Abstract
Background
Clonorchis sinensis was a food-borne zoonotic parasite in the worldwide and also an important risk factor of hepatic fibrosis. Excretory/secretion products of C. sinensis (CsESPs) are involved in parasite-host interactions and contribute to the development of hepatic damage. The aim of the present study was to investigate whether CsESPs and CsTP (adult protein) could induce autophagy of hepatic stellate cells (HSCs) and further activate HSCs so as to participate in the pathogenesis of hepatic fibrosis.
Methods and results
The human hepatic stellate cell line LX-2 was stimulated by CsESPs and CsTP. CsESPs showed the effect on cell proliferation in methyl thiazolyl tetrazolium (MTT) assay while CsTP failed. Autophagosomes and autolysosomes were observed after the transmission mRFP-EGFP-LC3 plasmid into the LX-2 cells. CsESPs had more powerful to induce the accumulation of autophagosomes and autolysosomes to enhance autophagic flux compared with CsTP. Western-blotting analysis confirmed that the ratio of LC3-II/I in LX-2 cells was up-regulated after CsESPs treatment for 6 h, which further proved that CsESPs could induce autophagy in LX-2 cells. Meanwhile, q-PCR results showed that the mRNA levels of collagen I, collagen III and α-SMA decreased in LX-2 cells after treatment with autophagy inhibitor chloroquine, whereas they increased when combination with CsESPs.
Conclusions
These results suggested that CsESPs-induced autophagy might be involved in the activation of HSCs, and consequently participate in the pathogenesis of hepatic fibrosis caused by C. sinensis infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonorchiasis, caused by Clonorchis sinensis (C. sinensis), is mainly prevalent in Asian countries and regions, especially Guangxi and Guangdong in China [1]. C. sinensis infection is closely related to hepatobiliary diseases, such as fibrosis and even cholangiocarcinoma, thus it is a serious public health problem in endemic areas. The proposed mechanisms of pathogenesis of C. sinensis infection include mechanical damage to bile duct epithelia resulting from the activities of the adult worm, inflammation and other pathological effects from excretory-secretory products (ESPs) [2].
Autophagy is a metabolic process that eukaryotic cells digest their own organelles and long-lived proteins. As a cellular house keeper, autophagy eliminates defective proteins and organelles, removes intracellular pathogens, and also prevents abnormal proteins from accumulating [3]. Therefore, autophagy plays active roles in the pathogenesis of many diseases, including hepatic diseases. Chronic hepatic injury initiates fibrogenic process by triggering inflammation and HSCs activation, during which quiescent HSCs reduce vitamin A droplets and acquire myofibroblastic features associated with the capacity to secrete extracellular matrix (ECM) and inhibition of matrix degradation [4]. Researches indicated that autophagy played a complex role in the liver fibrogenic process: it indirectly protects against hepatic fibrosis in hepatocytes, macrophages and endothelial cells, and it displays fibrogenic properties to promote the activation process in HSCs and influence other fibrogenic cells [5, 6].
Clinical information had shown that C. sinensis infection, as a chronic liver injury, could lead to hepatic fibrosis, the progress is different from that of the hepatic fibroplasias caused by hepatic virus and alcohol. Animal experiment proved that periductal fibroplasia occurred at an early stage of the infection and then developed into liver parenchyma [2]. ESPs are released continuously by adults into bile ducts and surrounding liver tissues to cause persistent injury. Hu et al. also found that HSCs activation and hepatic fibrosis occurred in the infected animal [7,8,9]. As components of ESPs, secretory phospholipase A(2) (CsPLA2), fructose-1, 6-bisphosphatase (CsFBPase), lysophospholipase (CslysoPLA), Fe heavychain protein (CsFHC) have been reported to directly activate human HSCs and other key cells in hepatic fibrosis process [7, 8, 10,11,12], suggesting that CsESPs play an important role in the progress of hepatic fibrosis in C. sinensis infection. However, the specific pathogenesis remains unclear, though the activated TGF-β/Smad signalling pathway contribution to fibrosis was reported [13].
In our previous study, hepatic fibrosis was observed in histological examination of livers from the rats infected by C. sinensis. Based on the H&E staining and Masson staining, histological analysis revealed that 4 rats were in stage F1 (Stellar enlargement of portal tracts without septa) and 6 rats were in stage F2 (Enlargement of portal tracts with rare septa) among 10 infected rats. According to the Metavir scoring system, significant fibrosis was considered when METAVIR stages were F ≥ 2 [14]. Meanwhile, in the same liver tissue, the ratio of LC3-II/I was elevated and the expression of p62 was decreased correspondingly. To date, microtubule associated protein light chain 3 (LC3) serves as a widely used marker for autophagosomes. Endogenous LC3 is detected as two bands by SDS-PAGE: LC3-I is present in the cytosol. When autophagy is induced, some LC3-I is converted into LC3-II, which is bound to the autophagosomal membrane. Thus the amount of LC3-II or LC3-II/I ratio correlates with the number of autophagosomes [15, 16]. Therefore, we hypothesized that autophagy might be triggered by C. sinensis infection and was involved in hepatic fibrosis. In the present study, we examined the autophagy flux and the activated markers expression of HSCs induced by CsESPs and CsTP to explore a possible link between autophagy and HSCs activation to further elucidate the role of autophagy in the pathogensis of C. sinensis–induced hepatic fibrosis.
Methods
Preparation ESPs and adult-derived total protein of C. sinensis
Pseudorasbora parvas, the second intermediate host of C. sinensis, naturally infected with metacercariae was collected from endemic areas. The fish were digested by using artificial gastric juice (0.5% pepsin, 1% HCl and 0.9% NaCl) at 37 °C overnight. Male New Zealand White rabbits (3000–3500 g) were purchased from Guangxi Medical University Laboratory Animal Center and raised carefully in accordance with National Institutes of Health on animal care and the ethical guidelines. Metacercaria were isolated under stereomicroscope and 800 metacercaria/each rabbit were administered intragastrically. After 6 weeks of infection, the rabbits were sacrificed after anesthesia and adult worms were collected from bile ducts, and then cultured in PBS (pH 7.4) at 37 °C under 5% CO2.
The culture medium was harvested after 6 h (named CsESP-6 h), then the adult worms were cultured in fresh PBS for another 6 h and the culture medium was harvested (named CsESP-12 h). They were centrifuged at 12,000×g for 10 min at 4 °C. The supernatant was sterilized with a 0.22 μm filter and stored at − 80 °C for later use. The homogenate of adult worms in PBS (pH 7.4) was centrifuged at 12,000×g for 10 min at 4 °C, and the supernatant (C. sinensis adult-derived total protein, CsTP) was stored at − 80 °C after sterilization. The concentrations of CsESP-6h, CsESP-12h and CsTP were detected using BCA method (Biosharp, China).
MTT assay
LX-2 cell was obtained from the Shanghai Cell Institute (Shanghai, China) and cultured in DMEM medium containing 10% fetal calf serum in 5% CO2 at 37 °C. The cells (3000/well) were seeded into a 96-well plate. After overnight incubation, culture medium was replaced with fresh medium supplemented with 25, 50, 100 µg/ml of CsESP-6h respectively. It was performed by the same way with the three concentrations of CsESP-12h and CsTP respectively. At 6, 12, 24, 36, 48 h respectively, 20 µl MTT solution (Solarbio, China) and 180 µl culture medium were added to each well after the medium was aspirated. The medium was aspirated and 150 µl DMSO was added to solubilize the formazan 4 h later. The absorbance at 490 nm was recorded with a microplate reader (Thermo, USA).
Measuring autophagy flux of LX-2 cells by fluorescent-tagged LC3
LX-2 cells were plated into the wells of a 24-well plate and incubated in the cell culture overnight. The cells were then transiently transfected with mRFP-EGFP-LC3 reporter plasmid (gift from Pro. Dong Hu, Anhui University of Science &Technology), performing with Lipofectamine 2000 transfection system (Effectene Transfection Kit, Qiagen, Germany) according to the manufacturer’s protocol. After 24 h transfection, the medium was aspirated and replaced with 100 µg/ml CsESP-6 h, CsESP-12 h and CsTP, 10 μM rapamycin (Sigma) respectively and maintained for 6 h. 100 cells/each group were randomly selected and taken photos by a fluorescence microscopy (× 400, Olympus, Japan) for counting the puncta. Then the amount of red and yellow puncta in the cells was observed and counted using ImageJ software. The results were presented as the average number of puncta per cell. The experiments were repeated 3 times.
Examining autophagy of LX-2 cells by western blotting analysis
LX-2 cells were plated into a 24-well plate and cultured for 24 h. Then the cells were treated with 25, 50, 100 µg/ml CsESP-6h; 25, 50, 100 µg/ml CsTP, and 20 μM CQ independently or simultaneously for 6 h. Then the cell pellets were lysed with RIPA buffer supplemented with protease inhibitors. Total soluble protein (40 µg) was subjected to 12% SDS-PAGE and electrotransferred to apolyvinylidenedifluoride (PVDF) membrane. The membranes were incubated overnight with primary antibodies for LC3 and glyceraldehyde 3-phosphatedehydrogenase (abcam) (1: 1000) at 4 °C, respectively. After washing procedures, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit/mouse secondary antibody (Abways Technology) for 2 h at room temperature and developed with Immobilon western chemiluminescent HRP substrate (Merck Millipore). Densitometry analysis was performed using ImageJ software, and the relative levels of protein in each group were normalized to the loading control. The experiments were performed 3 times.
Monitoring LX-2 cells activation by qPCR analysis
LX-2 cells were plated into a 24-well plate and cultured for 24 h. Then the cells were treated with 100 µg/ml CsESP-6h, and 20 μM CQ independently or simultaneously. After 48 h, total RNA was extracted from the cell line. Quantitative real-time PCR (qPCR) was performed by PrimeScript™RT Master Mix (Perfect Real Time) and TB Green®Premix Ex Taq™ (TliRNaseH Plus) (TaKaRa, China) according to the manufacturer’s instructions. Reverse transcription was carried out in a condition of 15 min at 37 °C and 5 s at 85 °C. The PCR cycling conditions were as follows: 95 °C for 5 min and 40 cycles of 95 °C for 5 s, 60 °C 40 s. The primers set used were listed as follows: for GAPDH (5′GAACGGGAAGCTCACTGG 3′, 5′GCCTGCTTCACCACCTTCT 3′), for Collagen-I (5′TCGGCGAGAGCATGACCGATGGAT 3′, 5′GACGCTGTAGGTGAAGCGGCTGTT 3′), for Collagen-III (5′ATGGTTGCACGAAACACACT3′, 5′CTTGATCAGGACCACCAATG 3′), for α-SMA (5′CCAGGGCTGTTTTCCCATCC 3′, 5′GCTCTGTGCTTCGTCACCCA 3′). Gene expression levels were calculated by the 2−△△CT method. The relative mRNA expression of each time point sample was calibrated to that of GAPDH internal control and reported as the fold change relative to the untreated control. The experiments were repeated 3 times.
Statistical analysis
Data are expressed as the means ± SD of three independent experiments and significant differences were assessed by Student’s t-test. Statistical significance was accepted with p < 0.05. Statistical analyses were performed using SPSS 22.0.
Results
MTT assay
MTT assay was used to detect the effects of different concentration of CsESP-6 h, CsESP-12h and CsTP on LX-2 cells proliferation. After incubation for 24 to 48 h, CsESP-6 h and CsESP-12h showed a dose-dependent effect on the proliferation of LX-2 cells (p < 0.05; Fig. 1a, b). Different concentration of CsTP had no effect to promote proliferation of LX-2 cells (p > 0.05; Fig. 1c).
MTT proliferation assay showing the effect of the CsESPs and CsTP on the viability and proliferation of LX-2 cells. Cells were grown in 96-well culture plates overnight following by incubation with different concentrations of CsESP-6h, CsESP-12h, CsTP (0, 25, 50, and 100 μg/ml), respectively. At 6, 12, 24, 36, and 48 h time points, MTT was added to cell culture medium, and cells were cultured for another 4 h followed by adding DMSO to each well. The absorbance at 490 nm was recorded with a microplate reader. a–c, showed the effect of the CsESP-6h, CsESP-12h, and CsTP on the viability and proliferation of LX-2 cells, respectively. Each bar represents the mean value from three experiments with standard deviation; *p < 0.05
Measurement autophagic flux of LX-2 cells by fluorescent-tagged LC3
In the fluorescent transfection experiment, the red puncta (autolysosomes) and yellow puncta (autophagosomes) significantly increased in LX-2 cells treated by rapamycin compared with the control (Fig. 2b), suggesting that the expression of mRFP-EGFP-LC3 in LX-2 cells could be used to evaluate autophagy flux (Fig. 2a). According to the result of MTT assay, 100 μg/ml CsESP-6h, CsESP-12h and CsTP were performed to treat LX-2 cell. As presented in Fig. 2a, c, the treatment of CsESP-6h, CsESP-12h and CsTP, led to a substantial number of yellow puncta as well as red puncta compared with the control (p < 0.01), suggesting that CsESPs and CsTP could enhance autophagic flux. Additionally, much more red and yellow puncta were observed after the treatment of CsESP than CsTP, especially CsESP-6h treatment. These results indicated the ability of induction of autophagy in LX-2 cells by CsESPs was more powerful than CsTP.
a LX-2 cells were transfected with mRFP-EGFP-LC3 plasmid and then treated by CsESP-6h, CsESP-12h, CsTP and rapamycin, respectively. Autophagosomes (yellow puncta) as well as autolysosomes (red puncta) were observed by a confocal fluorescent microscope (Leica, Germany). DAPI was used as a nuclear staining. Scale bar, 25 μm. b Autophagosomes as well as autolysosomes in the LX-2 cells treated by rapamycin were statistically analyzed. c Autophagosomes as well as autolysosomes in the LX-2 cells treated by CsESP-6h, CsESP-12h and CsTP were statistically analyzed respectively. 100 cells / each group were imaged and the number of red and yellow puncta in each cell was quantified. **(p < 0.01)
Monitoring LX-2 cells autophagy by western blotting
To further confirm the ability of CsESP-6h and CsTP to induce autophagy in LX-2 cells, the protein expression of LC3 was examined by using Western blotting. As shown in Fig. 3a, the ration of LC3-II/I elevated in a concentration-dependent manner after LX-2 cells exposure to CsESP-6h for 6 h. Combination with CQ, which disrupts the function of lysosome to result in the accumulation of LC3-II, further increased the amount of LC3-II (Fig. 3b). However, different concentrations of CsTP failed to elevate the ratio of LC3-II/I. This provided further evidence that CsESPs played more positive role in the induction of autophagy in LX-2 cells.
a The expression of LC3 was detected after LX-2 cells exposure to different concentration of CsESP-6h for 6 h by Western blotting. b The expression of LC3 was detected after treatment of 100 µg/ml CsESP-6 h with or without 20 μM chloroquine (CQ) for 6 h by Western blotting. *p < 0.05 vs control. Data represent the results of three independent experiments
Monitoring LX-2 cells activation by qPCR
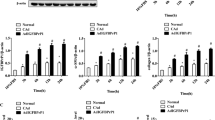
After treatment with autophagy inhibitor CQ for 48 h, the mRNA expression levels of collagen I, collagen III and α-SMA in LX-2 cells were significantly decreased (p < 0.05), and the mRNA expressions of the three genes were significantly increased after treatment by CQ combination with CsESP-6h (p < 0.05), suggesting that CsESPs-induced autophagy might contribute to the activation of human stellate cells (Fig. 4).
The mRNA expression of Collagen-I, Collagen-III and α-SMA in CsESPs-treated LX-2 cells were detected by qPCR. Cells were treated with 100 μg/ml CsESPs with or without 20 μM chloroquine (CQ) for 48 h and the mRNA levels were calculated as the fold change (2−△△CT) relative to the untreated control after normalization to GAPDH mRNA. Values were the means ± SD of three independent experiments (*p < 0.05, compared with the untreated control)
Discussion
Comprehensive analysis has demonstrated that autophagy could be triggered by bacteria, viruses [17,18,19,20]. Autophagy plays a double-edged sword during infection: autophagy involves by the formation of autophagosomes and their transport to lysosomes to degrade and dispose of the intracellular bacteria and viruses; on the other hand, bacteria and viruses combat autophagy for their survival by interfering with autophagy signalling [21, 22]. Autophagy research involving parasites especially protozoan also focused on host-autophagic responses to infections and their interaction with autophagy machinery in host cells to manipulate their virulence. It was reported that autophagy as a key process carried out during Trypanosoma cruzi differentiation and host cell infection [23]. Proxiredoxin of Entamoeba histolytica, as a critical molecule during the invasion of host tissues, could induce autophagy and cytotoxic effects in macrophages [24]. In the process of Leishmania infection, autophagy was inhibited at the early stage but was activated at later stages of infection, indicating that Leishmania engaged an alternative path way to induce host autopahgy to optimize its survival [25]. These studies revealed that the important functions of host autophagy trigged by protozoan in the battle between host and parasites. Even though less autophagy studies about helminth infection were reported, it also manifested that helminth-induced autophagy contributed to their pathogensis. Research presented that host macrophage autophagy was induced by Schistosoma japonicum egg antigen (SEA) to limit the development of pathology in host liver [26]. ESPs of Angiostrongylus cantonensis could activate autophagy in mouse brain astrocyte cell via Sonic hedgehog (Shh) signaling which had a protective potential for astrocytes [27]. Trichinella spiralis infection also induced autophagy in the host muscle cells and ESPs inhibited autophagy of myoblasts in vitro [28]. Studying the mechanism of autophagy in helminth infection and its interaction with host can help discover new pathogenic mechanism and accelerate the development of effective prevention and control strategies.
Sustained hepatic injury such as induced by C. sinensis infection maybe a major driving force of fibrosis progression. In the previous study, we successfully established the hepatic fibrosis rat model of C. sinensis infection for 6 months. In the hepatic tissues from the infected rats, the expression of fibrosis-related protein α-SMA increased in parallel to the up-regulated expression of the autophagy marker LC3-II/I and the down-regulated expression of p62. These results suggested that a potential link between autophagy and hepatic fibrous induced by C. sinensis infection. Adult worms of C. sinensis parasitize the secondary hepatobiliary duct of human and live for approximate 20 to 30 years. During the chronic infection, the adults experience a nutrient-deprived and oxygen-deficient state in the parasitic site, the probability of stimulation of autophagy exists. Moreover, the adults continuously excrete/secrete a complex mixture of proteins and other metabolites via the tegument and oral opening or excretory organs, which lead to hepatobiliary injury directly [29]. As the most abundant composition, the proteases and protein such as glutathione transferase, dehydrogenase, cysteine protease, basement membrane-specific heparan sulfate proteoglycan core protein, dynein, retinal dehydrogenase 1, and myoglobin, were found in the CsESP [30, 31], some of them are capable of potential chemical toxicity. In this study, we sought via cell experiment in vitro to investigate whether autophagy in LX-2 cells could be triggered by ESPs from C. sinensis and identify the possible relationship between the activated autophagy and activation of HSCs.
Previous studies reported that C. sinensis adult-derived total protein (CsTP) was involved in the pathogenesis by eliciting Th2 immune response [32]. It was also reported that most of tegument proteins of adult worms were oxidoreductases, hydrolases or transferases, which of them were predicted to be involved in liver disease [31]. Thus, in this study, we explored both CsESP and CsTP’s role in the autophagy-induced. Dual fluorescent mRFP-EGFP-LC3 system experiment result showed that CsTP was weaker capable of autophagy-induced in LX-2 cells compared with CsESP. However, it was demonstrated by Western blotting that different concentration of CsTP failed to upregulate the ratio of LC3-II/I in LX-2 cells. Taken together, these findings suggested that CsESPs could play more positive role in triggering autophagy of LX-2 cells. Obviously, it was due to the difference of the compositions between CsESP and CsTP. ESPs include more abundant proteases, antioxidant enzymes and metabolic enzyme, which lead to chemical stimulation to hepatic tissue. Zheng et al. reported that 267 proteins were identified in the CsESPs after adult culture for 0–6 h, only 103 proteins were identified in the CsESPs after culture for another 6 h [33]. In the present study, compared with CsESP-12 h, it was found that CsESP-6 h was more powerful to promote proliferation and induce autophagy in LX-2 cells with a dose-dependent effect. Obviously, it was due to the more secretion/excretion products of adult worms after culture for 0–6 h than 6–12 h, showing that the composition of CsESPs remained closer to the substance secreted/excreted by the parasitic state of adult worms in vivo as culture time shortened. It was suggested that ESPs used to stimulate cells were harvested in vitro in as short a time as possible to ensure the accuracy of the results.
A key step in the fibrogenic process is the activation of HSCs. Parenchymal injury and the resulting inflammatory reactions generate signals that promote a phenotypic switch from a lipid-rich to a myofibroblastic fibrogenic phenotype [34, 35]. Generally, HSCs are activated to lead to a large amount of ECM, especially the increase of collagen-I and III. And induction of α-SMA is also a reliable marker of HSCs activation. In HSCs, autophagy displays fibrogenic properties via p62 loss which impairs VDR-RXR (Vitamin D receptor-retinoid X receptor) interaction for maintaining HSC in a quiescent state, and via lipophagy which allow lipid droplet digestion and release of ATP required to promote the activation process. In qPCR analysis, the mRNA expression of collagen I in the LX-2 cells was up-regulated after the CsESPs treatment for 48 h, whereas, the mRNA expression of collagen-III and α-SMA was unchanged, which was not completely consistent with the previous study that the mRNA level of the collagen-III increased by using RT-PCR [9]. The difference may be mainly due to the method of identifying mRNA level and the time of CsESPs harvested in vitro. The previous report revealed that the degree of hepatic fibrosis could be alleviated through inhibiting autophagy [6]. In current study, the autophagy inhibitor chloroquine (CQ) inhibited the mRNA expression of collagen-I, collagen-III and α-SMA in LX-2 cells, which proved that autophagy participated in HSCs activation. More importantly, CQ combination with CsESPs compared with single CQ, the elevated level of mRNA of collagen-I, collagen-III and α-SMA was parallel to the elevated autophagy flux, indicating that CsESPs could alleviate the inhibition effect of autophagy inhibitor on HSCs activation. It implied that CsESPs could induce autophagy and then participate in the activation of HSCs that is the major driver hepatic fibrogenesis.
In conclusion, this is first study to show that CsESPs could induce autophagy in HSCs that might contribute to the activation of HSCs. It is important for elucidating hepatic fibrous pathogenesis caused by C. sinensis infection. Given an established relationship between HSCs activation and hepatic fibrosis, further research would focus on the mechanism of how C. sinensis-induced autophagy leads to hepatic fibrosis, which can point towards new targets for the treatment of the chronic liver disease.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Image analysis was performed using ImageJ software. Statistical analyses were performed using SPSS 22.0.
References
Zhou X (2018) Report on the national survey of important human parasitic diseases in China (2015). People’s Medical Publishing House, Beijing
Tang ZL, Huang Y, Yu XB (2016) Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect Dis Poverty 5(1):71. https://doi.org/10.1186/s40249-016-0166-1
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477. https://doi.org/10.1016/s1534-5807(04)00099-1
Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88(1):125–172. https://doi.org/10.1152/physrev.00013.2007
Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL (2012) Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142(4):938–946. https://doi.org/10.1053/j.gastro.2011.12.044
Thoen LF, Guimarães EL, Dollé L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA (2011) A role for autophagy during hepatic stellate cell activation. J Hepatol 55(6):1353–1360. https://doi.org/10.1016/j.jhep.2011.07.010
Hu F, Hu X, Ma C, Xu J, Yu X (2009) Excretory/secretory antigens from Clonorchis sinensis induces hepatic fibrosis in rats. Nan Fang Yi Ke Da Xue Bao 29(3):393–396 (in Chinese)
Hu F, Hu X, Ma C, Zhao J, Xu J, Yu X (2009) Molecular characterization of a novel Clonorchis sinensis secretory phospholipase A(2) and investigation of its potential contribution to hepatic fibrosis. Mol Biochem Parasitol 167(2):127–134. https://doi.org/10.1016/j.molbiopara.2009.05.003
Wang X, Hu F, Hu X, Chen W, Huang Y, Yu X (2014) Proteomic identificationof potential Clonorchis sinensis excretory/secretory products capable of binding and activating. Parasitol Res 113(8):3063–3071. https://doi.org/10.1007/s00436-014-3972-z
Liang P, Sun J, Huang Y, Zhang F, Zhou J, Hu Y, Wang X, Liang C, Zheng M, Xu Y, Mao Q, Hu X, Li X, Xu J, Lu G, Yu X (2013) Biochemical characterization and functional analysis of fructose-1,6-bisphosphatase from Clonorchis sinensis. Mol Biol Rep 40(7):4371–4382. https://doi.org/10.1007/s11033-013-2508-4
Mao Q, Xie Z, Wang X, Chen W, Ren M, Shang M, Lei H, Tian Y, Li S, Liang P, Chen T, Liang C, Xu J, Li X, Huang Y, Yu X (2015) Clonorchis sinensis ferritin heavy chain triggers free radicals and mediates inflammation signaling in human hepatic stellate cells. Parasitol Res 114(2):659–670. https://doi.org/10.1007/s00436-014-4230-0
Zhang F, Liang P, Chen W, Wang X, Hu Y, Liang C, Sun J, Huang Y, Li R, Li X, Xu J, Yu X (2013) Stage-specific expression, immunolocalization of Clonorchis sinensis lysophospholipase and its potential role in hepatic fibrosis. Parasitol Res 112(2):737–749. https://doi.org/10.1007/s00436-012-3194-1
Yan C, Wang L, Li B, Zhang BB, Zhang B, Wang YH, Li XY, Chen JX, Tang RX, Zheng KY (2015) The expression dynamics of transforming growth factor-β/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis. Parasit Vectors 8:70. https://doi.org/10.1186/s13071-015-0675-y
Calès P, Chaigneau J, Hunault G, Michalak S, Cavaro-Menard C, Fasquel JB, Bertrais S, Rousselet MC (2015) Automated morphometry provides accurate and reproducible virtual staging of liver fibrosis in chronic hepatitis C. J Pathol Inform 6:20. https://doi.org/10.4103/2153-3539.157782
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728. https://doi.org/10.1093/emboj/19.21.5720
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4(2):151–175. https://doi.org/10.4161/auto.5338
Chiramel AI, Best SM (2018) Role of autophagy in Zika virus infection and pathogenesis. Virus Res 254:34–40. https://doi.org/10.1016/j.virusres.2017.09.006
Jia K, Thomas C, Akbar M, Sun Q, Adams-Huet B, Gilpin C, Levine B (2009) Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc Natl Acad Sci USA 106(34):14564–14569. https://doi.org/10.1073/pnas.0813319106
Kim JK, Lee HM, Park KS, Shin DM, Kim TS, Kim YS, Suh HW, Kim SY, Kim IS, Kim JM, Son JW, Sohn KM, Jung SS, Chung C, Han SB, Yang CS, Jo EK (2017) MIR144 inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy 13(2):423–441. https://doi.org/10.1080/15548627.2016.1241922
Mattoscio D, Medda A, Chiocca S (2018) Human papilloma virus and autophagy. Int J Mol Sci 19(6):1775. https://doi.org/10.3390/ijms19061775
Choi Y, Bowman JW, Jung JU (2018) Autophagy during viral infection—a double-edged sword. Nat Rev Microbiol 16(6):341–354. https://doi.org/10.1038/s41579-018-0003-6
Huang J, Brumell JH (2014) Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol 12(2):101–114. https://doi.org/10.1038/nrmicro3160
Losinno AD, Martínez SJ, Labriola CA, Carrillo C, Romano PS (2021) Induction of autophagy increases the proteolytic activity of reservosomes during Trypanosoma cruzi metacyclogenesis. Autophagy 17(2):439–456. https://doi.org/10.1080/15548627.2020.1720428
Li X, Zhang Y, Zhao Y, Qiao K, Feng M, Zhou H, Tachibana H, Cheng X (2020) Autophagy activated by peroxiredoxin of Entamoeba histolytica. Cells 9(11):2462. https://doi.org/10.3390/cells9112462
Thomas SA, Nandan D, Kass J, Reiner NE (2018) Countervailing, time-dependent effects on host autophagy promotes intracellular survival of Leishmania. J Biol Chem 293(7):2617–2630. https://doi.org/10.1074/jbc.M117.808675
Zhu J, Zhang W, Zhang L, Xu L, Chen X, Zhou S, Xu Z, Xiao M, Bai H, Liu F, Su C (2018) IL-7 suppresses macrophage autophagy and promotes liver pathology in Schistosoma japonicum-infected mice. J Cell Mol Med 22(7):3353–3363. https://doi.org/10.1111/jcmm.13610
Chen KY, Cheng CJ, Cheng CC, Jhan KY, Chen YJ, Wang LC (2020) The excretory/secretory products of fifth-stage larval Angiostrongylus cantonensis induces autophagy via the Sonic hedgehog pathway in mouse brain astrocytes. PLoS Negl Trop Dis 14(6):e0008290. https://doi.org/10.1371/journal.pntd.0008290
Hu X, Liu X, Bai X, Yang L, Ding J, Jin X, Li C, Zhang Y, Li Y, Yang Y, Liu M (2021) Effects of Trichinella spiralis and its excretory/secretory products on autophagy of host muscle cells in vivo and in vitro. PLoS Negl Trop Dis 15(2):e0009040. https://doi.org/10.1371/journal.pntd.0009040
Won J, Cho Y, Lee D, Jeon BY, Ju JW, Chung S, Pak JH (2019) Clonorchis sinensis excretory-secretory products increase malignant characteristics of cholangiocarcinoma cells in three-dimensional co-culture with biliary ductal plates. PLoS Pathog 15(5):e1007818. https://doi.org/10.1371/journal.ppat.1007818
Madsen JA, Farutin V, Carbeau T, Wudyka S, Yin Y, Smith S, Anderson J, Capila I (2015) Toward the complete characterization of host cell proteins in biotherapeutics via affinity depletions, LC-MS/MS, and multivariate analysis. MAbs 7(6):1128–1137. https://doi.org/10.1080/19420862.2015.1082017
Shi Y, Yu K, Liang A, Huang Y, Ou F, Wei H, Wan X, Yang Y, Zhang W, Jiang Z (2020) Identification and analysis of the tegument protein and excretory-secretory products of the carcinogenic liver fluke Clonorchis sinensis. Front Microbiol 11:555730. https://doi.org/10.3389/fmicb.2020.555730
Zhao L, Shi M, Zhou L, Sun H, Zhang X, He L, Tang Z, Wang C, Wu Y, Chen T, Shang M, Zhou X, Lin Z, Li X, Yu X, Huang Y (2018) Clonorchis sinensis adult-derived proteins elicit Th2 immune responses by regulating dendritic cells via mannose receptor. PLoS Negl Trop Dis 12(3):e0006251. https://doi.org/10.1371/journal.pntd.0006251
Zheng M, Hu K, Liu W, Li H, Chen J, Yu X (2013) Proteomic analysis of different period excretory secretory products from Clonorchis sinensis adult worms: molecular characterization, immunolocalization, and serological reactivity of two excretory secretory antigens-methionine aminopeptidase 2 and acid phosphatase. Parasitol Res 112(3):1287–1297. https://doi.org/10.1007/s00436-012-3264-4
Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A (2005) Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 145:605–628
Mallat A, Lotersztajn S (2013) Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am J Physiol Cell Physiol 305(8):C789–C799. https://doi.org/10.1152/ajpcell.00230.2013
Acknowledgements
This research was funded by the Natural Science Foundation of Guangxi (No. 2020GXNSFAA159068 and 2019GXNSFAA245069), Guangxi First-class Discipline Project for Basic Medicine (No. GXFCDP-BM-2018), Guangdong Province University Student Innovation and Entrepreneurship Training Programs (No. S201910570080), Guangzhou Medical University Student Science and Technology Innovation Programs (No. 2018A094) China
Funding
This research was funded by the Natural Science Foundation of Guangxi (No. 2020GXNSFAA159068 and 2019GXNSFAA245069), Guangxi First-class Discipline Project for Basic Medicine (No. GXFCDP-BM-2018), Guangdong Province University Student Innovation and Entrepreneurship Training Programs (No. S201910570080), Guangzhou Medical University Student Science and Technology Innovation Programs (No. 2018A094).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. Conception, design, manuscript modification, and team coordination, etc. (CM and YL), Methodology (BZ, ZG, LL, YL, YK, and WC); Material preparation, data collection and analysis (KL, WC, JM); The first draft of the manuscript (BZ and ZG). All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Ethical approval
All animal procedures in this study were approved by the Animal Care & Welfare Committee of Guangxi Medical University.
Consent to participate
All authors participated in the informed consent.
Consent for publication
All authors approved publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, B., Gao, Z., Liang, L. et al. Autophagy of hepatic stellate cell induced by Clonorchis sinensis. Mol Biol Rep 49, 1895–1902 (2022). https://doi.org/10.1007/s11033-021-07001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07001-9