, 22]. Likewise, excessive sodium (Na+) accumulation in plant cytoplasm causes potassium (K+) deficiency and disrupts photosynthetic processes, enzymatic activities and biosynthesis of proteins [3, 23]. Moreover, salinity induces oxidative stress that produce ROS which are highly cyto-toxic and cause huge damage to lipids, proteins and nucleic and resulting in DNA mutation, lipid peroxidation and denatuartion of proteins [19]. Additionally, SS also reduces the synthesis of chlorophyll contents, soluble proteins and free amino acids and increases the membrane damage, malondialdehyde (MDA) and hydrogen peroxide (H2O2) accumulation which in turn causes a huge reduction in growth and productivity [24].
Plant use different defense mechanisms to ensure their survival and maintain growth under different abiotic stresses [25,26,27,28], especially SS [29]. One of the most important protective mechanisms is an anti-oxidant system which comprises of different enzymatic and non-enzymatic antioxidant which counter the salinity induced oxidative damage by increasing the scavenging of ROS [30,31,32,33]. Indeed, higher anti-oxidant activities provide resistance to plants and reduce the salinity induced oxidative damages [33]. The second most important defense mechanism is an osmotic adjustment which is vital process that induce stress tolerance in the plant by synthesis of different organic solutes [34]. Plants accumulate different solutes including proline, glyciene-betaine, amino acids and different sugars that maintain ionic balance of vacuole and protect plant organelles by scavenging ROS [31, 35]. Moreover, osmolytes also improve the photosynthetic efficiency and improve the anti-oxidant activities resulting in appreciable improvement in growth and production under stress conditions [36, 37].
Trehalose (Tre) is non-reducing sugar that synthesized in plants and it plays a protector role under different stresses [38]. This sugar has white color with no odor and it 45% times sweeter as compared to sucrose [39]. Tre play a significant role in plant functioning and it is considered to be potential osmo-protectant [31, 40]. Trehalose stabilized cellular membranes, enzymes, proteins and protects the biological system from damaging impacts of SS [31, 40]. It also works as an elicitor of genes involved in stress responses and detoxification ROS [41,42,43]. Tre protects the biological structures from SS by working as signaling molecule and increasing anti-oxidant activities [44]. The application of Tre has been found to increase the plant growth and yield by overcoming adverse of SS [45]. However, production of Tre is not sufficient to mitigate the adverse of different stresses. Therefore, exogenous application of Tre increased endogenous Tre and it is considered as an alternate strategy to improve stress tolerance [41, 46]. Therefore, in this review we discussed the physiological and biochemical roles of Tre under SS. We have also presented the Tre mediated improvement in anti-oxidant activities, its cross talk with different osmo-regulating compounds and success stories of engineering Tre to induce salinity tolerance in plants. Additionally, we also highlighted the research gaps where future research must be conducted to explore the role of this important osmo-protectant under SS.
Plant responses to salinity stress
Seed germination is most critical stage of plant life [47]. Generally, SS delays or decrease the seed germination [48] by reducing water uptake and decreasing soil osmotic potential [48, 49]. Excessive Na+ and chloride (Cl-) concentration in growing medium also cause toxicity to seed embryo, alter protein synthesis, energy production, hormonal and nutrient balance which in turn delay the seed germination [47, 50]. Reducing enzymatic activities (α-amylase) during germination reduce the translocation of sugar to growing embryo which reduce and delay in germination [51, 52]. SS also negatively affect growth and development of plants however, response of plants can different due to plant growth stage, plant species, duration and intensity of SS [53]. Generally, SS decrease the plant growth in two phases. In first phase plant growth is reduced within a few minutes where higher concentration of salts reduces growth rate and water uptake owing to osmotic stress [54]. SS reduces the expansion of both root and shoots cells [55], induce stomata closure, and reduce the CO2 assimilation which resultantly cause reduction in growth [56, 57].
Further, plants are grown under SS also face changes in plant physiological and metabolic processes, enzymatic activities, plant water relations, carbon dioxide (CO2) assimilation, synthesis of proteins and efficiency of PS-II which also contribute toward a reduction in growth [53, 58]. In second phase growth is reduced due to the accumulation of salts in plant leaves which reach to threshold levels and cause toxicity. This phase is a long phase and it can take a few days to weeks or months to complete [53]. During this phase higher Na+ accumulation in plant body inhibit enzymatic activities. For instance, the activities of different enzymes involved in the synthesis of starch, glycolysis, polyamine, phenylpropanoid pathway and Calvin cycle are disturbed under SS which causes a reduction in growth [59, 60].
Plants also show chlorosis, necrosis, senescence and reduced leaf area owing to a reduction in photosynthetic activities which also contributed to a reduction in growth in the second phase [53]. The threshold levels of ionic toxicity vary from among plant species, cultivars and genotypes. For instance magnitude of reduction in grain yield was maximum (67%) in sensitive cultivars as compared to adapted wheat cultivar (41%) at a salinity level of 120 mM [54]. The availability of moisture significantly affects physiological and metabolic processes taking place within the plant body. During, SS plants undergo osmotic stress that decreases cell water potential and reduces the water uptake [54]. Relative water contents (RWC) are considered to be important criteria to select plants for salinity tolerance [61]. For instance, Nassar et al. [62] noted a reduction of 3.5% in RWC of salt tolerant wheat cultivar, whereas these authors noted a reduction of 6.7% in RWC of salt sensitive wheat cultivar. Salinity stress also reduced membrane stability and increases the loss of important substances [63] (Table 1).
SS induced the production of ROS that cause membrane damage and increase the production of MDA and lipid peroxidation [24]. The ability of a plant to maintain a normal transpiration rate under SS reflects their stress however, SS reduced the transpiration rate [72]. Chlorophyll plays a significant role in processes of photosynthesis however, SS causes significant reduction in chlorophyll contents [73]. Salinity stress reduced the chlorophyll contents by the disintegration of chlorophyll structure and excessive ROS production that denature enzymes needed for the synthesis of chlorophyll [74]. SS also reduced the photosynthetic rate which is associated with reduced stomatal conductance, rubisco activities and efficiency of PS-II [75]. A higher concentration of salts also induces early leaf senescence which reduced the leaf area and consequently reduced the rate of photosynthesis and leads to a reduction in biomass production [76].
SS also disturbs nutrient homeostasis, and plant metabolic processes and increases the production of ROS that damage DNA, lipids and membranes and cause leakage of cell solutes [54]. However, plants possess an excellent defense system comprising enzymatic and non-enzymatic antioxidant enzymes to scavenge ROS [77]. The increase in anti-oxidant activities is considered to be vital process to improve salinity tolerance. For instance, the activities of ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutathione reductase (GR) and superoxide dismutase (SOD) were significantly increased under a SS of 100 mM [78]. SS also cause osmotic stress which induces a reduction in plant growth. However, plants minimize the deleterious impacts of osmotic stress by a mechanism known as osmoregulation [54]. In this mechanism plants accumulate various osmolytes (proline, glyciene-betaine, amino acids and different sugars) which regulate plant water relations and antioxidant activities to improve salinity tolerance [79].
Ionic homeostasis is considered as an essential mechanism to mitigate toxic effects of SS. Keeping a higher ration of K+/Na+ in cytosol prevent the cellular damages and increase the salinity tolerance in plants [80, 81]. However, SS significantly increased the accumulation Na+ and cause reduction in calcium (Ca), magnesium (Mg) and K uptake and accumulation [24]. Plants also accumulate various hormones that regulate the plant responses under stress conditions. For instance, in water stress different hormones including abscisic acid (ABA), gibberellic acid (GA), jasmonic acid (JS) and salicylic acid (SA) acted together to regulate the plant responses in A. thaliana [82]. Moreover, ABA and JA showed synergistic impacts in signaling pathways to regulate each other responses against salinity stress [83].
Salinity stress also negatively reduced the yield and final quality of crops. For example, SS (4 dSm−1) reduced the grain weight, number of grains and grain yield of rice [84]. SS also causes flower abortion, reduce the growth of pollen tube grain filling duration and cause significant yield losses [79]. The concentration of salts reduces the concentration of starch and amylase and modulates the texture of grains [85, 86]. Moreover, in the maize crop SS increased the carbohydrate concentration while SS decreased the grain protein contents [85]. In another study it was noted that SS decreased the rice yield by 36% however, it did not affect the texture of grain as compared to the control [87]. Additionally, in barley crop SS reduced grain size and grain carbohydrate concentration but increase the protein contents [88], conversely, in wheat crop, SS significantly reduced the grain protein content [89].
Trehalose biosynthesis, metabolism and structural properties
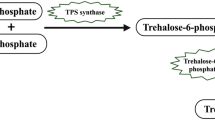
Tre biosynthesis in plants involves the production of trehalose-6-phosphate (T6P) from glucose-6-phosphate and UDP-glucose by trehalose-6-phosphate synthase (TPS), and the subsequent dephosphorylation of T6P to Tre by trehalose-6-phosphate phosphatase (TPP) [90]. Two molecules of uridine-diphospho-glucose (UDP-Glc) and glucose-6-phosphate (Glc-6-P) are used for biosynthesis of Tre in plants. The enzyme TSP catalyzed UDP-Glc and Glc-6-P into T6P [91, 92], whereas enzyme trehalose-6-phosphate phosphatase (TPP) catalyzed the T6P into Tre as the final product [93]. The presence of Tre has been reported in many plants including Selaginella lepidophylla and Myrothamnus flabellifolius, tobacco and rice [41, 94, 95].
A lower concentration of Tre is not solely due to the action of Tre but also owing to tight regulation of TPS and TPP genes expression and enzymatic activities [96]. For instance addition of validamycin A to the growing medium increased Tre accumulation by inhibiting Tre however, it did not stimulate Tre biosynthesis [97]. The regulation of Tre genes increased the Tre biosynthesis which in turn conferred the stress tolerance [96]. For instance expression of yeast and E. coli derived Tre genes made plants tolerant against, cold, salinity and drought stress [98]. In rice plants higher expression of TSP genes increased the plant acclimation against, cold, drought and salt stress [99]. Similarly, up-regulation of AtTPS1 in Arabidopsis plants caused an increase in Tre biosynthesis which in turn increased the cold [100]. Moreover, in A. thaliana high temperature increased the Tre levels by two folds whereas the levels of Tre were increased by eight folds under cold stress (4 °C) [101]. The expression of Tre transgenes also activates biosynthetic pathways in plant organs exposed to stress conations. For example, in cotton crop TPS1 genes were expressed only in leaves and roots whereas in the maize crop TPS1 genes were expression occurred in ears under water deficiency [102]. Sometimes, Tre degradation regulates its levels in different plant tissues. For instance in Medicago expression of the Tre gene MtTRE1 was blocked under SS and Tre concentration was increased in plant nodules [103]. Moreover, a microarray showed that in A. thaliana the instance of abiotic stress caused a marked increase in the expression of genes involved in Tre metabolism [98]. Tre is effectively involved in stress tolerance and transgenic plants with improved Tre biosynthesis and stress tolerance support this logic [41, 104].
Tre is a non-reducing disaccharide sugar comprising two glucose subunits linked by an alpha, alpha-1,1 glycosidic bond. Tre has special characteristics as compared to other disaccharides owing to the fact both reducing subunits in Tre are used in making the glycosidic bond [39]. Tre has a substantiated resistance against hydrolysis and remains durable insoluble at a very temperature [105]. The α–α linkage of Tre is very stable and it possesses higher hydrophilicity owing to its inability for internal hydrogen bonding [106]. All these properties make Tre a useful molecule for the protection of membranes and proteins [107]. Moreover, Tre also has excellent dehydrating and vitrification ability [41] and in case of dehydration, Tre forms hydrogen boding with molecules and membranes by replacing the water molecules [108]. It also crystallized into glassy appearance under dehydration which preserves molecules from de-naturation [109, 110]. Tre is an inert sugar and it has low bond energy (1 kcal mol−1) as compared to sucrose (27 kcal mol−1) [95]. Lastly, Tre does not break down into reducing monosaccharide until it is exposed to extreme hydrolytic conditions or Tre action [107].
Trehalose a potential osmolyte to improve plant performance under salinity stress
Salinity stress is serious abiotic stress negatively affecting crop growth and productivity across the globe. Salt stress induces serious alterations in plant physiological, biochemical and metabolic functioning and causes serious growth and yield losses. Tre has emerged as an excellent osmo-protectant that substantially improved plant growth and subsequent performance under salinity stress [40, 111, 112]. Moreover, Tre also strengthens the antioxidant defense system and protects the plants from salinity induced oxidative damage [41, 46]. Here we systematically presented the different roles of Tre in inducing the salinity stress in plants.
Trehalose maintains membrane stability and plant water relationships under salinity stress
Generally, SS causes the production of ROS that damages cell membranes and increased lipid per-oxidation, and leading to an increase electrolyte leakage (EL) and loss of membrane permeability [24, 29]. Salinity induced increase in lipoxygenases (LOX) activity increased the oxidation of polyunsaturated fatty acids and thus increases lipid per-oxidation under stress conditions [113, 114]. However, Tre appreciably protects the membranes and improve the plant performance under SS. The application of Tre reduced the H2O2 production by activating antioxidant defense system which protects the membranes and reduces the MDA accumulation and membranes damage and EL [44, 115]. Tre application decreases H2O2 productions by increasing the anti-oxidant activities APX, catalase (CAT), peroxides (POD) and SOD that alleviate salinity induced damage to the membrane and improve membrane stability [116]. Moreover, Tre application also increased the accumulation of phenolic compounds which activate anti-oxidant defense system for ROS scavenging therefore, improve membrane stability and reducing the EL and lipid peroxidation [29, 116].
The increased activity of LOX causes lipid peroxidation and increased in MDA contents. However, Tre pretreatment reduced the LOX activities and MDA accumulation by scavenging ROS, stabilizing membranes and modulating the antioxidant activities [117, 118]. Tre application also improved the plant water accumulation under SS. The application of Tre improved the root growth and reduce the salinity inducing, osmotic stress by improving water uptake, therefore, maintain higher RWC under SS [118]. Tre reduced the inhibitory effects of SS on plant growth by improving the water status of plant tissues by stomata closing and osmoregulation [44, 119]. In another study, it was reported that SS decreased the leaf RWC by 25%, however, Tre foliar spray appreciably increase the leaf RWC by 34% as compared to the control [117]. Therefore, all aforementioned findings suggested that Tre improves the membrane stability by increasing antioxidant activities, and reducing LOX activity, therefore, reduce the MDA accumulation and maintain higher RWC under salinity stress.
Trehalose improves nutrient uptake under salinity stress
Nutrients play an important role in plant growth and development however, SS significantly disrupts the nutrient uptake and causes a reduction in growth and yield [24, 120, 121]. Tre appreciably improves nutrient uptake and cause significantly improved growth and productivity under SS. For instance, Tre supplementation significantly improves the nitrogen (N), phosphorus (P) and potassium (K) uptake under SS and cause marked improvement in the growth and yield of cowpea plants [122]. In another study, Shahbaz et al. [123] reported that Tre supplementation reduced the adverse impacts of SS by increasing the uptake of calcium in rice plants [123]. Tre works as a source of energy and the exogenous application of Tre significantly improved the Ca and K accumulation while reduced the Na+ accumulation in rice plants grown under SS [123]. Moreover, Zeid [119] reported Tre supply increase the K+ uptake and maintains the optimum K+/Na+ ratio to confer salinity tolerance in maize plants. In another study, it was recorded that Tre supplementation (10 and 30 mM) increased the K contents by three folds as compared to control plants [124]. Tre plays a beneficial role in nutrient uptake and it reduces the P deficiency by increasing P uptake in maize plants grown under SS and P deficiency [111]. Additionally, Tre also retains higher K+ in plant stem and leaves that improves the salinity tolerance and plant responses against SS [125]. These are the limited information available in the literature about the role of Tre on nutrient uptake. Moreover, studies are needed to explore the role of Tre on nutrient uptake under SS.
Trehalose protects the photosynthetic apparatus and improves photosynthesis under salinity stress
Salinity stress causes a significant reduction in plant photosynthetic efficiency mainly due to the closing of stomata, limited CO2 assimilation and reduction in chlorophyll synthesis. Nonetheless, Tre supplementation significantly improves plant photosynthetic efficiency under SS. SS negatively affects gas exchange characteristics including CO2 assimilation rate (Ci), transpiration rate (Tr) and stomatal conductance [123]. However, Tre application improved all these gas exchange characteristics by improving anti-oxidant activities and leads to an appreciable increase in photosynthetic performance [123]. Moreover, the exogenous supply of Tre also decreased the activity of NPQ and increased the electron transport, photochemical quenching (qP) thereby appreciably improves plant photosynthetic efficiency [119].
Tre pre-soaking (25 mM) significantly increased the chlorophyll and cartenoid contents which are also major reasons of Tre induced increase in photosynthetic efficiency under SS [45]. Moreover, the Tre supply also increased the Rubisco activity owing to an increase in the amount of Rubisco protein, chlorophyll synthesis and protection of photosynthetic apparatus [45]. Likewise, Ali, Ashraf [126] also noted the same results and they concluded that Tre induced increase in biomass production is linked with an increase in Rubisco activity and an increase in anti-oxidant activities. Maintenance of chlorophyll contents and plant water status play a crucial role in salinity tolerance [90]. Tre preserves the stability of chloroplast and maintains chloroplast, osmotic potential, therefore, improve the chlorophyll synthesis and subsequent plant growth under SS [44]. Similarly, different authors also noted a substantial increase in chlorophyll synthesis and photosynthetic efficiency with Tre under SS [5, 118].
Because of the protective impact of carotenoid in energy dissipation with PS-II and its function as a non-enzymatic anti-oxidant during stress conditions [135, 136], this higher concentration of carotenoid is considered to be protective mechanism [131]. Tre application (30 mM) significantly improved the leaf carotenoid contents under SS and lead to a significant increase in salinity tolerance [131]. Tre supplementation improves the performance of PS-II, electron transport and increases the synthesis of chlorophyll contents which contribute to improvement in photosynthetic efficiency in strawberry plants under SS [131]. The foliar application of regulates plant photosynthetic efficiency by improving chlorophyll synthesis and bringing favorable anatomical changes in plant body under SS [41, 122]. In conclusion, Tre protects the photosynthetic apparatus by increasing antioxidant activities and improves chlorophyll synthesis, Rubisco activity, and efficiency of PS-II resulting in a significant increase in photosynthesis under salt stress.
Trehalose maintains osmolytes accumulation and hormones crosstalk under salinity stress
Osmo-regulation is an important practice used by plant to counter the effects of SS. Plant accumulates different osmotic substances that protect macromolecules and stabilize the protein structure by scavenging ROS. Proline is an important osmolyte accumulated by plants under SS that stabilize cellular structure and proteins and improve plant performance under SS [137]. The controversial reports are available in literature about the effect of Tre on proline accumulation under SS. Likewise, Tre application reduced the accumulation of proline in salt stresses plants indicating low demand for proline or a compensating mechanism for Tre since both Tre and proline works as osmo-protectant [115]. However, some authors also noted significant increase in proline accumulation with Tre application under SS. The application of Tre significantly improved the accumulation of proline which indicates that Tre being a sugar stimulated proline synthesis [138]. Similarly, Feng et al. [132] reported that Tre supply increased the photosynthetic pigments and proline synthesis in plants grown under SS [132]. Moreover, Sadak [44] also suggested that Tre increased the biosynthesis of proline that prevented the wheat plants from adverse impacts of SS [44].
Tre application significantly improved the level of glycine betaine (GB) and total soluble proteins (TSP) under SS which markedly improved the plant tolerance against SS [123]. There is also interplay between Tre and amino acids accumulation under SS. The exogenous supply of Tre also significantly increased the total essential amino acids (valine, leucine, lysine, arginine, isoleucine, phenylalanine, histidine, and methionine) and non essential amino acids (proline, tyrosine, serine, glycine, cysteine, alanine, glutamic acid) which improved the plant stress tolerance [130]. Moreover, the exogenous supplementation of Tre also markedly improved the accumulation of different sugars (glucose, fructose, galactose and xylose, sucrose ant total soluble sugars) in wheat and contributes significantly towards improvement in salinity tolerance [124, 130]. Tre application reduced the accumulation of starch, and promotes the conversion of starch into soluble sugars, therefore improved concentration of total soluble sugars under SS [132]. Tre being a non-reducing sugar regulate sugar accumulation and distribution by affecting the activities of sugar transporters and regulating sugar metabolism and ABA metabolism therefore, significantly improved SS tolerance [132]. The interplay between Tre and ABA plays a significant role under SS. Tre application increased ABA accumulation to induced salinity responses by regulating the genes linked with ABA synthesis and metabolism [132]. In crux, Tre application improves osmolytes accumulation and maintains hormonal crosstalk which in turn improves the plant performance under salinity stress.
Trehalose improves accumulation of secondary metabolites under salinity stress
Phenolic compounds possess an excellent anti-oxidant activity and they play a significant role in stress tolerance [45, 139]. The exogenous supplementation of Tre significantly increased phenolic compounds in cowpea leaves [122]. Similarly, a medical alkaloid (vinblastine) and its two precursors vindoline and catharanthine also showed a significant increase in Tre treated plants as compared to control plants [140, 141]. The exogenous supply of Tre (10 and 30 mM) significantly increased the alkaloid content under SS by its ability to control C/N metabolism pathway [124]. Tre supplementation significantly improved the phenolic contents under SS which appreciably reduced the deleterious impacts of salinity stress on quinoa in plants [142]. The beneficial effects of Tre in increasing the phenolic contents are results from its signaling function through the induction of diverse metabolic pathways [114]. Additionally, Tre foliar supply also causes an increase in flavonoids and anthocyanins content under SS. Since phenolics substances provide photo-protection therefore Tre mediated increase in these substances can lead to the preservation of photosystem under SS [131]. Anthocyanin regulates ROS accumulation and maintains photosynthetic efficiency [143, 144], therefore, Tre mediated increase in anthocyanin content decreases damage to the photosynthetic apparatus and improves the plant’s photosynthetic efficiency and overall productivity [131]. Trehalose mediated increase in secondary metabolites improves antioxidant activities and protects the photosynthetic apparatus which therefore improves the plant growth under salinity stress.
Trehalose strengthens anti-oxidant activities under salinity stress
The major effect of SS is the production of ROS that damage cellular structures, proteins, lipids and DNA. Nonetheless, Tre appreciably scavenges the ROS by improving anti-oxidant activities to reduce salinity induced damages. Tre signaling contributes to plant adaption under SS by modulating anti-oxidant activities. For instance, exogenous Tre has no impact on CAT activity however, it significantly increased the activities of POD and SOD to counter the negative effects of SS [45, 115, 126]. Luo et al. [145] also documented that Tre plays a major role in scavenging O2− by modulating the SOD activity. CAT has a low affinity for H2O2 and it is considered a bulk removal of excessive H2O2 produced under SS [146]. The exogenous supply of Tre significantly enhanced the CAT activity in rice plants which indicates an efficient mechanism of Tre to scavenge ROS under SS [45] (Figs 1, 2, 3).
Role of Tre under salinity stress. Tre application maintains optimum K+/Na+, diverts excessive Na+, improves electron transport, efficiency of PS-11, osmolytes accumulation, genes expression, anti-oxidant activities, and reduces the MDA accumulation, ROS production and LOX therefore improve plant growth under salinity stress
Tre improves the expression of stress responsive genes and transcription factors and leading to a significant increase in salinity tolerance. Tre up-regulated the genes linked with proline and soluble sugars, and sucrose accumulation and ABA regulation and it also improved the activities of genes linked with anti-oxidant activities and leading to significant increase against salinity tolerance
The exogenous application of Tre (2.5 and 5 mM) significantly reduced the salt induced oxidative injuries in quinoa plants by increasing the activities of APX, CAT, POD and SOD [134]. In other studies, conducted on rice and wheat crop it was noted that Tre application alleviated adverse effects of SS by decreasing H2O2 production through enhanced anti-oxidant activities (APX, CAT, POD and SOD) [118, 123, 145]. According to Feng et al. [132] exogenous supply of Tre (2 mM) reduced the salinity induced osmotic and oxidative injuries by increasing the activities of CAT, POD and SOD under SS. In another study conducted on rice it was recorded that Tre application reduced the MDA and H2O2 accumulation by increasing activities of potential anti-oxidant including APX, CAT and POD [115]. Conversely, Tre decreased the O2- accumulation and SOD activity in salt stressed rice seedlings which suggested that Tre may also take in direct scavenging of O2- therefore reduce the activity of SOD under SS [117].
AsA and GSH are the two major non-enzymatic antioxidants that significantly improved the stress tolerance in plants by scavenging ROS [147, 148]. Tre pre-treatment in wheat plants showed a significant ascorbic acid (AsA) and glutathione (GSH) content and their redox ratios as compared to control. Tre mediated increase in activities of AsA and GSH play a synergistic role in preventing ROS induced oxidative damage [149]. The exogenous supply of Tre favorably modulates glycosyltransferase GTS however, it had a little impact on glutathione peroxidase (GPX) activity and confer salinity tolerance [117]. Moreover, Tre also increased activity of GSH and maintain a higher GSH/GSSG ratio in salt stress rice plants which reduced the salinity induced oxidative damages [117]. Interestingly, Tre application increased the activities of Gly-I and Gly-II which play an important role in detoxification of methylglyoxal (MG) thereby improved SS tolerance in maize plants [111]. Moreover, in maize crop Tre supplementation considerably increased the activities DHAR, MDHAR, GR and GPX and ameliorated the salinity induced oxidative damages by scavenging ROS [111]. The strawberry plants treated with Tre also showed a marked increase in CAT, GSH and SOD activities which protected the membranes and bio-molecules by scavenging the ROS [131]. In conclusion, Tre mediated increase in antioxidant activities substantially scavenge the ROS and protect the plants from salinity induced oxidative damage.
Trehalose improves expression of stress responsive genes under salinity stress
The increase in expression of stress responsive genes improves the plant tolerance against stress conditions. The application of Tre increases the expression of AOX genes which retarded the H2O2 accumulation and increased the accumulation of phenolic substances to confer salinity tolerance [116]. Tre supply also up-regulated the expression of NHX1 genes and this up-regulation is linked with an increase in growth recovery and improvement in K+ and K+/Na+ ratio [116]. Moreover, Tre mediated increase in NHX1 also alleviated Na+ induced toxicity in wheat crop [116]. On the other hand, Tre also up-regulates expression of SOS1 genes which significantly decreased the Na+ concentration and maintain a higher K+ and K+/Na+ ratio to confer salt tolerance [116]. The vacuolar Na+/H+ antiporter activity play a critical role in salt tolerance [150, 151]. Tre restricts the Na+ transportation and alleviate the salinity induced ROS production and cell death with an increase in AOX expression [116]. Additionally, an increase in expression of AOX, NHX1, and SOS1 concomitant with the regulation of proline and soluble sugars can highlight the important osmo-protective role of Tre [116].
The exogenous supplementation of Tre significantly enhanced the expression of SPS genes under SS and increases the accumulation of sucrose in tomato plants. Tre also increased the expression of sucrose synthase gene (SUS3) and acid invertase gene (Wiv-1) which maintained higher sucrose contents and contributed to a significant improvement in salinity tolerance [132]. Moreover, exogenous Tre increased the endogenous Tre contents in tomato plants, resulted in negative feedback regulation, inhibited the TPP expression, enhanced Tre gene expression, and promoted the conversion of excess Tre into glucose to maintain the stability of its content. Tre mediated differential expression of the aforementioned genes can directly affect the salt tolerance in plants [132]. NCED1 and NCED2 are important genes involved in ABA synthesis. The exogenous supply of Tre up-regulated the expression of these genes (NCED1 and NCED2) under salt stress and which indicates that exogenous Tre application has a positive effect on ABA synthesis. CYP707A1 and CYP707A2 are important genes involved in ABA metabolic pathway. The exogenous Tre application increased the expression of CYP707A1 and reduced the expression of CYP707A2 under SS [132]. In another study it was noted that exogenous supply of Tre up-regulated the expression of Cu/ZnSOD and MnSOD and CytAPX transcription and APX activity [152] which in turn improved anti-oxidant activities linked with genes resultantly improved the salt tolerance in rice [153]. Similarly, in another study Nounjan, Theerakulpisut [115] also found a significant increase in CytAPX and CatC expression following Tre application which resulted in significant improvement in anti-oxidant activities and SS tolerance. Tre also improves expression of different antioxidant genes (SlCu/Zn-SOD, SlFe-SOD, SlMn-SOD, SlPOD, and SlCAT) which counter the effects of SS and improve plant performance [154]. The transcription levels of Tre genes (TaTPPs and TaTPP1) also significantly increased under SS which improved the growth and development and plant tolerance against SS [155, 156]. In conclusion, Tre mediated increase in genes expression regulates proline and soluble sugars accumulation and antioxidant activities which therefore improve the plant performance under salinity stress.
Trehalose brought ultra-structure changes to induce salinity stress
Tre application also brought ultra-structural changes to induce SS tolerance in plants. For instance the exogenous application of Tre significantly increased the stem diameter (5.30%) epidermis thickness (6.91%) cortex thickness (19.62%), phloem tissue thickness (24.28%) xylem tissue thickness (45.45%) xylem rows in vascular cylinder (82.31%) and diameter of xylem vessel (21.21%) under SS. This increase in anatomical changes by Tre is linked with its ability to alleviate the deleterious impacts of SS [122]. Similarly, Akram et al. [157] also reported that Tre foliar spray and pre-treatment significantly improved thickness of leaf epidermis, vascular bundles, midrib thickness and thickness of vascular bundles in radish crop. Salt stress cause a significant reduction in thickness of leaf blades, and reduce the length and width vascular bundle and diameter of xylem vessels and mesophyll tissue in wheat plants. The reduction in aforementioned traits reduced assimilates translocation to plant parts therefore cause reduction in growth and yield [158]. However, Tre supplementation maintains vascular bundle and their thickness, diameter of xylem cells, mesophyll tissues and improved the xylem and phloem areas and ensure the better translocation of assimilates and resulting in significant improvement in growth and yield under SS stress [130].
Success stories of engineered trehalose for inducing salinity stress
The recent development in genetic engineering has provided the opportunities to build stress tolerant crops. A significant number of plants have been developed by using engineering approaches having strong tolerance against a wide range of stresses [41]. The accumulation of different osmolytes, sugars and hormones significantly improves the salt tolerance in plants. Because of the promising characteristics of Tre, efforts are being made across the globe to develop the genotypes with increased levels of Tre to confer salt tolerance. For instance, the fusion of Tre gene from E. coli significantly increased the Tre biosynthesis and reduced the salinity induced toxic effects by increasing RWC, chlorophyll contents, stomatal conductance and maintaining optimum K+/Na+ ratio as compared to control plants [159]. Similarly, transgenic rice plants having over-expression of OsTRE1 showed a significant increase in Tre activity. The over-expression of OsTRE1 significantly enhanced the salt tolerance however, it did not impose any morphological alteration or any growth defect [160]. Similarly, transgenic rice plants with over-expression of OsTPS1 showed a significant tolerance against cold, drought and SS. The over-expression of OsTPS1 significantly improved the accumulation of Tre and proline and stress responsive genes (WSI18, RAB16C, HSP70, and ELI) and contributes to improvement in salinity tolerance [99]. Another work showed that in transgenic rice plants over expression of OsTPS8 improved SS tolerance by controlling the concentration of sugars and regulating the genes expression involved in ABA signaling via SAPK9 regulation [161]. In another study, transgenic tomato plants were produced by introducing a gene encoding a bi-functional fusion of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase genes from E. coli. The developed transgenic plants showed significantly higher levels of Tre with higher photosynthetic rates and salt and drought tolerance [6]. Therefore, the development of salt tolerant cultivars could be a promising approach to improve SS tolerance in plants. However, engineering Tre biosynthesis pathway without creating any negative impacts is a major challenge. The genetic cascades of Tre can be investigated by comparing Tre enriched and deficient plants under both control and stressed conditions. Moreover, by figuring out the key nodes in Tre biosynthesis pathway whose modulation can be effective without any negative effect of SS. Lastly, it will depend on the degree of success to use those pathways for engineering salt tolerant crops.
Trehalose improves growth, yield and quality under salinity stress
Salinity stress causes a significant reduction in growth by inducing ionic, osmotic and oxidative stress [162]. However, Tre significantly improved the growth and salinity tolerance in plants (Table 2). Tre being non-toxic and compatible solute accumulates at higher concentration in the cytoplasm and maintains the cell turgor which contributes toward improvement in water uptake and subsequent plant growth [119]. Tre also stabilizes the membrane structure and prevent the loss the important solutes and resulting in significant improvement in growth and biomass production [134]. The exogenous supplementation of Tre improves anti-oxidant activities, and osmolytes accumulation and reduced the ROS production leading to significant improvement in growth and biomass production in maize, Arabidopsis and rice [45, 125]. In another study Tre application (10 mM) significantly increased the leaf fresh and dry weights by 30% and 42% under SS (50 mM) which was linked with improved proline and Tre accumulation and maintenance of anti-oxidant activities and photosynthetic performance [131].
Tre application improves water relations and stomata opening and leads to significant improvement in growth and biomass production under SS (250 mM) [124]. In another study, it was noted that Tre application resulted in more production of seeds/pod (6.89), pods/plant (9.33) seeds weight/plant (12.67) and 1000 seed weight (16.93 g) whereas the lowest values of seeds/pod (5.56), pods/plant (7.67) seeds weight/plant (8.56) and 1000 seed weight (15.66 g) was recorded in SS with Tre application [122]. Similarly, Shahbaz et al. [123] also found significant improvement in seeds/plant, 1000 seed weight, tillers and grain yield of rice following Tre application as compared to control. Soaking of wheat seeds in Tre (10 mM) ameliorated the adverse impacts of SS and improves the growth and biomass by increasing genes expression, osmolytes accumulation, K+/Na+ ratio and reducing Na+ accumulation [116]. Moreover, Mohamed et al. [130] also reported a significant increase in wheat yield and yield contributing traits including grains/spike, grains weight, spikelet’s and tillers of wheat with foliar spray of Tre (10 mM) grown under SS as compared to no Tre application. Tre mediated increase in growth and yield is linked with higher K + concentration, higher photosynthetic pigments, dry matter production, WUE, and reduced senescence and production of ROS, membrane damage, MDA and H2O2 accumulation [111]. In addition, Tre application also significantly improved the quality of crops grown under SS [130]. The exogenous application of increased the endogenous Tre contents, protein and amino acid contents of salt tolerant cultivars of wheat as compared to sensitive ones [130]. Tre being a carbon sugar significantly improved the concentration of sugars in tomatoes which contributed to a significant reduction in deleterious impacts of SS [132]. To summarize, Tre improves plant growth by increasing antioxidant activities, genes expression, osmolytes accumulation, K+/Na+ ratio and decreasing Na+, MDA and H2O2 accumulation.
Conclusion and future prospective
Trehalose application modulates plant growth and development under salinity stress. Salinity stress induces a serious reduction in plant growth and development by disturbing the plant’s physiological, biochemical and molecular functioning. However, trehalose supplementation improves the plant performance under salinity stress by improving membrane integrity, water uptake, nutrient uptake, photosynthetic efficiency, and protecting the photosynthetic apparatus from salinity induced oxidative damages. Trehalose supply also improves the accumulation of osmolytes and secondary metabolites and hormonal crosstalk thereby improving the plant performance under salinity stress. Besides this, trehalose also strengthens antioxidant activities, expression of stress responsive genes, and brought ultra-structural changes in the plant body for inducing salinity stress. Additionally, an increase in endogenous trehalose by engineering approaches also improve the genes expression, plant water relations, stomata conductance and photosynthetic efficiency and lead to a significant increase in salt tolerance.
Globally, efforts have been made to clarify trehalose role in plant responses under salinity stress, however, there are still many unanswered questions. The role of Tre in seed germination is not well explored, and it is interesting to explore the role of Tre in different processes and mechanisms involved in seed germination. The roles of Tre on nutrient uptake are also not well explored and limited studies are conducted on this aspect. Therefore, it is necessary to explore the role of Tre on nutrient uptake under salinity stress. Moreover, it would also be fascinating to explore the role of Tre in nutrient signaling and its effects on nutrient and ionic transporters. Moreover, anatomical changes taking place owing to Tre application also needed to be explored further for making it an important osmo-protectant. The role of Tre on stomata movements is poorly studied and it is necessary to explore the effect of Tre on stomata movements under salinity stress. The role of Tre on seed quality, composition and activities of antioxidant genes must also be explored under SS. Moreover, Tre signaling mechanisms and functions in different signal crosstalks at cell, tissue and organ levels are not fully explained and it is direly needed to acknowledge Tre role in signaling crosstalk under salinity stress at plant cell, tissue and organ levels.
There is missing information related to Tre cross talk with different hormones (abscisic acid auxins, cytokinins, ethylene, gibberellic acid and salicylic acid) and omsolytes (proline and glycine betaine) under salinity stress. Thus, it would be useful to unfold and discover the role of Tre in increasing the endogenous hormones to counter the effects of salinity stress. The complex relationship of Tre with these hormones and osmolytes must also be explored at transcriptomic level under salinity stress. It would also be fascinating to reveal the effect of Tre on genes and enzymes linked with synthesis of aforementioned hormones and osmolytes. Moreover, it is also crucial to explore the potential of modern techniques to identify the Tre related genes, metabolites and proteins for development of salt tolerant cultivars. The discovery of Tre mediated regulatory and metabolic pathways can provide new insights to understand the signaling network under salinity stress. The engineered Tre mediating metabolic pathways and signaling can open new vision into present knowledge to explore the Tre mediated salt tolerance mechanism in plants. Additionally, the role of Tre is mostly explored in lab studies and it is direly needed to conduct field studies under different climatic conditions and cropping system to make it an important osmolyte. Lastly, it is direly needed to optimize the rates of Tre applied by different application methods for different crops keeping in mind the crop, climate and soil conditions.
Abbreviations
- SS:
-
Salinity stress
- ROS:
-
Reactive oxygen species
- Na+ :
-
Sodium
- K+ :
-
Potassium
- MDA:
-
Malondialdehyde
- H2O2 :
-
Hydrogen peroxide
- Tre:
-
Trehalose
- Cl-:
-
Chloride
- CO2 :
-
Carbon dioxide
- RWC:
-
Relative water contents
- PS-II:
-
Photo system-II
- APX:
-
Ascorbate peroxidase
- GXP:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxides
- CAT:
-
Catalase
- ABA:
-
Abscisic acid
- GA:
-
Gibberellic acid
- JA:
-
Jasmonic acid
- SA:
-
Salicylic acid
- EL:
-
Electrolyte leakage
- LOX:
-
Lipoxygenases
- MG:
-
Methylglyoxal
References
Talaat NB, Shawky BT (2022) Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L.) plants by enhancing root h+-pump activity. Plants 11(3):416
Ismail LM, Soliman MI, Abd El-Aziz MH, Abdel-Aziz HM (2022) Impact of silica ions and nano silica on growth and productivity of pea plants under salinity stress. Plants 11(4):494
Talaat NB (2019) Role of reactive oxygen species signaling in plant growth and development. Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Wiley, Hoboken, pp 225–266
Tavakkoli E, Rengasamy P, McDonald GK (2010) High concentrations of Na+ and Cl–ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61(15):4449–4459
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276
Lyu JI, Min SR, Lee JH, Lim YH, Kim J-K, Bae C-H et al (2013) Overexpression of a trehalose-6-phosphate synthase/phosphatase fusion gene enhances tolerance and photosynthesis during drought and salt stress without growth aberrations in tomato. Plant Cell Tissue Organ Culture 112(2):257–262
Batool M, El-Badri AM, Hassan MU, Haiyun Y, Chunyun W, Zhenkun Y et al (2022) Drought stress in Brassica napus: effects, tolerance mechanisms, and management strategies. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10542-9
Dustgeer Z, Seleiman MF, Imran K, Chattha MU, Alhammad BA, Jalal RS et al (2021) Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49(1):12248
Imran K, Zafar H, Chattha MU, Mahmood A, Maqbool R, Athar F et al (2022) Seed priming with different agents mitigate alkalinity induced oxidative damage and improves maize growth. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 50(1):12615
Sultan I, Khan I, Chattha MU, Hassan MU, Barbanti L, Calone R et al (2021) Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital J Agron. https://doi.org/10.4081/ija.2021.1810
Umair Hassan M, Aamer M, Umer Chattha M, Haiying T, Shahzad B, Barbanti L et al (2020) The critical role of zinc in plants facing the drought stress. Agriculture 10(9):396
Batool M, El-Badri AM, Wang Z, Mohamed IA, Yang H, Ai X et al (2022) Rapeseed morpho-physio-biochemical responses to drought stress induced by PEG-6000. Agronomy 12(3):579
Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M et al (2019) Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities—a review. Environ Sci Pollut Res 26(13):12673–12688
Hassan MU, Chattha MU, Khan I, Chattha MB, Barbanti L, Aamer M et al (2021) Heat stress in cultivated plants: nature, impact, mechanisms, and mitigation strategies—a review. Plant Biosyst 155(2):211–234
Imran K, Seleiman MF, Chattha MU, Jalal RS, Mahmood F, Hassan FA et al (2021) Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49(2):12303
Umer Chattha M, Arif W, Khan I, Soufan W, Bilal Chattha M, Hassan MU et al (2021) Mitigation of cadmium induced oxidative stress by using organic amendments to improve the growth and yield of mash beans [Vigna mungo (L.)]. Agronomy 11(11):2152
Nafees M, Fahad S, Shah AN, Bukhari MA, Ahmed I, Ahmad S, Hussain S (2019) Reactive oxygen species signaling in plants. In Plant abiotic stress tolerance. Springer, Cham, pp. 259–272
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77
Ashraf MA, Asma HF, Iqbal M (2019) Exogenous menadione sodium bisulfite mitigates specific ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench). Acta Physiol Plant 41(12):1–12
Seleiman MF, Aslam MT, Alhammad BA, Hassan MU, Maqbool R, Chattha MU et al (2022) Salinity stress in wheat: effects, mechanisms and management strategies. Phyton 91(4):667
Talaat NB (2015) Effective microorganisms improve growth performance and modulate the ROS-scavenging system in common bean (Phaseolus vulgaris L.) plants exposed to salinity stress. J Plant Growth Regul 34(1):35–46
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Abbasi H, Jamil M, Haq A, Ali S, Ahmad R, Malik Z et al (2016) Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: a review. Zemdirbyste-Agriculture 103(2):229–238
Rasheed A, Fahad S, Aamer M, Hassan M, Tahir M, Wu Z (2020) Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice. A review. Appl Ecol Environ Res 18(3):4005–4023
Rasheed A, Gill RA, Hassan MU, Mahmood A, Qari S, Zaman QU et al (2021) A critical review: recent advancements in the use of CRISPR/Cas9 technology to enhance crops and alleviate global food crises. Curr Issues Mol Biol 43(3):1950–1976
Rasheed A, Hassan MU, Aamer M, Batool M, Sheng F, Ziming W et al (2020) A critical review on the improvement of drought stress tolerance in rice (Oryza sativa L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 48(4):1756–1788
Rasheed A, Ilyas M, Khan T, Nawab NN, Ahmed I, Mazhar M et al (2017) Genetic association and path coefficient analysis among yield and yield related traits in tomato (Solanum lycopersicon MILL.). Int J Biosci 11(5):21–26
Nedjimi B (2014) Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochem Syst Ecol 52:4–13
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky E-SM (2019) Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J Hortic Sci Biotechnol 94(6):777–789
Rehman AU, Bashir F, Ayaydin F, Kóta Z, Páli T, Vass I (2021) Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiol Plant 172(1):7–18
Shen W, Liu D, Zhang H, Zhu W, He H, Li G et al (2021) Overexpression of β-cyanoalanine synthase of Prunus persica increases salt tolerance by modulating ROS metabolism and ion homeostasis. Environ Exp Bot 186:104431
Berwal MK, Kumar R, Prakash K, Rai GK, Hebbar K (2021) Antioxidant defense system in plants against abiotic stress. Abiotic stress tolerance mechanisms in plants. CRC Press, Boca Raton, pp 175–202
Azad N, Rezayian M, Hassanpour H, Niknam V, Ebrahimzadeh H (2021) Physiological mechanism of salicylic acid in Mentha pulegium L. under salinity and drought stress. Braz J Bot 44(2):359–369
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Bio/Technol 14(3):407–426
Dey S, Biswas A, Huang S, Li D, Liu L, Deng Y et al (2021) Low temperature effect on different varieties of Corchorus capsularis and Corchorus olitorius at seedling stage. Agronomy 11(12):2547
Khan MT, Ahmed S, Shah AA, Noor Shah A, Tanveer M, El-Sheikh MA et al (2021) Influence of zinc oxide nanoparticles to regulate the antioxidants enzymes, some osmolytes and agronomic attributes in Coriandrum sativum L. Grown Under Water Stress Agron 11(10):2004
Almeida AM, Silva AB, Araujo SS, Cardoso LA, Santos DM, Torné JM et al (2007) Responses to water withdrawal of tobacco plants genetically engineered with the AtTPS1 gene: a special reference to photosynthetic parameters. Euphytica 154(1):113–126
Jain NK, Roy I (2009) Effect of trehalose on protein structure. Protein Sci 18(1):24–36
Luo Y, Xie Y, Li W, Wei M, Dai T, Li Z et al (2021) Physiological and transcriptomic analyses reveal exogenous trehalose is involved in the responses of wheat roots to high temperature stress. Plants 10(12):2644
Kosar F, Akram NA, Sadiq M, Al-Qurainy F, Ashraf M (2019) Trehalose: a key organic osmolyte effectively involved in plant abiotic stress tolerance. J Plant Growth Regul 38(2):606–618
Jinhua S, Weixiong W, Rasul F, Munir H, Huang K, Albishi TS et al (2022) Trehalose induced drought tolerance in plants: physiological and molecular responses. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 50(1):12584
Rehman S, Chattha MU, Khan I, Mahmood A, Hassan MU, Al-Huqail AA et al (2022) Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing Cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 11(6):822
Sadak MS (2019) Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent 43(1):1–10
Abdallah M-S, Abdelgawad Z, El-Bassiouny H (2016) Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S Afr J Bot 103:275–282
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W et al (2021) Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 10(6):1078
Debez A, Ben Slimen ID, Bousselmi S, Atia A, Farhat N, El Kahoui S et al (2020) Comparative analysis of salt impact on sea barley from semi-arid habitats in Tunisia and cultivated barley with special emphasis on reserve mobilization and stress recovery aptitude. Plant Biosyst 154(4):544–552
Lokupitiya E, Agrawal M, Ahamed T, Mustafa N, Ahmed B, Vathani A et al (2020) Evaluation of best management practices with greenhouse gas benefits for salt-affected paddy soils in South Asia. APN Sci Bull. https://doi.org/10.30852/sb.2020.1042
Mwando E, Han Y, Angessa TT, Zhou G, Hill CB, Zhang X-Q et al (2020) Genome-wide association study of salinity tolerance during germination in barley (Hordeum vulgare L.). Front Plant Sci 11:118
Bilkis A, Islam M, Hafiz M, Hasan M (2016) Effect of NaCl induced salinity on some physiological and agronomic traits of wheat. Pak J Bot 48(2):455–460
El-Hendawy S, Elshafei A, Al-Suhaibani N, Alotabi M, Hassan W, Dewir YH et al (2019) Assessment of the salt tolerance of wheat genotypes during the germination stage based on germination ability parameters and associated SSR markers. J Plant Interact 14(1):151–163
Bagwasi G, Agenbag GA, Swanepoel PA (2020) Effect of salinity on the germination of wheat and barley in South Africa. Crop Forage Turfgrass Manag 6(1):e20069
Al-shareef NO, Tester M (2019) Plant salinity tolerance. eLS. Wiley, Hoboken, pp 1–6
Alkharabsheh HM, Seleiman MF, Hewedy OA, Battaglia ML, Jalal RS, Alhammad BA et al (2021) Field crop responses and management strategies to mitigate soil salinity in modern agriculture: a review. Agronomy 11(11):2299
Fricke W, Akhiyarova G, Veselov D, Kudoyarova G (2004) Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J Exp Bot 55(399):1115–1123
Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso S, Shabala S (2011) Sequential depolarization of root cortical and stelar cells induced by an acute salt shock–implications for Na+ and K+ transport into xylem vessels. Plant Cell Environ 34(5):859–869
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT et al (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172(4):2445–2458
Parkash V, Singh S (2020) Potential of biochar application to mitigate salinity stress in eggplant. HortScience 55(12):1946–1955
Benito B, Haro R, Amtmann A, Cuin TA, Dreyer I (2014) The twins K+ and Na+ in plants. J Plant Physiol 171(9):723–731
Wu H, Zhang X, Giraldo JP, Shabala S (2018) It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 431(1):1–17
Saeed Z, Naveed M, Imran M, Bashir MA, Sattar A, Mustafa A et al (2019) Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 14(3):e0213016
Nassar R, Kamel HA, Ghoniem AE, Alarcón JJ, Sekara A, Ulrichs C et al (2020) Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 9(2):237
Senadheera P, Tirimanne S, Maathuis FJ (2012) Long term salinity stress reveals variety specific differences in root oxidative stress response. Rice Sci 19(1):36–43
Saddiq MS, Iqbal S, Hafeez MB, Ibrahim AM, Raza A, Fatima EM et al (2021) Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 11(6):1193
Zahra N, Raza ZA, Mahmood S (2020) Effect of salinity stress on various growth and physiological attributes of two contrasting maize genotypes. Braz Arch Biol Technol. https://doi.org/10.1590/1678-4324-2020200072
Yan Q, Zhang J, Li X, Wang Y (2019) Effects of salinity stress on seed germination and root growth of seedlings in island cotton. Acta Agron Sin 45(1):100–110
Sheteiwy MS, Shao H, Qi W, Daly P, Sharma A, Shaghaleh H et al (2021) Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J Sci Food Agric 101(5):2027–2041
Alzahrani SM, Alaraidh IA, Migdadi H, Alghamdi S, Khan MA, Ahmad P (2019) Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak J Bot 51(3):786–798
Rahneshan Z, Nasibi F, Moghadam AA (2018) Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J Plant Interact 13(1):73–82
Ahanger MA, Agarwal R (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460
Hassan A, Amjad SF, Saleem MH, Yasmin H, Imran M, Riaz M et al (2021) Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J Biol Sci 28(8):4276–4290
Kamran M, Malik Z, Parveen A, Huang L, Riaz M, Bashir S et al (2020) Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J Plant Growth Regul 39(1):266–281
Singh SK, Hoyos-Villegas V, Ray JD, Smith JR, Fritschi FB (2013) Quantification of leaf pigments in soybean (Glycine max (L.) Merr.) based on wavelet decomposition of hyperspectral features. Field Crop Res 149:20–32
Rangani J, Parida AK, Panda A, Kumari A (2016) Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte Salvadora persica L. Front Plant Sci 7:50
Yang C, Wang P, Li C, Shi D, Wang D (2008) Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 46(1):107–114
Abrar MM, Saqib M, Abbas G, Atiq-ur-Rahman M, Mustafa A, Shah SAA et al (2020) Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants 9(11):1574
Yang Z, Li J-L, Liu L-N, Xie Q, Sui N (2020) Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01722
Wahid I, Kumari S, Ahmad R, Hussain SJ, Alamri S, Siddiqui MH et al (2020) Silver nanoparticle regulates salt tolerance in wheat through changes in ABA concentration, ion homeostasis, and defense systems. Biomolecules 10(11):1506
Al-Ashkar I, Alderfasi A, El-Hendawy S, Al-Suhaibani N, El-Kafafi S, Seleiman MF (2019) Detecting salt tolerance in doubled haploid wheat lines. Agronomy 9(4):211
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217(2):523–539
Bhatt T, Sharma A, Puri S, Minhas AP (2020) Salt tolerance mechanisms and approaches: future scope of halotolerant genes and rice landraces. Rice Sci 27(5):368–383
Ding Y, Liu N, Virlouvet L, Riethoven J-J, Fromm M, Avramova Z (2013) Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol 13(1):1–11
Wang J, Song L, Gong X, Xu J, Li M (2020) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21(4):1446
Thitisaksakul M, Tananuwong K, Shoemaker CF, Chun A, Tanadul O-U-M, Labavitch JM et al (2015) Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J Agric Food Chem 63(8):2296–2304
Li J, Chen J, Jin J, Wang S, Du B (2019) Effects of irrigation water salinity on maize (Zea may L.) emergence, growth, yield, quality, and soil salt. Water 11(10):2095
Raza MM, Ullah S, Tariq A, Abbas T, Yousaf MM, Altay V et al (2019) Alleviation of salinity stress in maize using silicon nutrition. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 47(4):1340–1347
Kongpun A, Jaisiri P, Rerkasem B (2020) Impact of soil salinity on grain yield and aromatic compound in Thai Hom Mali rice cv. Khao Dawk Mali 105. Agric Nat Res 54(1):74–78
Jamshidi A, Javanmard H (2018) Evaluation of barley (Hordeum vulgare L.) genotypes for salinity tolerance under field conditions using the stress indices. Ain Shams Eng J 9(4):2093–2099
Abbas G, Saqib M, Rafique Q, Rahman A, Akhtar J, Haq M et al (2013) Effect of salinity on grain yield and grain quality of wheat (Triticum aestivum L.). Pak J Bot 50:185–189
Ponnu J, Wahl V, Schmid M (2011) Trehalose-6-phosphate: connecting plant metabolism and development. Front Plant Sci 2:70
Blazquez MA, Santos E, Cl F, Martínez-Zapater JM, Salinas J, Gancedo C (1998) Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 13(5):685–689
Zentella R, Mascorro-Gallardo JO, Van Dijck P, Folch-Mallol J, Bonini B, Van Vaeck C et al (1999) A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiol 119(4):1473–1482
Vogel G, Aeschbacher RA, Müller J, Boller T, Wiemken A (1998) Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J 13(5):673–683
Gechev TS, Hille J, Woerdenbag HJ, Benina M, Mehterov N, Toneva V et al (2014) Natural products from resurrection plants: potential for medical applications. Biotechnol Adv 32(6):1091–1101
Schwarz S, Van Dijck P (2017) Trehalose metabolism: a sweet spot for Burkholderia pseudomallei virulence. Virulence 8(1):5–7
Delorge I, Janiak M, Carpentier S, Van Dijck P (2014) Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front Plant Sci 5:147
Goddijn OJ, Verwoerd TC, Voogd E, Krutwagen RW, De Graff P, Poels J et al (1997) Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol 113(1):181–190
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50(10):1223–1229
Li H-W, Zang B-S, Deng X-W, Wang X-P (2011) Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234(5):1007–1018
Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R (2008) The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 283(14):9269–9275
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N et al (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136(4):4159–4168
Zhuang Y, Ren G, Yue G, Li Z, Qu X, Hou G et al (2007) Effects of water-deficit stress on the transcriptomes of developing immature ear and tassel in maize. Plant Cell Rep 26(12):2137–2147
López M, Tejera NA, Iribarne C, Lluch C, Herrera-Cervera JA (2008) Trehalose and trehalase in root nodules of Medicago truncatula and Phaseolus vulgaris in response to salt stress. Physiol Plant 134(4):575–582
Bae H, Herman E, Sicher R (2005) Exogenous trehalose promotes non-structural carbohydrate accumulation and induces chemical detoxification and stress response proteins in Arabidopsis thaliana grown in liquid culture. Plant Sci 168(5):1293–1301
Teramoto N, Sachinvala ND, Shibata M (2008) Trehalose and trehalose-based polymers for environmentally benign, biocompatible and bioactive materials. Molecules 13(8):1773–1816
Paul S, Paul S (2014) Trehalose induced modifications in the solvation pattern of N-methylacetamide. J Phys Chem B 118(4):1052–1063
López-Gómez M, Lluch C (2012) Trehalose and abiotic stress tolerance. Abiotic stress responses in plants. Springer, Cham, pp 253–265
Crowe JH (2007) Trehalose as a “chemical chaperone”. Molecular aspects of the stress response: chaperones, membranes and networks. Springer, New York, pp 143–158
Cesaro A, De Giacomo O, Sussich F (2008) Water interplay in trehalose polymorphism. Food Chem 106(4):1318–1328
Einfalt T, Planinšek O, Hrovat K (2013) Methods of amorphization and investigation of the amorphous state. Acta Pharm 63(3):305–334
Rohman M, Islam M, Monsur MB, Amiruzzaman M, Fujita M, Hasanuzzaman M (2019) Trehalose protects maize plants from salt stress and phosphorus deficiency. Plants 8(12):568
Tanveer M, Shah AN (2017) An insight into salt stress tolerance mechanisms of Chenopodium album. Environ Sci Pollut Res 24(19):16531–16535
Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100(11):6849–6854
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Trehalose-induced drought stress tolerance: a comparative study among different Brassica species. Plant Omics 7(4):271–283
Nounjan N, Theerakulpisut P (2012) Effects of exogenous proline and trehalose on physiological responses in rice seedlings during salt-stress and after recovery. Plant Soil Environ 58(7):309–315
Mn ALLA, Badran E, Mohammed F (2019) Exogenous trehalose alleviates the adverse effects of salinity stress in wheat. Turk J Bot 43(1):48–57
Mostofa MG, Hossain MA, Fujita M (2015) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252(2):461–475
Theerakulpisut P, Phongngarm S (2013) Alleviation of adverse effects of salt stress on rice seedlings by exogenous trehalose. Asian J Crop Sci 5(4):405
Zeid I (2009) Trehalose as osmoprotectant for maize under salinity-induced stress. Res J Agric Biol Sci 5(5):613–622
Shah AN, Wu Y, Iqbal J, Tanveer M, Bashir S, Rahman SU, Hafeez A, Ali S, Ma X, Alotaibi SS, El-Shehawi A (2021a) Nitrogen and plant density effects on growth, yield performance of two different cotton cultivars from different origin. J King Saud Univ-Sci 33(6):101512
Shah AN, Wu Y, Tanveer M, Hafeez A, Tung SA, Ali S, Khalofah A, Alsubeie MS, Al-Qthanin RN, Yang G (2021b) Interactive effect of nitrogen fertilizer and plant density on photosynthetic and agronomical traits of cotton at different growth stages. Saudi J Biol Sci 28(6):3578–3584
Eisa G, Ibrahim SA (2016) Mitigation the harmful effects of diluted sea water salinity on physiological and anatomical traits by foliar spray with trehalose and sodium nitroprusside of cowpea plants. Middle East J Gric 5(4):672–686
Shahbaz M, Abid A, Masood A, Waraich EA (2017) Foliar-applied trehalose modulates growth, mineral nutrition, photosynthetic ability, and oxidative defense system of rice (Oryza sativa L.) under saline stress. J Plant Nutr 40(4):584–599
Chang B, Yang L, Cong W, Zu Y, Tang Z (2014) The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol Biochem 77:140–148
Yang L, Zhao X, Zhu H, Paul M, Zu Y, Tang Z (2014) Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in Arabidopsis seedlings. Front Plant Sci 5:570
Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci 197(4):258–271
Shahzad AN, Qureshi MK, Ullah S, Latif M, Ahmad S, Bukhari SAH (2020) Exogenous trehalose improves cotton growth by modulating antioxidant defense under salinity-induced osmotic stress. Pak J Agric Res 33(2):270–279
Shah AN, Yang G, Tanveer M, Iqbal J (2017) Leaf gas exchange, source–sink relationship, and growth response of cotton to the interactive effects of nitrogen rate and planting density. Acta Physiologiae Plantarum 39(5):1–10
Abdelgawad Z, Hathout T, El-Khallal S, Said E, Al-Mokadem A (2014) Accumulation of trehalose mediates salt adaptation in rice seedlings. Am Eurasian J Agric Environ Sci 14(12):1450–1463
Mohamed HI, Akladious SA, El-Beltagi HS (2018) Mitigation the harmful effect of salt stress on physiological, biochemical and anatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Environ Bull 27(10):7054–7065
Samadi S, Habibi G, Vaziri A (2019) Exogenous trehalose alleviates the inhibitory effects of salt stress in strawberry plants. Acta Physiol Plant 41(7):1–11
Feng Y, Chen X, He Y, Kou X, Xue Z (2019) Effects of exogenous trehalose on the metabolism of sugar and abscisic acid in tomato seedlings under salt stress. Trans Tianjin Univ 25(5):451–471
Tian L, Qu D, Bi W, Xie T, Li J (2017) Trehalose alleviates the negative effects of salinity on the growth and physiological characteristics of maize seedlings. Acta Pratacul Sin 26(8):131–138
Abdallah MM-S, El Sebai TN, Ramadan AAE-M, El-Bassiouny HMS (2020) Physiological and biochemical role of proline, trehalose, and compost on enhancing salinity tolerance of quinoa plant. Bull Natl Res Cent 44(1):1–13
Habibi G, Ajory N (2015) The effect of drought on photosynthetic plasticity in Marrubium vulgare plants growing at low and high altitudes. J Plant Res 128(6):987–994
Hossain MA, Mostofa MG, Fujita M (2013) Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed 4(7):50–70
Mansour MMF, Ali EF (2017) Evaluation of proline functions in saline conditions. Phytochemistry 140:52–68
Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ et al (2012) Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol 158(3):1241–1251
Hassanein R, Bassuony F, Baraka D, Khalil R (2009) Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. 1-Changes in growth, some relevant metabolic activities and oxidative defense systems. Res J Agric Biol Sci 5(1):72–81
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163(1):11–18
Zhonghua T, Yanju L, Xiaorui G, Yuangang Z (2011) The combined effects of salinity and nitrogen forms on Catharanthus roseus: the role of internal ammonium and free amino acids during salt stress. J Plant Nutr Soil Sci 174(1):135–144
Sadak MS, El-Bassiouny HMS, Dawood MG (2019) Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull Natl Res Cent 43(1):1–11
Xu Z, Mahmood K, Rothstein SJ (2017) ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol 58(8):1364–1377
Xu Z, Rothstein SJ (2018) ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav 13(3):1364–1377
Luo Y, Li F, Wang G, Yang X, Wang W (2010) Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol Plant 54(3):495–501
Mishra P, Bhoomika K, Dubey R (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250(1):3–19
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18
Ma C, Wang Z, Kong B, Lin T (2013) Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regul 70(3):275–285
Feki K, Tounsi S, Masmoudi K, Brini F (2017) The durum wheat plasma membrane Na+/H+ antiporter SOS1 is involved in oxidative stress response. Protoplasma 254(4):1725–1734
Badran EG, Abogadallah GM, Nada RM, Nemat Alla MM (2015) Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 252(3):835–844
Hong C-Y, Kao CH (2008) NaCl-induced expression of ASCORBATE PEROXIDASE 8 in roots of rice (Oryza sativa L.) seedlings is not associated with osmotic component. Plant Signal Behav 3(3):199–201
Kim D, Shibato J, Agrawal GK, Fujihara S, Iwahashi H, Kim DH et al (2007) Gene transcription in the leaves of rice undergoing salt-induced morphological changes (Oryza sativa L.). Mol Cells 24(1):45
Yang Y, Yao Y, Li J, Zhang J, Zhang X, Hu L, Ding D, Bakpa EP, Xie J (2022) Trehalose alleviated salt stress in tomato by regulating ROS metabolism, photosynthesis, osmolyte synthesis, and trehalose metabolic pathways. Front Plant Sci 13:772948
Du L, Li S, Ding L, Cheng X, Kang Z, Mao H (2022) Genome-wide analysis of trehalose-6-phosphate phosphatases (TPP) gene family in wheat indicates their roles in plant development and stress response. BMC Plant Biol 22(1):1–20
Islam MA, Rahman MM, Rahman MM, Jin X, Sun L, Zhao K et al (2021) In silico and transcription analysis of trehalose-6-phosphate phosphatase gene family of wheat: trehalose synthesis genes contribute to salinity. Drought Stress Leaf Senescence Genes 12(11):1652
Akram NA, Shafiq S, Ashraf M, Aisha R, Sajid MA (2016) Drought-induced anatomical changes in radish (Raphanus sativus L.) leaves supplied with trehalose through different modes. Arid Land Res Manag 30(4):412–420
Khafagy M, Arafa A, El-Banna M (2009) Glycinebetaine and ascorbic acid can alleviate the harmful effects of NaCl salinity in sweet pepper. Aust J Crop Sci 3(5):257–267
Joshi R, Sahoo KK, Singh AK, Anwar K, Pundir P, Gautam RK et al (2020) Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J Exp Bot 71(2):653–668
Islam MO, Kato H, Shima S, Tezuka D, Matsui H, Imai R (2019) Functional identification of a rice trehalase gene involved in salt stress tolerance. Gene 685:42–49
Vishal B, Krishnamurthy P, Ramamoorthy R, Kumar PP (2019) Os TPS 8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytol 221(3):1369–1386
Taha SR, Seleiman MF, Alhammad BA, Alkahtani J, Alwahibi MS, Mahdi AH (2020) Activated Yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 11(1):74
Acknowledgements
The authors are thankful to Dr. Muhammad Aamer for proof reading of paper and valuable suggestions to improve quality of work. The authors also extend their appreciation to the Deanship of Scientific Research, King Khalid University for supporting this work through research groups program under Grant Number R.G.P. 2/17/43.
Author information
Authors and Affiliations
Contributions
Conceptualization: MN, MUH and ANS; writing-original draft preparation: MN, MUH and ANS; writing-review: MUC, AM, MH, SA, MB, AR, MAT, HASA and SHQ. Figures prepare: MB and AR.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nawaz, M., Hassan, M.U., Chattha, M.U. et al. Trehalose: a promising osmo-protectant against salinity stress—physiological and molecular mechanisms and future prospective. Mol Biol Rep 49, 11255–11271 (2022). https://doi.org/10.1007/s11033-022-07681-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07681-x