Abstract
Salinity, being a major environmental constraint, impedes plant growth and productivity worldwide. Menadione sodium bisulfite (MSB) was previously studied as activator of plant defense responses against pathogens. We further studied the potential of MSB in salt tolerance. MSB compound derived from vitamin K is soluble in water and possesses the potential to mediate plant defense responses to abiotic stress such as salinity. In the present experiment, foliar application of MSB (0, 50, 100, 150 and 200 µM) markedly mitigated salinity (100 mM) effects on two okra cultivars (Shabnam-786 and Arka Anamika). Salinity stress significantly decreased growth, chlorophyll and K+ content, but increased the tissue contents of Na+ and Ca2+ as well as the cellular levels of hydrogen peroxide (H2O2) and malondialdehyde (MDA). Plants with MSB treatment manifested minimal oxidative injury in the form of lower H2O2 and MDA accumulation. This decrease was ascribed to MSB-mediated improvement in the accumulation of antioxidant compounds (anthocyanins, ascorbate, flavonoids, and phenolics) alongside enhanced activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). MSB-treated plants exhibited a maximal improvement in the accretion of total free amino acids and proline under salinity. Foliar spray of MSB at 50 µM effectively protected plants from salinity-induced oxidative damage and specific ion toxicity. Higher salinity tolerance in cv. Shabnam-786 was ascribed to better antioxidant system, lower oxidative damage, and minimal tissue Na+ contents compared with cv. Arka Anamika.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The estimated increase in the world population by the year 2050 is 9.3 billion (Ishikawa and Shabala 2018). There is dire need to increase agricultural production by 50% to fulfill the food requirements of the increasing human population (Rengasmay 2006). Plant growth and yield is significantly impeded in saline soils. Therefore, mitigation of salinity is central to increase agricultural productivity in sustainable way (Heuer 2003). Yield reductions are significant in crop plants when soil EC reaches 4 dS m−1. The agriculture sector faces annual losses worth more than US$ 27 billion due to soil salinization (Qadir et al. 2014). According to FAO, more than 800 million ha land is saline with 32 million ha of dryland agriculture. Soil salinization occurs due to anthropogenic and natural factors such as poor agricultural practices, salt fluctuations due to seasonal variability and soil processes in soils with deep water tables (Greene et al. 2016).
Crop plants are usually glycophytes with limited potential to tolerate salinity compared with halophytes. Osmotic damage and specific ion toxicity, being the major salinity components, impedes yield production in glycophytes (Munns and Tester 2008). Plant growth rate, cell expansion, and water relation are impaired due to the initial exposure of salinity (Ferchichi et al. 2018). Plants under long-term salinity face ionic stress that leads to oxidative injury and nutritional imbalance (Munns and Tester 2008). Osmotic factor of salinity inhibits photosynthesis, enzyme activities, and protein synthesis that lead to chlorosis, necrosis, and premature senescence of old leaves (Ferchichi et al. 2018). Glycophytes accumulate substantial amounts of organic osmolytes. Compatible solutes are low molecular weight nitrogen-containing compounds such as betaines, amines, amino acids, and sugars (Zhu et al. 2015; Gharbi et al. 2017; Nguyen et al. 2017). These compatible compounds also mitigate oxidative damage in addition to their function as osmotic adjustment in cytosol (Annunziata et al. 2017). Salinity causes oxidative stress, nutrient imbalance, osmotic damage, as well as specific ion injury (Ashraf and Ashraf 2012; Ashraf et al. 2015). Oxidative damage is induced by enhanced production of ROS (Ashraf and Ashraf 2016; Ashraf et al. 2018). Plants possess antioxidant defense system which includes compounds with antioxidant potential (anthocyanins, ascorbate, tocopherols, flavonoids, glutathione, and phenolics) and antioxidant enzymes [APX (ascorbate peroxidase), GR (glutathione reductase), POD (peroxidase), CAT (catalase), and SOD (superoxide dismutase)] (Ahmad et al. 2011; Akram et al. 2018; Ashraf et al. 2018). This antioxidant system is upregulated under salinity enabling plants to scavenge ROS more efficiently. Enhanced detoxification of ROS reduces oxidative damage which can be measured in terms of H2O2 and MDA levels (Akram et al. 2018; Kaya et al. 2018a, b).
Menadione sodium bisulfite (MSB) being derived from vitamin K displays significant solubility in the aqueous medium. MSB is used to study oxidative stress in plants due to its redox-active nature (Borges et al. 2014). The redox properties of vitamin K enable this compound to perform physiological functions. Naphthoquinones (menadione), quinones, and benzoquinones are able to either donate or attract electrons and these properties render them active in biological systems (Borges et al. 2014). However, these properties depend upon the concentration of vitamin K (Castro et al. 2007). Vitamin K induces the production of ROS that in turn result in enhanced accumulation of ROS-scavenging proteins (Borges et al. 2014). Vitamin K is an important element of photosystem I and functions as an essential electron carrier. Vitamin K protects plants from environmental constraints through its functions as mobile electron carrier and maintenance of redox state of membrane proteins (Lüthje et al. 2013). Phylloquinone (vitamin K1) is also an important metabolite produced during the shikimate pathway. Vitamin K can be oxidized and reduced in cycles through enzyme pools and several substances (Lüthje et al. 1998). Vitamin K derivative increases the activity of H+-ATPase and thereby maintains acid pH and negative membrane potential. This electrochemical gradient across plasma membrane mediates transport of amino acids and sucrose through symporters as well as regulates cell turgor and stomatal closure (Elmore and Coaker 2011). Exogenous MSB increases growth, chlorophyll, tissue K+ and Ca2+ contents and decrease tissue Na+ contents in Arabidopsis challenged with salinity (Jiménez-Arias et al. 2015).
Okra (Abelmoschus esculentus Moench) is an important nutritious vegetable crop extensively grown around the globe (Abid et al. 2002). It is rich in nutrients including unsaturated fatty acids (oleic and linolenic acids), carbohydrates, calcium, potassium, and vitamins considered essential for human health (Asare et al. 2016). It is ranked as salt-sensitive vegetable crop by Qureshi and Barret-Lennard (1998). However, Esan et al. (2017) reported that response of okra could be variable from very salt sensitive to moderately salt sensitive. In addition, Jeyapraba et al. (2016) reported that okra is particularly sensitive to salinity at the initial developmental stages. Okra suffers significant yield losses due to salinity (Tanji and Neeltje 2002). For example, Minhas and Gupta (1993) reported 90%, 75%, and 50% fruit reduction in okra subjected to 6.7, 3.9, and 2.1 dS m−1, respectively. Keeping in view the adverse effects of salinity in vegetables, this study was undertaken to alleviate salinity effects in okra through foliar-applied MSB. Our hypothesis is that exogenous application of MSB may enhance plant salt tolerance by improving oxidative defense system and ion homeostasis. We also assessed the role of exogenous MSB in regulating key metabolic processes in okra subjected to salinity. This study was executed to appraise the influence of exogenous MSB on okra growth, oxidative defense system, and specific ion toxicity under salinity.
Materials and methods
A pot experiment was performed under natural environmental conditions to appraise the role of MSB in mitigating salinity effects in okra. The climatic conditions during the entire course of experiment were: average photosynthetically active radiation (PAR) 1110–1395 µmol m−2 s−1, 34% relative humidity, 33 °C temperature, and photoperiod of 12 h. Two okra cultivars (Arka Anamika and Shabnam-786), provided by AARI (Ayub Agricultural Research Institute, Faisalabad) Pakistan, were disinfected with 10% sodium hypochlorite for 5 min followed by thorough washing with distilled water. Seeds were sown in plastic pots (27 cm in diameter and 30 cm in height) filled with 8 kg of washed river sand. Thirteen seeds were sown in each pot and thinned to three plants per pot after 7 days of germination. Hoagland’s nutrient solution (full strength) was used to supplement nutrition in the sand medium. Salinity stress treatment (100 mM of NaCl) was applied 23 days after seed germination. Two liters of the nutrient solution with 100 mM NaCl was applied for the salt-treated plants every 5 days, while control plants were treated with the same nutrient solution without NaCl. Salinity treatments were applied using NaCl (analytical grade, Merck, Germany). Menadione sodium bisulfite (MSB) (analytical grade, Sigma-Aldrich) was applied as foliar spray. Different doses of MSB [CK (control), WS (water spray), 50, 100, 150 and 200 µM MSB] were prepared in distilled water with 0.1% Tween-20 (v/v) for foliar application. Ten days after the imposition of salt stress, MSB doses were applied as foliar application until drenching of leaves. After 15 days of treatment, data for morphological and biochemical attributes were recorded.
Growth attributes
Plants were carefully uprooted, washed with distilled water, and blotted dry. Shoot and root fresh weights and lengths were measured. Then plants were placed in an oven at 70 °C for 74 h to measure shoot and root dry weights. Leaf area was calculated with the help of the following formula: maximum leaf length × maximum leaf width × 0.68, where 0.68 is a correction factor (Hussain et al. 2017).
Chlorophyll
Fresh leaf tissue (0.1 g) was homogenized with aqueous acetone (80%) to measure chlorophyll (a and b) and carotenoids. The absorbance of the extract was read at 480, 645, and 663 nm with the help of a spectrophotometer (U-4000, Hitachi, Japan) (Arnon 1949).
Hydrogen peroxide (H2O2)
The method of Velikova et al. (2000) was followed to measure H2O2. 5 mL of 0.1% TCA (trichloroacetic acid) was used to grind fresh leaf (0.5 g). The resultant ground material was centrifuged at 10,000×g for 10 min to collect the supernatant. 1 mL of potassium iodide and 0.5 mL of 100 mM potassium phosphate buffer (pH 7.5) were added in 0.5 mL of the supernatant. The mixture was allowed to remain at 25 °C for 20 min before reading the absorbance at 390 nm with a spectrophotometer.
Malondialdehyde (MDA)
Heath and Packer (1968) method was used to determine the MDA contents. Fresh leaf material (0.5 g) was ground in 5 mL TCA (6%). The supernatant was collected after centrifugation at 10,000×g for 10 min. Then, 0.5 mL supernatant was mixed with 2 mL TBA (thiobarbituric acid) and the resulting reaction was incubated at 95 °C for 45 min. When the reaction solution reached 25 °C, its absorbance was read at 532 and 600 nm with a spectrophotometer.
Phenolics
Julkunen-Tiitto (1985) procedure was followed to measure total phenolics from fresh leaf. 5 mL aqueous acetone (80%) was used to homogenize 0.5 g fresh leaf. The homogenized material was centrifuged (10,000×g for 10 min) to get the supernatant. The Folin–Ciocalteu phenol reagent (1 mL) and distilled water (2 mL) were mixed with the supernatant (0.1 mL). The volume of the mixture was raised to 10 mL with distilled water after the addition of 5 mL Na2CO3 (20%). The reaction solution was thoroughly shaken before getting its absorbance at 720 nm with a spectrophotometer.
Anthocyanin contents
10 mL of 100 mM potassium phosphate buffer with 7.5 pH was used to grind 0.5 g fresh leaf. The ground material was centrifuged (10,000×g for 10 min) to take out the supernatant whose absorbance was directly read at 600 nm with a spectrophotometer (Kubo et al. 1999).
Flavonoids
The method of Marinova et al. (2005) was used to measure flavonoids. One mL of the supernatant (the one used for phenolics determination) was mixed with AlCl3 and NaNO2 (300 uL of each). The reaction solution was incubated at 25 °C for 5 min and 1 M NaOH (2 mL) was added. The mixture was used to get absorbance at 510 nm after incubation at 25 °C for 10 min. A spectrophotometer (the model already mentioned) was used to measure flavonoids.
Ascorbic acid
Mukherjee and Choudhuri’s (1983) method was followed for the measurement of ascorbic acid from fresh leaf material (0.5 g) extracted in 10 mL of 6% TCA. The homogenate was filtered and 1 mL of 2% dinitrophenylhydrazine was added to 2 mL filtrate. Then one drop of 10% thiourea was added to the reaction mixture. The reaction solution was incubated at 45 °C for 20 min. Then mixture was allowed to reach room temperature. Afterward, 2.5 mL of 80% H2SO4 was added to the reaction mixture. The absorbance of the mixture was read at 530 nm.
Total soluble proteins and antioxidant enzyme assay
Total soluble proteins were extracted from 0.5 g fresh leaf in 10 mL chilled potassium phosphate buffer (100 mM) with 7.5 pH. The supernatant was collected after centrifugation of homogenate at 10,000×g for 15 min. The centrifugation of the homogenate was done at 4 °C. Bradford (1976) method was followed to determine total soluble proteins from the supernatant. This supernatant was also used for measuring the antioxidant enzyme activities.
Catalase (CAT) and peroxidase (POD) activities were measured with the help of the method by Chance and Maehly (1955). The reaction mixture containing 100 mM potassium phosphate buffer (pH 7.5), 5.9 mM H2O2, and enzyme extract (0.1 mL) was used for measuring CAT activity. Change in absorbance was read every 20 s at 240 nm. The reaction mixture for POD consisted of 100 mM potassium phosphate buffer (pH 7.5), H2O2 (40 mM), and guaiacol (20 mM). Change in absorbance was read every 20 s at 470 nm. SOD activity was measured following the method of Giannopolitis and Ries (1977). The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.5), riboflavin (1.3 µM), methionine (13 mM), NBT (50 µM), and enzyme extract (0.1 mL). The activities of the antioxidant enzymes were expressed in U mg−1protein.
Leaf free proline
10 mL sulfosalicylic acid (3%) was used to grind 0.5 g fresh leaf. The extract was filtered and glacial acetic acid and ninhydrin (2 mL each) were added to 2 mL filtrate. The mixture was heated for an hour at 100 °C. The mixture was allowed to get to room temperature and then toluene (4 mL) was added to the mixture. The absorbance was read at 520 nm with a spectrophotometer (Bates et al. 1973).
Total free amino acids
Potassium phosphate buffer extract of 0.5 g fresh leaf was used to measure this parameter. The supernatant (1 mL) was mixed with acid ninhydrin and 10% pyridine (1 mL each). The mixture was incubated at 95 °C for 30 min. Distilled water was added to make the volume to 7.5 mL and the absorbance of the mixture was obtained at 570 nm with a spectrophotometer (Hamilton and Van Slyke 1943).
Determination of Na+, K+, and Ca2+
The oven-dried leaves, stem, and roots (0.1 g) were acid digested (Wolf 1982). Concentrated H2SO4 (2 mL) was added to the digestion flask containing dry plant material and incubated for 24 h. After incubation, 0.5 mL of H2O2 (35%) was added to the digestion mixture and then heated at 250 °C. The flasks were then removed from heating and cooled down at room temperature and put on heat after adding 0.5 mL H2O2. The procedure was repeated until a clear transparent solution was obtained. Sodium, calcium, and potassium in the digested solutions were measured with flame photometer (PFP-7, Jenway Ltd. Felsted, UK).
Statistical analysis
The experiment was laid out in a completely randomized design with factorial arrangement. There were four replicates of each treatment. Analysis of variance (ANOVA) of data was computed with the help of statistical software Costat, version 6.303 (Table 1S). The difference between means was compared with the help of least significant difference (LSD) at P ≤ 0.05.
Results
Plant growth
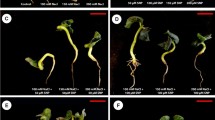
Growth characteristics (shoot and root lengths and fresh and dry masses) considerably (P ≤ 0.001) diminished in two cultivars subjected to salinity stress. There was also a noticeable lessening (P ≤ 0.001) in leaf area in okra plants challenged with salinity. However, exogenous MSB significantly increased various growth attributes in okra cultivars under salinity. Of different doses of MSB, it was seen that a lower dose of MSB (50 µM) showed maximum improvement in growth attributes under salinity, while higher MSB dose (200 µM) had negative effect on plant growth under saline conditions. Cultivar Shabnam-786 manifested higher biomass accumulation compared with cv. Arka Anamika when 50 µM MSB was applied as foliar spray under salinity (Figs. 1 and 1S; Table 1S).
Effect of menadione sodium bisulfite on growth characteristics and photosynthetic pigments of salinity-stressed okra (Abelmoschus esculentus Moench) plants (n = 4; mean ± S.E.). Different lowercase letters in each attribute are significantly different at P ≤ 0.05. CK control, WS water stress, different concentrations of MSB (50, 100, 150, and 200 µM)
Photosynthetic pigments
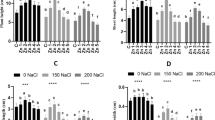
Decrease in growth attributes was associated with decrease in photosynthetic pigments. Salinity caused marked (P ≤0.001) decline in photosynthetic pigments (a and b and total chlorophyll contents) in two cultivars. However, changes in carotenoids were not significant under salinity. Plants with MSB treatment displayed higher photosynthetic pigments (carotenoid content, chlorophyll a and b, and total chlorophyll). Plants fed 50 µM MSB as foliar application had maximal values of these pigments. Higher dose of MSB (200 µM) had non-significant effect on these variables under salinity. The differences between cultivars were not significant except for chlorophyll b where cv. Shabnam-786 exhibited more chlorophyll b over Arka Anamika under salinity (Fig. 1; Table 1S).
H2O2 contents
As a result of salinity exposure, endogenous levels of H2O2 markedly (P ≤0.001) increased in the two cultivars. However, exogenous MSB noticeably decreased (P ≤0.001) H2O2 contents. The cultivars did not display noticeable difference with respect to H2O2 contents and MSB doses. Plants with 50 µM MSB treatment manifested a noticeable reduction in H2O2 contents over other MSB concentrations in two cultivars under salinity. The endogenous H2O2 contents in cv. Shabnam-786 treated with 50 µM MSB was slightly lower compared with cv. Arka Anamika. The higher concentration of MSB resulted in further increase in H2O2 contents in the two cultivars under salinity (Fig. 2; Table 1S).
Effect of menadione sodium bisulfite on the biochemical attributes of salinity-stressed okra (Abelmoschus esculentus Moench) plants. (n = 4; mean ± S.E.). Different lowercase letters in each attribute are significantly different at P ≤ 0.05. CK control, TSP total soluble proteins, AsA ascorbic acid, TFAA total free amino acids, WS water stress, different concentrations of MSB (50, 100, 150, and 200 µM)
MDA contents
Imposing salinity stress in the plant-growing media led to a remarkable (P ≤ 0.001) rise in MDA content which is taken as an indirect measure of lipid peroxidation. The MDA contents were greater (P ≤ 0.05) in cv. Arka Anamika compared with cv. Shabnam-786. Foliar-applied MSB significantly altered endogenous MDA contents. For instance, MSB applied at 50 µM concentration caused significant reduction in this attribute compared with other MSB treatments [CK (control), WS (water spray), 100, 150, and 200 µM MSB] in both cultivars under salinity stress. The maximal values for MDA contents were recorded in plants with 200 µM MSB treatment under salinity (Fig. 2; Table 1S).
Non-enzymatic antioxidants
Ascorbic acid
There was a prominent increasing effect of salinity (P ≤ 0.001) on ascorbic acid contents in okra plants. Both okra cultivars significantly differed (P ≤ 0.001) with respect to this variable. In this context, a more considerable increase in ascorbic acid was recorded in cv. Shabnam-786 over cv. Arka Anamika under salinity. Exogenous supplementation of varying MSB doses (0, 50, 100, 150 and 200 µM) exhibited significant effect (P ≤ 0.001) on this variable. For instance, exogenous MSB (50 µM) showed maximal values of ascorbic acid contents over higher MSB dose (200 µM) which had negative impact on this attribute under salinity stress (Fig. 2; Table 1S).
Phenolics
Phenolics accumulation in okra plants enhanced considerably (P ≤ 0.001) with the imposition of salinity. There existed marked differences (P ≤ 0.01) between the two cultivars for phenolics accumulation. Higher phenolics contents were present in cv. Shabnam-786 over Arka Anamika. The exogenous MSB 50 µM resulted in a more considerable (P ≤ 0.001) increase in phenolics over other MSB doses under salinity. The elevated dose of MSB (200 µM) resulted in minimal values of phenolics under salinity (Fig. 2; Table 1S).
Flavonoids
A considerable rise in flavonoids accumulation (P ≤ 0.001) was observed in okra plants under salinity. Cultivar Shabnam-786 accumulated more flavonoid contents (P ≤ 0.01) than cv. Arka Anamika. Plants displayed variable (P ≤ 0.001) flavonoid accumulation in response to foliar application of MSB. In this context, maximal flavonoids were present in plants sprayed with 50 µM MSB, whereas plants treated with 200 µM MSB showed minimal flavonoid accumulation under salinity (Fig. 2; Table 1S).
Anthocyanins
In the present study, there existed significant variation (P ≤ 0.001) in anthocyanin contents in okra plants. Both cultivars displayed different anthocyanin contents (P ≤ 0.01) in response to salinity and foliar MSB applications. Cultivar Arka Anamika had more anthocyanins compared with cv. Shabnam-786 under salinity. Plants accumulated significantly higher anthocyanins when MSB was applied at 50 µM. The elevated level of exogenous MSB (200 µM) showed decreasing effect on this variable under salinity (Fig. 1; Table 1S).
Total soluble protein, leaf free proline and total free amino acids
Total soluble protein
Total soluble proteins increased remarkably (P ≤ 0.001) in plants exposed to salinity in the growth medium. Both cultivars had slightly different (P ≤ 0.05) total soluble protein contents. For example, foliar MSB (50 µM) resulted in more endogenous levels for this variable in cv. Shabnam-786 than that of cv. Arka Anamika. The exogenous MSB considerably affected (P ≤ 0.001) this variable in both cultivars under salinity. MSB-induced changes in total soluble protein were conspicuous in plants sprayed with 50 µM of plant growth regulator under salinity (Fig. 2; Table 1S).
Leaf free proline
There existed noticeable variation (P ≤ 0.05) in both okra cultivars for accumulation of leaf free proline under salinity stress and varying doses of foliar-applied MSB (0, 50, 100, 150 and 200 µM). Higher proline contents were observed in cv. Shabnam-786 over Arka Anamika under salinity. The exogenous MSB markedly affected (P ≤ 0.001) proline levels in plants under salinity. In this regard, more proline accumulation was found in plants when 50 µM MSB was applied as foliar spray under salinity. By contrast, exogenous application of higher concentration of MSB (200 µM) led to minimal values of proline under salinity stress (Fig. 2; Table 1S).
Total free amino acids
Exposure of okra plants to salinity resulted in a more considerable improvement (P ≤ 0.001) in total free amino acids. Cultivar Shabnam-786 accumulated more total free amino acids than cv. Arka Anamika under salinity. However, maximal increase in this variable was seen only in those plants sprayed with 50 µM MSB, while other concentrations of MSB did not influence the endogenous levels of this variable (Fig. 2; Table 1S).
Antioxidant enzyme activities
SOD activity
Exogenous supplementation of MSB significantly affected (P ≤ 0.001) SOD activity in two cultivars under salinity. Cultivar Shabnam-786 had markedly higher SOD activity over Arka Anamika when MSB was applied at 50 µM under salinity. Foliar application of lower MSB dose (50 µM) had marked increasing effect on this variable compared with those of other MSB doses under salinity stress (Fig. 2; Table 1S).
POD activity
When two okra cultivars were subjected to salinity stress, a noticeable enhancement (P ≤ 0.001) in POD activity was recorded. Foliar-applied MSB-induced noticeable (P ≤ 0.001) changes in this variable under salinity. In this context, plants with MSB treatment (50 µM) showed maximal POD activity in cv. Shabnam-786 compared to Arka Anamika under salinity. Higher MSB doses (100, 150 and 200 µM) did not affect POD activity under stress conditions (Fig. 2; Table 1S).
CAT activity
Salinity considerably enhanced (P ≤ 0.001) CAT activity in okra plants. There existed marked differences (P ≤ 0.001) between two cultivars with reference to this variable. Cultivar Shabnam-786 displayed maximal CAT activity over cv. Arka Anamika under salinity. Foliar-applied varying doses of MSB (0, 50, 100, 150 and 200 µM) markedly (P ≤ 0.001) influenced CAT activity under salinity. Plants administered MSB (50 µM) exhibited more CAT activity than those of other MSB doses under salinity (Fig. 2; Table 1S).
Uptake of Na+, K+ and Ca2+
To examine the interactive effect of MSB and salinity on nutrient uptake, we measured Na+, Ca2+ and K+ in leaf, stem and root tissues. Figure 3 shows that salinity caused a noticeable accretion (P ≤ 0.001) in the accumulation of toxic Na+ in the root, stem and leaf tissues between the cultivars. However, cv. Shabnam-786 manifested lower Na+ content in different plant tissues compared to cv. Arka Anamika. Plants fed with 50 µM MSB showed remarkable (P ≤ 0.001) abatement in tissue Na+ content of roots, stem, and leaf under saline condition (Fig. 3; Table 1S).
Effect of menadione sodium bisulfite on nutrient contents of salinity-stressed okra (Abelmoschus esculentus Moench) plants (n = 4; mean ± S.E.). Different lowercase letters in each attribute are significantly different at P ≤ 0.05. CK control, WS water stress; different concentrations of MSB (50, 100, 150, and 200 µM)
Salinity caused distinguishable (P ≤ 0.001) enhancement in the accumulation of Ca2+ in root, stem, and leaf of both okra cultivars. The accumulation of Ca2+ was more in cv. Shabnam-786 compared to Arka Anamika under salinity. We further recorded the maximal values of tissue Ca2+ in plants treated with 50 µM MSB and this effect was more pronounced (P ≤ 0.001) in cv. Shabnam-786 (Fig. 3; Table 1S).
Tissue K+ content in root, stem, and leaf diminished considerably (P ≤ 0.001) due to salinity stress. Decrease in tissue K+ was many folds greater in cv. Arka Anamika over Shabnam-786 under salinity. Plants administered with MSB (50 µM) manifested increase (P ≤ 0.001) in root, stem and leaf K+ content under salinity. Foliar application of 200 µM MSB had negative effect on tissue K+ content under salinity stress (Fig. 3; Table 1S).
Discussion
Vitamins are important due to their functions as enzyme cofactors and as regulators of metabolism in plants. Vitamins, both lipid soluble (A, E and K) and water soluble (B and C), act as antioxidants (Asensi-Fabado and Munné-Bosch 2010). In our study, we evaluated the functions of water-soluble derivative compound of vitamin K, menadione sodium bisulfite (MSB), in mediating important metabolic reactions in okra plants under salinity. MSB was first reported as important regulator of plant growth in 1985 (Rama Rao et al. 1985). However, until recently, very few reports are present in the literature showing MSB-mediated improvement in plant defense responses under salinity (Borges et al. 2014; Jiménez-Arias et al. 2015). In the present study, salinity led to a noticeable decrease in growth of okra plants. Salt-induced growth suppression was related to a decrease in chlorophyll and increase in MDA and H2O2 levels along with enhanced tissue Na+ and limited tissue K+ concentration. Foliar MSB (50 µM) application defended plants from salinity-induced oxidative injury by increasing the concentrations of antioxidant compounds (ascorbic acid, phenolics, flavonoids, and anthocyanins) and antioxidant enzyme activities (CAT, POD, and SOD). Better antioxidant system readily scavenged ROS (H2O2) and protected membranes, which is evident in terms of minimal MDA accumulation. Consequently, plants with minimal oxidative damage, lower Na+ content, and higher chlorophyll had better growth under saline condition. Our results manifested minimal tissue Na+ level in plants treated with MSB (50 µM) under salinity. Likewise, MSB-treated plants had more K+ and Ca2+ content that could have contributed to better plant growth under salinity.
Salinity reduces plant growth in several other plants such as wheat (Siddiqui et al. 2017; Wang et al. 2018), honeysuckle (Cheng et al. 2018), Acacia gerrardii Benth (Abd-Allah et al. 2018), maize and rice (Ashraf et al. 2018; Yasmeen and Siddiqui 2017), tomato (Siddiqui et al. 2017), and okra (Habib et al. 2012). Root growth is inhibited first due to the ionic and osmotic effects of salinity. Consequently, water and mineral absorption, distribution, and accumulation are retarded (Cheng et al. 2018). Plants challenged with salinity showed growth suppression due to buildup of higher Na+ and lower K+ contents which inhibit important metabolic reactions (Ashraf et al. 2018). Plants require optimum chlorophyll for maintaining normal photosynthesis. However, salinity considerably diminished chlorophyll contents (Kaya et al. 2018a) as evident in the present study. Salt-induced decrease in chlorophyll could have been due to altered chlorophyll biosynthesis, enhanced degradation of chlorophyll by chlorophyllase enzyme, and changes in chloroplast membranes and chlorophyll protein complex (Ashraf and Harris 2013; Kaya et al. 2018a). Decline in chlorophyll content is positively associated with suppression in plant growth as found in maize (Ashraf et al. 2018), wheat (Ashraf and Ashraf 2012), sunflower (Santos 2004), bean (Taïbi et al. 2016), and tomato (Li et al. 2015). In our study, MSB-treated plants had greater chlorophyll contents due to lower oxidative damage. It is reported that chlorophyll content usually enhanced in plants with minimum oxidative damage (Taïbi et al. 2016). Similar to our results, exogenous application of MSB was reported to improve growth by increasing chlorophyll and proline concentration under salinity (Jiménez-Arias et al. 2015). Our results manifested greater proline in plants administered exogenously applied MSB. Moreover, enhanced proline levels regulate the activity of chlorophyllase enzyme and thereby more chlorophyll concentration is found in plants with greater endogenous levels of proline (Elsheery and Cao 2008). In our study, more chlorophyll contents due to exogenous application of MSB could be attributed to MSB-induced increased proline accumulation.
Salinity-mediated accretion in H2O2 contents caused peroxidation of lipids measured as MDA contents, thereby disturbing membrane permeability (Ashraf et al. 2018; Mandhania et al. 2006). In our study, we recorded enhanced H2O2 levels that in turn increased MDA accumulation and thus degraded membranes. Salt-induced greater oxidative injury in the form of more H2O2 and MDA generation has been documented in various plant species such as maize (Kaya et al. 2015), cucumber (Wang et al. 2016), tomato (Siddiqui et al. 2017), sorghum (Surender Reddy et al. 2015), and rice (Habib et al. 2016). A better antioxidant system in the form of non-enzymatic and enzymatic antioxidants protects plants from oxidative damage (Abid et al. 2018; Ali et al. 2018; Pehlivan 2018). In our study, we found minimal MDA and H2O2 levels in MSB-treated plants under salinity stress. This could have been due to higher concentration of non-enzymatic compounds (ascorbic acid, phenolics, flavonoids and anthocyanins) and activities of antioxidant enzymes (SOD, POD and CAT) in plants fed with foliar MSB. Our results are corroborated with previous report in Arabidopsis where exogenous MSB enhanced the expression of ROS-scavenging genes (Jiménez-Arias et al. 2015). MSB supplementation as foliar spray or seed priming induced a slight oxidative burst in the form of ROS production which in turn triggered the accumulation of antioxidant compounds and enhanced the activities of enzymes involved in ROS detoxification, thereby conferring salinity tolerance to plants (Borges et al. 2014). In the present study, MSB application resulted in greater accumulation of ascorbic acid, phenolics, flavonoids, and anthocyanins along with enhanced activities of SOD, POD, and CAT. This change in oxidative defense system could have been attributed to a slight oxidative burst caused by MSB (Borges et al. 2014).
Plants challenged with salinity face ionic imbalance due to buildup of Na+ content which in turn interfered with the uptake and accumulation of K+ and Ca2+. In the present study, we recorded increased tissue Na+ that decreased tissue K+. However, we have noted increase in tissue Ca2+. Salt-induced increase in Na+ and interference of toxic Na+ with the accumulation of K+ and Ca2+ have been documented in wheat (Ashraf and Ashraf 2016), maize (Ashraf et al. 2018), okra (Habib et al. 2012), and Arabidopsis (Jiménez-Arias et al. 2015). In the present study, exogenous application of MSB significantly diminished Na+ with concomitant accretion in tissue K+ and Ca2+. Our results are in keeping with earlier study where MSB-treated Arabidopsis plants had shown less Na+ and more tissue K+ and Ca2+ concentration (Jiménez-Arias et al. 2015). More Na+ in growth medium actively competes with K+ uptake and resultantly causes decline in tissue K+ (Ashraf and Harris 2004; Ashraf and Ashraf 2016). In contrast, salinity stress significantly increased Ca2+ which was maximal in plants administered foliar MSB. This could have been due to the function of Ca2+ as a signaling molecule in plants under salinity stress (Kong et al. 2015; Tuteja and Mahajan 2007).
Conclusion
In the current study, salinity considerably diminished growth, chlorophyll, and tissue K+ alongside the increase in tissue Na+ and Ca2+. Reduction in growth was associated with salt-induced decline in chlorophyll and increase in tissue Na+. Moreover, H2O2 and MDA accumulation also revealed greater intensity of oxidative damage that in turn degraded chlorophyll and suppressed plant growth. However, MSB application as foliar spray defended plants from oxidative injury by arousing the antioxidant system and altering nutrient uptake. Plants treated with MSB accumulated increasing concentrations of phenolics, flavonoids, ascorbic acid, and anthocyanins under salinity. Furthermore, the activities of antioxidant enzymes (SOD, POD, CAT) substantially increased in plants sprayed with MSB. Better antioxidant system in MSB-treated plants resulted in lower oxidative damage that in turn increased chlorophyll and improved growth. We measured Na+, K+, and Ca2+ in MSB-treated plants and found more tissue K+ and Ca2+ and lower Na+ under salinity. MSB protected plants from specific ion toxicity effect of salinity. Among MSB doses (0, 50, 100, 150, and 200 µM), 50 µM MSB was found to be effective as it protected plants from oxidative damage and specific ion toxicity under salinity stress.
Author contribution statement
MAA and MI planned the experiment and prepared the manuscript. MI supervised the whole experiment; MAA and HFA did sampling and analyses. All authors read and approved the manuscript.
Abbreviations
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- Na+ :
-
Sodium
- K+ :
-
Potassium
- Ca2+ :
-
Calcium
- ROS:
-
Reactive oxygen species
References
Abd-Allah EF, Alqarawi AA, Hashem A, Wirth S, Egamberdieva D (2018) Regulatory roles of 24-epibrassinolide in tolerance of Acacia gerrardii Benth to salt stress. Bioengineered 9:61–71
Abid M, Malik SA, Bilal K, Wajid RA (2002) Response of okra (Abelmoschus esculentus L.) to EC and SAR of irrigation water. Intl J Agric Biol 4:311–314
Abid M, Hakeem A, Shao Y, Liu Y, Zahoor R, Fan Y, Suyu J, Ata-Ul-Karim ST, Tian Z, Jiang D, Snider JL, Dai T (2018) Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ Exper Bot 145:12–20
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. and Coss.] plants can be alleviated by salicylic acid. S Afr J Bot 77:36–44
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2018) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255:163–174
Ali E, Hussain N, Shamsi IH, Jabeen Z, Siddiqui MH, Jiang L-X (2018) Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J Zhejiang Univ Sci B 19:130–146
Annunziata MG, Ciarmiello LF, Woodrow P, Maximova E, Fuggi A, Carillo P (2017) Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front Plant Sci 7:2035
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Asare AT, Asare-Bediako E, Agyarko F, Taah K, Osei EO (2016) Phenotypic traits detect genetic variability in okra (Abelmoschus esculentus L. Moench). Afr J Agr Res 11:3169–3177
Asensi-Fabado MA, Munné-Bosch S (2010) Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci 15:582–592
Ashraf MA, Ashraf M (2012) Salt-induced variation in some potential physiochemical attributes of two genetically diverse spring wheat (Triticum aestivum L.) cultivars: photosynthesis and photosystem II efficiency. Pak J Bot 44:53–64
Ashraf MA, Ashraf M (2016) Growth stage-based modulation in physiological and biochemical attributes of two genetically diverse wheat (Triticum aestivum L.) cultivars grown in salinized hydroponic culture. Environ Sci Pollut Res 23:6227–6243
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, Harris P (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Ashraf M, Iqbal M, Hussain I, Rasheed R (2015) Physiological and biochemical approaches for salinity tolerance. Managing salt tolerance in plants: molecular and genomic perspectives. CRC Press, London
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Borges AA, Jiménez-Arias D, Expósito-Rodríguez M, Sandalio LM, Pérez JA (2014) Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00642
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castro FA, Herdeiro RS, Panek AD, Eleutherio EC, Pereira MD (2007) Menadione stress in Saccharomyces cerevisiae strains deficient in the glutathione transferases. Biochim Biophys Acta 1770:213–220
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods in Enzymology, vol 2. Academic Press, Cambridge, pp 764–775
Cheng S-J, Tang D-Q, Miller WB, Shi Y-M (2018) Evaluation of salinity tolerance in honeysuckle (Lonicera japonica) using growth, ion accumulation, lipid peroxidation, and non-enzymatic and enzymatic antioxidants system criteria. J Hortic Sci Biotechnol 93:185–195
Elmore JM, Coaker G (2011) The Role of the Plasma Membrane H + -ATPase in Plant-Microbe Interactions. Mol Plant 4:416–427
Elsheery NI, Cao K-F (2008) Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol Plant 30:769–777
Esan AM, Masisi K, Dada FA, Olaiya CO (2017) Comparative effects of indole acetic acid and salicylic acid on oxidative stress marker and antioxidant potential of okra (Abelmoschus esculentus) fruit under salinity stress. Sci Hort 216:278–283
Ferchichi S, Hessini K, Dell’Aversana E, D’Amelia L, Woodrow P, Ciarmiello LF, Fuggi A, Carillo P (2018) Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct Plant Biol. https://doi.org/10.1071/FP18046
Gharbi E, Martínez J-P, Benahmed H, Hichri I, Dobrev PI, Motyka V, Quinet M, Lutts S (2017) Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci 258:77–89
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Greene R, Timms W, Rengasamy P, Arshad M, Cresswell R (2016) Soil and aquifer salinization: toward an integrated approach for salinity management of groundwater. In: Jakeman AJ, Barreteau O, Hunt RJ, Rinaudo JD (eds) In ‘integrated ground water management. Springer, Basel
Habib N, Ashraf M, Ali Q, Perveen R (2012) Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. S Afr J Bot 83:151–158
Habib N, Akram M, Javed M, Azeem M, Ali Q, Shaheen H, Ashraf M (2016) Nitric oxide regulated improvement in growth and yield of rice plants grown under salinity stress: antioxidant defense system. Appl Ecol Env Res 14:91–105
Hamilton P, Van Slyke D (1943) Amino acid determination and metal accumulation by Brassica juncea L. Int J Plant Prod 3:1735–8043
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heuer B (2003) Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci 165:693–699
Hussain I, Siddique A, Ashraf MA, Rasheed R, Ibrahim M, Iqbal M, Akbar S, Imran M (2017) Does exogenous application of ascorbic acid modulate growth, photosynthetic pigments and oxidative defense in okra (Abelmoschus esculentus (L.) Moench) under lead stress? Acta Physiol Plant 39(6):144
Ishikawa T, Shabala S (2018) Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity tolerance. Physiol Plant. https://doi.org/10.1111/ppl.12758
Jeyapraba J, Mahendran S, Sujirtha N (2016) Growth physiology and membrane permeability of okra (Abelmoschus esculentus L.) seedlings as affected by salinity. Int J Plant Soil Sci 9(5):1–5
Jiménez-Arias D, Pérez JA, Luis JC, Martín-Rodríguez V, Valdés-González F, Borges AA (2015) Treating seeds in menadione sodium bisulphite primes salt tolerance in Arabidopsis by inducing an earlier plant adaptation. Environ Expe Bot 109:23–30
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Kaya C, Ashraf M, Sönmez O, Tuna AL, Aydemir S (2015) Exogenously applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance. Turk J Agric For 39:909–919
Kaya C, Akram N, Ashraf M, Sonmez O (2018a) Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun 46:67–78
Kaya C, Ashraf M, Sonmez O (2018b) Combination of nitric oxide and thiamin regulates oxidative defense machinery and key physiological parameters in salt-stressed plants of two maize cultivars differing in salinity tolerance. Adv Agric Sci 6:34–44
Kong D, Ju C, Parihar A, Kim S, Cho D, Kwak JM (2015) Arabidopsis glutamate receptor homolog3. 5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol 167:1630–1642
Kubo A, Aono M, Nakajima N, Saji H, Tanaka K, Kondo N (1999) Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J Plant Res 112:279–290
Li J, Hu L, Zhang L, Pan X, Hu X (2015) Exogenous spermidine is enhancing tomato tolerance to salinity–alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol 15:303
Lüthje S, Van Gestelen P, Córdoba-Pedregosa MC, González-Reyes JA, Asard H, Villalba JM, Böttger M (1998) Quinones in plant plasma membranes: a missing link? Protoplasma 205:43–51
Lüthje S, Möller B, Perrineau FC, Wöltje K (2013) Plasma membrane electron pathways and oxidative stress. Antioxid Redox Signal 18:2163–2183
Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedlings. Biol Plant 50:227–231
Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metallurgy 40:255–260
Minhas PS, Gupta RK (1993) Using high salinity and SAR waters for crop production–Some Indian experiences. In: Leith H, Al–Masoom A (eds) Towards the rational use of high salinity tolerant plants. Kluwer Academic Publishers, Amsterdam
Mukherjee S, Choudhuri M (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munns R, Tester M (2008) Mechanism of salinity tolerance. Annu Rev Plant Physiol 35:299–319
Nguyen HT, Meir P, Sack L, Evans JR, Oliveira RS, Ball MC (2017) Leaf water storage increases with salinity and aridity in the mangrove Avicennia marina: integration of leaf structure, osmotic adjustment and access to multiple water sources. Plant Cell Environ 40:1576–1591
Pehlivan N (2018) Salt stress relief potency of whortleberry extract biopriming in maize. 3 Biotech 8:89
Qadir M, Quillerou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD (2014) Economics of salt-induced land degradation and restoration. Nat Resour Forum 38:282–295
Qureshi RH, Barrett-Lennard EG (1998) Saline agriculture for irrigated land in Pakistan: a handbook. Monographs, Australian Centre for International Agricultural Research (ACIAR), number 117728. https://doi.org/10.22004/ag.econ.117728
Rama Rao AV, Ravichandran K, David SB, Ranade S (1985) Menadione sodium bisulphite: a promising plant growth regulator. Plant Growth Regul 3:111–118
Rengasmay P (2006) Wolrd salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic 103:93–99
Siddiqui MH, Alamri SA, Al-Khaishany MY, Al-Qutami MA, Ali HM, AL-Rabiah H, Kalaji HM (2017) Exogenous application of nitric oxide and spermidine reduces the negative effects of salt stress on tomato. Hortic Environ Biotechnol 58:537–547
Surender Reddy P, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kavi Kishor PB (2015) Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem 94:104–113
Taïbi K, Taïbi F, Ait Abderrahim L, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Tanji KK, Neeltje CK (2002) Agricultural drainage water management in arid and semi-arid areas. FAO, Rome, pp 135–160
Tuteja N, Mahajan S (2007) Calcium signaling network in plants. Plant Signal Behav 2:79–85
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang LY, Liu JL, Wang WX, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54:19–27
Wang W, Tian F, Hao Q, Han Y, Li Q, Wang X, Wang W, Wang Y, Wang W (2018) Improved salt tolerance in a wheat stay-green mutant tasg1. Acta Physiol Plant 40:39
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13:1035–1059
Yasmeen R, Siddiqui ZS (2017) Ameliorative effects of Trichoderma harzianum on monocot crops under hydroponic saline environment. Acta Physiol Plant 40:4
Zhu M, Zhou M, Shabala L, Shabala S (2015) Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct Plant Biol 42:252–263
Acknowledgements
Funds were provided by GCUF project No. 52-Bot-11 and HEC project 21-188/SRGP/R&D/HEC/2014 for the execution of this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ashraf, M.A., Asma, H.F. & Iqbal, M. Exogenous menadione sodium bisulfite mitigates specific ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench). Acta Physiol Plant 41, 187 (2019). https://doi.org/10.1007/s11738-019-2978-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2978-7