Abstract
Lysophospholipase, belonging to the complex family of phospholipases, is supposed to play a vital role in virulence and pathogenesis of parasites and fungi. In the current study, the potential role of Clonorchis sinensis lysophospholipase (CslysoPLA) in hepatic fibrosis induced by C. sinensis was explored for the first time. In the liver of the cat infected with C. sinensis, CslysoPLA was recognized in the lumen between adult worms and surrounding bile duct epithelia together with some inside the cells by means of immunolocalization. Both Cell Counting Kit-8 (CCK-8 assay) and cell cycle analysis of human hepatic stellate cell line LX-2 showed that a higher percentage of cells were at proliferation phase after incubation with lower concentrations of recombinant CslysoPLA (rCslysoPLA). Quantitative real-time polymerase chain reaction (RT-PCR) demonstrated an upregulation in fibrogenic genes of smooth muscle α-actin, collagen III, matrix metalloproteinase 2 and tissue inhibitors of metalloproteinase II in LX-2 treated with rCslysoPLA. Moreover, human biliary epithelial cell line 5100 proliferated significantly in response to rCslysoPLA. Notably, CslysoPLA was localized in the adenomatoid hyperplastic tissue within the intrahepatic bile duct of experimentally infected rats by immunolocalization analysis. In addition, quantitative RT-PCR implied that CslysoPLA was differentially expressed at the developmental stages of C. sinensis (metacercariae, adult worms and eggs), with the highest level at metacercariae stage. Immunolocalization analysis showed that CslysoPLA was distributed in the intestine, vitelline gland, tegument and eggs in the adult worms and in the tegument and vitelline gland in the metacercariae, respectively. Collectively, it suggests that CslysoPLA might be involved in the initiation and promotion of C. sinensis-related human hepatic fibrosis and advance future studies on its promotion to C. sinensis-induced cholangiocarcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonorchiasis, triggered by infection with Clonorchis sinensis, is one of the most prevalent food-borne parasitic diseases in East Asia. It is estimated that about 35 million people have been infected with C. sinensis globally, of which 15 million are in China (Lun et al. 2005). The adult worms dwelling in bile ducts can lead to cholelithiasis, pyogenic cholangitis, cholecystitis, hepatic fibrosis, or even liver tumour and cholangiocarcinoma. In addition, C. sinensis is one of the etiological factors of cholangiocarcinoma (Bouvard et al. 2009). Previous studies have centred on enzymes which are important in the process of glycometabolism and survival of the parasite (Zheng et al. 2011; Hu et al. 2012). However, the molecular mechanisms of clonorchiasis remain obscure.
It has been revealed that C. sinensis infection in experimental animals could give rise to hepatic fibrosis, initiating at the hepatic sinusoids at early stage of clonorchiasis (Gao et al. 1994), suggesting that hepatic fibrosis may be attributed, at least in part, to excretory/secretory products (ESPs) released from the parasite. Reportedly, the effect of C. sinensis infection on change in gene expression of a human cholangiocarcinoma cell line HuCCT1 treated with ESPs is evaluated, using complementary DNA microarrays involving 23,920 human genes of known function. Of the identified 435 genes altered by ESPs, 31 were upregulated and 35 were downregulated more than twofold in a time-dependent manner, which are involved in the cell cycle, oncogenesis, protein modification, immunity, signal transduction, cell structure and developmental processes (Pak et al. 2009). Additionally, proliferative effect of C. sinensis ESPs on the human epithelial cell line HEK293 was clarified (Kim et al. 2008). It was also reported that C. sinensis ESPs increased proliferation of the cholangiocarcinoma cell line HuCCT1 and augmented the expression of cyclooxygenase-2, and the cells pretreated with ESPs were resistant to parthenolide-induced apoptosis (Kim et al. 2009). Lately, increasing evidence has shown that hepatic stellate cells (HSCs) may be of great importance in the progression of parasite-induced diseases and that the interaction between parasites or parasite antigens (Bartley et al. 2006; Chang et al. 2006; Anthony et al. 2010). It is noteworthy that phospholipases can exert physiological, pathophysiological and toxic effects on cell proliferation and migration (Mora et al. 2005). Briefly, lysophospholipase (lysoPLA) is an essential enzyme in regulating lysophospholipids (lysoPLs), which are cytotoxic at higher concentrations but function like growth factor at lower non-toxic concentrations (Wang and Dennis 1999).
In the present study, we demonstrated expression pattern and immunolocalization of CslysoPLA in the developmental stages of C. sinensis and its interplay with host cells including human hepatic stellate cell line LX-2 and human biliary epithelial cell line 5100. We aimed to seek out the potential role of CslysoPLA as one of the pathogenic factors of C. sinensis-related human hepatic fibrosis.
Materials and methods
Preparation of parasites and infected liver sections
C. sinensis metacercariae were isolated from freshwater fish (Pseudorasbora parve), which were experimentally infected in our established ecologic pool (Liang et al. 2009). The fishes were digested with acid pepsin solution (0.2 % HCl, 0.6 % pepsin and pH 2.0). The cats that naturally infected with C. sinensis were obtained from Guangdong Province of China, where is of high morbidity of C. sinensis infection (Lun et al. 2005). Ether was used to conduct mercy killing. Adult parasites were obtained from cats’ livers. After being freshly extracted from bile ducts, adult worms were washed several times with normal saline to prevent contamination from host. Meanwhile, eggs were separated from the supernatant. Adult worms, metacercariae and eggs were stored at −80 °C in Trizol reagent (Invitrogen, USA) for RNA extraction. Additionally, adult worms, metacercariae and the liver of a seriously infected cat were fixed and cut by microtome into sections for immunolocalization analysis. Sprague–Dawley (SD) rats were experimentally infected orally with 60 metacercariae each. Infected at 1 day and 10 weeks, livers were extracted from SD rats, respectively, and prepared for immunolocalization analysis. SD rats and the following BALB/c mice were purchased from animal centre of Sun Yat-sen University and raised carefully in accordance with National Institutes of Health on animal care and the ethical guidelines. All experimental procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University (permit number: SCXK (Guangdong) 2009-0011).
Expression and purification of rCslysoPLA
CslysoPLA isolated from our complementary DNA (cDNA) plasmid library of adult C. sinensis was used as template for polymerase chain reaction (PCR) amplification. Induced by isopropyl-β-d-thiogalactopyranoside (IPTG), the protein was expressed at 28 °C for 7 h in Luria–Bertani medium and purified with His Bind Purification kit (Novagen, USA). The final concentration of purified rCslysoPLA was determined by bicinchoninic acid (BCA) protein assay kit (Novagen, USA). Components of the kit are as follows: BCA solution (bicinchoninic acid, sodium carbonate, sodium tartrate and sodium bicarbonate in 0.1 M NaOH, pH 11.25), 4 % cupric sulphate and bovine serum albumin standard (2 mg/ml).
Preparation of anti-CslysoPLA sera
Each SD rat (approximately 80 g in weight) and BALB/c mouse (4 weeks old) were immunized with 200 μg and 50 μg rCslysoPLA in equivalent complete Freund’s adjuvant, respectively, and boosted with 100 and 25 μg rCslysoPLA in equivalent incomplete Freund’s adjuvant at 2-week interval. Serum specificity and antibody titre were measured by enzyme-linked immunosorbent assay. Antiserum was obtained at the eighth week for use.
Quantitative RT-PCR analysis of CslysoPLA at different life stages of C. sinensis
Total RNA from adult worms, metacercariae and eggs was extracted in Trizol regent following the manufacturer’s protocol. The concentration and purity of RNA were detected by nucleic acid/protein analyser (Beckman Coulter, USA) and agarose gel electrophoresis. The reaction of reverse transcription was conducted with a final volume of 20 μl involving 1 μg of total RNA, using avian myeloblastosis virus (AMV) reverse transcriptase (TaKaRa, Japan). Then the mixture reacted at 42 °C for 1 h. In order to analyse the expression level of CslysoPLA in the life stages of C. sinensis, quantitative RT-PCR was carried out to amplify the transcripts of CslysoPLA from cDNA of adult worms, metacercariae and eggs, using SYBR Premix Ex Taq (TaKaRa, Japan) in a volume of 20 μl. The forward and reverse primers for CslysoPLA were 5′-GGAATGGGGAATAGCGTTGACGGGG-3′ and 5′-AGACCGTCATCGCCAAGGCCGTGAA-3′, and primers for C. sinensis β-actin (GenBank accession number EU109284) were 5′-ACCGTGAGAAGATGACGCAGA-3′ and 5′-GCCAAGTCCAAACGAAGAATT-3′. The constitutively expressed β-actin gene served as the internal standard (Yoo et al. 2009). PCR conditions were as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 20 s, with an incremental increase of 0.1 °C/s from 60 to 95 °C. For graphical representation of the data, the method was applied according to the reference (Pfaffl 2001). The ΔC t value of each developmental stage was obtained by subtracting the C t value of β-actin at corresponding stage from that of adult worms, metacercariae and eggs, respectively. Subsequently, the ΔΔC t value of each life stage was obtained by subtracting the ΔC t value of adult worms from that of adult worms, metacercariae and eggs, respectively. For graphical representation of the data, \( {2^{{-\varDelta \varDelta {C_t}}}} \) was calculated as the final unit of Y-axis. The reaction was conducted by LC480 Software (Light Cycler 480, Switzerland).
Immunolocalization of CslysoPLA in parasites and infected livers
Sectioned parasites and infected cat’s liver in paraffin wax were deparaffinised in xylene and hydrated in gradient alcohol. Then the sections were blocked with normal goat serum for 1 h at room temperature and incubated in the rat anti-CslysoPLA serum (1:100 dilutions with 0.1 % bovine serum albumin (BSA) in phosphate-buffered saline (PBS)) in a humid chamber at 4 °C overnight. Rat preimmune serum was applied as negative control. Subsequently, the sections were probed with goat anti-rat IgG labelled with red-fluorescence Cy3 (1:400 dilutions with 0.1 % BSA in PBS, Molecular Probe, USA) for 1 h at room temperature in the darkness and imaged under fluorescence microscope (Carl Zeiss, Germany).
Sectioned rats’ livers were deparaffinised in xylene and hydrated in a series of ethanol. Endogenous peroxidase was blocked in 3 % (v/v) H2O2 in PBS for 15 min, and immunoreactivity was assessed by retrieving antigens in 10 mM citrate buffer at 95 °C for 20 min using water bath. Non-specific staining was blocked by incubation in normal goat serum for 1 h at room temperature. The sections were incubated with mouse anti-CslysoPLA serum (1:100 dilutions with 0.1 % BSA in PBS) in a humid chamber at 4 °C overnight, with mouse preimmune serum as negative control. After washing process, the sections were probed with horse radish peroxidase-conjugated goat anti-mouse IgG (1:400 dilutions with 0.1 % BSA in PBS, ProteinTech Group, USA) for 1 h at room temperature. The immunoreactive signal was developed by diaminobenzidine tetrahydrochloride. Then the sections were counterstained with Mayer’s haematoxylin, dehydrated, cleared in xylene and imaged by light microscopy (Carl Zeiss, Germany).
Reagents for cell proliferation assay
To remove endotoxin from rCslysoPLA, triton X-114 (AMRESCO, China) was utilized as follows: Induced by IPTG, the bacteria were harvested by centrifugation at 4 °C and suspended using 5 mM imidazole. The suspension containing rCslysoPLA was dealt with triton X-114 according to the manufacturer’s instructions. After the endotoxin was eliminated, tachypleus amebocyte lysate (Bo Kang, China) detection was performed to determine the amount of endotoxin in rCslysoPLA. One hundred microliters rCslysoPLA and 100 μl sterile detection water were mixed with tachypleus amebocyte lysate, and the mixture was incubated at 37 °C for 1 h. Two hundred microliters sterile detection water was used as negative control. Before being included in cell culture, the endotoxin-eliminated rCslysoPLA was filtered under sterile conditions with 0.22 μm syringe filters (Millipore, USA). Dilutions were carried out in Dulbecco modified Eagle medium (DMEM; Thermo Hyclone, USA) with 2 % foetal bovine serum (FBS; NQBB, Australia). Protein concentrations described hereafter refer to the final concentration in cell culture after dilution in media.
Assessment of cell viability and proliferation by CCK-8 assay
The human hepatic stellate cell line LX-2 was maintained according to standard procedure, which was well validated as a faithful representation of primary human HSCs (Xu et al. 2005). Briefly, cells were maintained with regular splitting, using 0.25 % trypsin every 2–5 days in DMEM with 10 % FBS and at 37 °C under 5 % CO2. To determine the proliferative effect of rCslysoPLA on LX-2, cell viability and proliferation by CCK-8 assay (DOJINDO, Japan) was performed. 2-(4-Methphenyl)-3-(4-ni-trophenyl)-2H-5-tetrazolium-2,4-benzene disulfonate monosodium salt is the major component of CCK-8 regent and can be deoxidized by cellular dehydrogenase. The deoxidized product, formazan, is a measure of metabolic activity of cells, which is routinely used to measure cell numbers over time. The cells were removed from cell-culture plastic bottle by trypsin and seeded into 96-well culture plates (5,000 cells/well, Eppendorf, Germany) in DMEM containing 10 % FBS. Diluted in DMEM with 2 % FBS (serum-free medium), different concentrations of rCslysoPLA were included with cells. After 48 h or 72 h, the cells were incubated with 10 μl of CCK-8 reagent at 37 °C for 2 h in the darkness. The cell proliferation was determined by measuring the absorbance at 450 nm using Benchmark Plus plate reader (BioRad, USA). Cells cultured in serum-free medium were used as negative control.
Human biliary epithelial cell line 5100 was also maintained according to the standard procedure mentioned above, except that it grew in epithelial cell medium (EpiCM; ScienceCell, USA). EpiCM consists of 90 ml of basal medium, 10 ml of FBS, 1 ml of epithelial cell growth supplement and 1 ml of penicillin/streptomycin solution. The following experimental procedures were the same as mentioned above.
Cell cycle analysis by flow cytometry
Cell cycle distribution experiments were conducted by DNA staining using propidium iodide (PI; Sigma, USA). LX-2 cells were seeded in cell-culture plastic bottles. After 24 h, the medium was replaced with DMEM (serum-starved medium) to arrest cells in the quiescent stage of cell cycle. The cells were treated with different concentrations of rCslysoPLA (1, 2, 5, 10, 20 μg/mL) for 48 h or 72 h, respectively. Cells cultured in serum-free medium were applied as negative control. The cells were pelleted by centrifugation at 1,000×g and washed twice with PBS. Cell pellets were suspended and fixed with 1 ml of cold 70 % ethanol at −20 °C overnight. Then the cells were centrifuged and subjected to PI staining, and DNA contents were analysed by a FACSCalibur flow cytometry (BectonDickinson, USA). The distribution of cells in each cell cycle stage (G1, S, or G2/M) was calculated with the computer program ModFit LT (Verity Software House). The peak preceding the normal G1 region was the apoptotic peak. In contrast to apoptotic cells, necrotic cells do not show immediate reduction in DNA stainability.
Quantitative RT-PCR analysis of activation markers of LX-2
Total RNA, extracted from LX-2 cells stimulated with different concentrations of rCslysoPLA for 48 h by RNA extraction kit (Fastagen, China), was reversely transcribed by AMV reverse transcriptase, respectively. Using SYBR Premix Ex Taq, the cDNA was used as template for PCR amplification for human smooth muscle α-actin (α-SMA) (GenBank accession number NM_001141945) with the sense primer 5′-CCAGGGCTGTTTTCCCATCC-3′ and anti-sense primer 5′-GCTCTGTGCTTCGTCACCCA-3′, human collagen III (GenBank accession number NM_000090) with the sense primer 5′-GGTCCTCCTGGAACTGCCGGA-3′ and anti-sense primer 5′-GAGGACCTTGAGCACCAGCGTGT-3′, human matrix metalloproteinase (MMP) 2 (GenBank accession number NM_004530) with the sense primer 5′-CAGCGATGGCTTCCTCTGGTGCT-3′ and anti-sense primer 5′-CTTGCAGGGCTGTCCTTCAGCGTT-3′, human tissue inhibitors of metalloproteinase (TIMP) I (GenBank accession number NM_003254) with the sense primer 5′-GCCTTAGGGGATGCCGCTGACAT-3′ and anti-sense primer 5′-CAGGCTGTTCCAGGGAGCCACA-3′, human TIMP II (GenBank accession number NM_003255) with the sense primer 5′-GAGACGTGGGTCCAAGGTCCTCA-3′ and anti-sense primer 5′-GCTTGGCATCTGTGACGGTGCCA-3′ and human β-actin (GenBank accession number BC013835) with the sense primer 5′-GGCACTCTTCCAGCCTTCCTTCCT-3′ and anti-sense primer 5′-GCCAGACAGCACTGTGTTGGCGT-3′. The human β-actin served as the internal standard. Cells cultured in DMEM with 2 % FBS were used as negative control. PCR conditions were the same as mentioned above.
Statistics and software
SPSS version 13.0 software was applied in this study for all statistical analysis. Three independent experiments were performed, and the results were presented as the mean value from three experiments with standard deviation. The Student’s t test was used to determine the statistical significance, and P value of less than 0.05 is classified as statistically significant (*P < 0.05).
Results
Expression, endotoxin removal and purification of rCslysoPLA
The concentration of the recombinant His tag-CslysoPLA fusion protein was about 462 μg/ml, while the concentration of endotoxin-free rCslysoPLA was about 383 μg/ml. The tachypleus amebocyte lysate detection showed that the regent containing rCslysoPLA did not become colloidal but in water shape, indicating that the amount of endotoxin in rCslysoPLA was very small and the endotoxin-free rCslysoPLA could then be included in cells, compared with sterile detection water as negative control (in water shape).
Transcriptional level of CslysoPLA at different life stages of C. sinensis
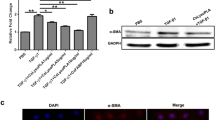
At different life stages of C. sinensis, CslysoPLA was highly expressed in metacercariae, followed by adult worms and eggs (Fig. 1).
Developmental stage expression level of CslysoPLA by quantitative RT-PCR. The expression level of CslysoPLA during the developmental stages of C. sinensis was analysed by means of the \( {2^{{-\Delta \Delta {C_t}}}} \) method, with C. sinensis β-actin as internal standard. The ΔC t value of each developmental stage was obtained by subtracting the C t value of β-actin at corresponding stage from that of adult worms, metacercariae and eggs, respectively. Subsequently, the ΔΔC t value of each life stage was obtained by subtracting the ΔC t value of adult worms from that of adult worms, metacercariae and eggs, respectively. For graphical representation of the data, \( {2^{{-\Delta \Delta {C_t}}}} \) was calculated as the final unit of Y-axis
Immunolocalization of CslysoPLA in parasites and infected cat’s liver
In the adult worms (Fig. 2a), specific fluorescence of CslysoPLA was detected in the vitelline gland, tegument, intestine and eggs (panels d and h), while could not be detected in negative control (panels b and f). In the metacercariae (Fig. 2a), CslysoPLA was also distributed in the tegument and vitelline gland (panel l), compared with negative control (panel j). In the intrahepatic bile duct of infected cat (Fig. 2b), specific fluorescence of CslysoPLA was observed in the lumen between adult worms and surrounding bile duct epithelia together with some inside the cells (panel o), compared with negative control (panel p).
Immunolocalization of CslysoPLA in adult worms and metacercariae of C. sinensis and infected cat’s liver. Rat anti-CslysoPLA serum was used as primary antibody and goat anti-rat IgG as secondary antibody. Rat preimmune serum was applied as negative control. a b, d, f, h, j, l Under fluorescence microscope and the same parts (a, c, e, g, i, k) under white light. b, f Negative control in adult worms; d, h localization of CslysoPLA in adult worms; j negative control in metacercariae; l localization of CslysoPLA in metacercariae. b o, p Under fluorescence microscope and the same images (m, n) under white light. p Negative control; o localization of CslysoPLA in intrahepatic bile duct of infected cat. v vitelline gland, t tegument, i intestine, e intrauterine egg, l lumen, w within the cells. The images were magnified at ×100 for adult worms and the cat’s liver and ×400 for metacercariae
Effects of rCslysoPLA on human hepatic stellate cell line LX-2 and human biliary epithelial cell line 5100
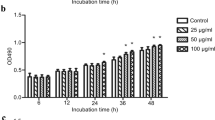
To be expected, rCslysoPLA stimulated significant growth of LX-2 at concentrations of 1 and 2 μg/ml for 48 h and at concentrations of 1 to 5 μg/ml for 72 h and induced apoptosis at concentrations of 10 and 20 μg/ml, compared with negative control (Fig. 3a, b). However, with the increasing concentration of rCslysoPLA (1 to 20 μg/mL), the biliary epithelial cells proliferated more and more significantly, in comparison with negative control (Fig. 3c, d).
Effects of rCslysoPLA on human hepatic stellate cell line LX-2 and human biliary epithelial cell line 5100 by CCK-8 assay. a, b LX-2 cells were treated with different concentrations of rCslysoPLA for 48 or 72 h. c, d Five thousand one hundred cells were treated with different concentrations of rCslysoPLA for 48 or 72 h. Growth is expressed as a ratio of cell numbers after incubation with rCslysoPLA compared with negative control (growth rate = 1.0). Different concentrations of rCslysoPLA were diluted in serum-free medium. Cells cultured in serum-free medium were applied as negative control. Each bar represents the mean value from three experiments with standard deviation (*P < 0.05)
Effects of rCslysoPLA on cell cycle distribution of LX-2
When LX-2 cells were treated with rCslysoPLA at concentrations of 1 and 2 μg/ml for 48 h and at concentrations of 1 to 5 μg/ml for 72 h, the cell number in the G1 phase decreased. Contrastly, the cell number in the S phase and G2/M phase, in which cells duplicated and proliferated rapidly, increased significantly, compared with negative control. However, when the cells were treated with rCslysoPLA at concentrations of 10 and 20 μg/ml for 48 or 72 h, the cells underwent apoptosis. The apoptotic peak was observed preceding the normal G1 region (Fig. 4).
Effects of rCslysoPLA on cell cycle distribution in LX-2 by flow cytometry. a Cell cycle distribution of LX-2 incubated with rCslysoPLA at concentrations of 1 and 10 μg/ml for 48 or 72 h. b Percentage of cells in each phase (G1, S and G2/M) of LX-2 treated with lower concentrations (1 to 5 μg/ml) of rCslysoPLA for 48 or 72 h. Different concentrations of rCslysoPLA were diluted in serum-free medium. Cells cultured in serum-free medium were used as negative control. Each bar represents the mean value from three experiments with standard deviation (*P < 0.05)
Effects of rCslysoPLA on LX-2 activation
The expression of activation markers normalized by β-actin was detected in LX-2, which was incubated with a series of concentrations of rCslysoPLA (1 to 5 μg/ml) for 48 h. The cells exposed to rCslysoPLA showed progressively increased expression in fibrogenic genes of α-SMA, collagen III, MMP 2 and TIMP II, in comparison with negative control. However, TIMP I displayed a downregulation (Fig. 5).
Effects of rCslysoPLA on LX-2 activation by quantitative RT-PCR. The expression levels of α-SMA, collagen III, MMP 2, TIMP I and TIMP II were detected in LX-2 after treatment with lower concentrations (1 to 5 μg/ml) of rCslysoPLA for 48 h. Different concentrations of rCslysoPLA were diluted in serum-free medium. Cells cultured in serum-free medium were applied as negative control. Each bar represents the mean value from three experiments with standard deviation (*P < 0.05)
Immunolocalization of CslysoPLA in infected rats’ livers
Mouse anti-CslysoPLA serum was used to recognize CslysoPLA in the livers of SD rats experimentally infected with metacercariae at 1 day and 10 weeks (Fig. 6). CslysoPLA was present in the adenomatoid hyperplastic tissue within intrahepatic bile duct of rat’s liver infected at 10 weeks (panel b), in comparison with negative control (panel a). Additionally, mouse anti-CslysoPLA serum showed no affinity for host lysoPLA as indicated by a lack of staining in rat’s liver infected at 1 day (panel c).
Immunolocalization of CslysoPLA in infected rats’ livers. a Probed with mouse preimmune serum. b A liver section from a rat infected at 10 weeks that was incubated with mouse anti-CslysoPLA serum. c A liver section from a rat infected at 1 day that was probed with mouse anti-CslysoPLA serum. Peroxidase staining was revealed as a yellow/rust coloured deposit and Mayer’s haematoxylin counterstained the nuclei in light purple. Black arrows highlight the regions of intrahepatic bile duct tissue and the tissue that stained positive for CslysoPLA. Original magnification of ×400
Discussion
Recently, parasite-derived chemical products have been manifested to promote proliferation of host cells and be involved in pathogenesis of parasitic diseases (Kim et al. 2008, 2009; Pak et al. 2009; Smout et al. 2009; Hu et al. 2009). Research by our laboratory illuminated that C. sinensis ESPs could promote significant proliferation of human hepatic stellate cell line LX-2 (unpublished results). Secretory type phospholipases, comprising lysoPLA, play key roles in virulence and pathogenesis of parasites and fungi (Wright et al. 2004; de Oliveira et al. 2004). In this study, CslysoPLA was cloned according to the previous paper (Ma et al. 2007), and its expression in the developmental stages and tissues of C. sinensis and potential contribution to hepatic fibrosis were investigated for the first time.
LysoPLA plays a pivotal role in modulating a variety of cellular reactions in phospholipid metabolism and, particularly, in regulating the cytotoxic lysoPLs concentrations in the cells by converting lysoPLs to glycerolphosphate derivative and fatty acids. Generally, lysoPLs are at very low concentrations (0.5–6 % of total membrane lipid weight) in biological membranes and are multifunctional phospholipid messengers, which have been proven to be cytotoxic at high concentrations but act as lipid second messengers at low non-toxic concentrations (Wang and Dennis 1999). Lysophosphatidic acid, the simplest naturally occurring lysoPL, is responsible for diverse biological responses, including platelet aggregation, cell proliferation and differentiation, smooth muscle contraction, focal adhesion assembly and stress fibre formation, growth cone collapse and neurite retraction (Moolenaar 1995). Thus, CslysoPLA is probably a safeguard to ensure the safety levels of lysoPLs and yet provides enough for a variety of biological functions.
In view of the fundamental part in regulating lysoPLs, CslysoPLA was supposed to express widely in developmental stages and tissues of C. sinensis. In the present study, quantitative RT-PCR experiments showed that CslysoPLA was detected in adult worms, metacercariae and eggs. Particularly, CslysoPLA presented the highest expression pattern at metacercariae stage. Metacercariae parasitize cold-blooded intermediate hosts, which provide a complete different environment from that encountered during adult stage. Metacercariae inhabiting the muscles of freshwater fishes are in a resting stage wherein they simply maintain a basal metabolic status. Three key enzymes involved in anaerobic glycolysis expressed lower at metacercariae stage (unpublished data). It is widely acknowledged that, except for gluconeogenesis, organisms mainly rely on fat mobilization to supply free fatty acids (FFA) as crucial energy source. Furthermore, it is likely that considerable FFA are needed to provide metacercariae with enough energy for development and growth, especially during the period of parasites’ invasion into definitive host when they are in need of a large amount of energy to transform into juvenile flukes and adults (Huang et al. 2011). Besides, in our recent paper on genome of C. sinensis (Wang et al. 2011), genes for the complete pathways for fatty acid metabolism were found, but key genes in fatty acid biosynthesis are missing from the genome. In other words, this parasite could not synthesize fatty acids de novo. Indeed, during the life cycle of C. sinensis, adult worms are unable to synthesize most of their own lipids de novo, particularly the long-chain fatty acids and cholesterol molecules (McManus and Bryant 1986), entailing the fact that the parasite has to depend on its host for these lipids. Adult worms thrive in bile juice, which is abundant in a variety of nutritions such as phospholipids, cholesterol and bile salt. It is known that phospholipids are components of blood or membrane of host cells. Previously, we confirmed that CslysoPLA, exhibiting both phospholipase A2 and lysoPLA activity, can catalyze both phospholipids and lysoPLs (Ma et al. 2007). In this case, CslysoPLA is able to convert phospholipids into FFA which are necessary for the development of parasites.
Relative high expression level of CslysoPLA at metacercariae stage consists with its immunolocalization in the cyst wall of metacercariae. Trematode tegument is a dynamic organ involved in host–parasite interaction and participates in nutrition, immune evasion and modulation, excretion, osmoregulation and signal transduction (Mulvenna et al. 2010). What is more, given the importance of cyst wall in protecting juveniles from being attacked by immune response of hosts (Wang et al. 2012), it seems that CslysoPLA might play a critical role in the parasite’s self-defence mechanism, owing to its possible involvement in the host cell plasma membrane disruption leading to penetration of host cells and cell lysis (Ghannoum 2000). In trematode, intestine is not only a major source of secretory protein but also a place for nutrition digestion and absorption (Lv et al. 2011). Coupled with the localization in the tegument as a feeding structure, CslysoPLA might participate in digesting phospholipids from host into FFA for energy supply. Moreover, CslysoPLA was observed in the vitelline gland and the eggs in the uterus. The trematode vitelline gland plays a key role in egg production by supplying eggshell materials, relevant enzymatic activity and nutrients to the fertilized ovum (Cai et al. 2008), indicative of the possible participation of CslysoPLA in egg production.
Intriguingly, CslysoPLA was localized not only in the lumen between adult worms and surrounding bile duct epithelia but also within the epithelial cells. The possibility that the parasite could secret CslysoPLA into the environment is supposed to occur in several ways. One potential role of CslysoPLA involves digesting phospholipids from host for nutritional purpose. Another possible function of CslysoPLA might be penetration and desquamation of mucosa of bile ducts, as adult parasites suck the blood of the host through mucosa of bile ducts (Rim 2005). However, it is still unclear about the mechanism of uptake or internalization of C. sinensis ESPs by host cells. It may be by simple diffusion or endocytosis (Ishii et al. 1990). In Schistosoma japonicum, cellular internalization of glutathione transferase (GST) occurs from the medium into mammalian cells via an endocytotic pathway based on clathrin-coated pits (Morris et al. 2009). Likewise, CslysoPLA might enter host cells via a similar mechanism along with GST. The presence of CslysoPLA within host cells is possibly due to the putative roles which internalized ESPs play in transforming host cells (Sripa et al. 2007). In Helicobactor pylori, by interacting with a kinase involved in cell polarity, the Cag A protein can drive gastric cells to proliferate and lead to malignant transformation in gastric cancer (Saadat et al. 2007). Similarly, internalized CslysoPLA might interfere with cellular signalling in order to modulate normal cellular signal transduction in the contaminated host cells. In the cellular reaction, phospholipase A2 regulates the provision of arachidonic acid to cyclooxygenase- and lipoxygenase-derived eicosanoids (Dong et al. 2006), and the upregulation of cyclooxygenase-2 is thought to be an important feature of cholangiocarcinogenesis in both human and experimental rodent models (Sirica et al. 2002). Considering the possibility in regulating cellular response and penetration of host cells, CslysoPLA might stimulate pathogenic pathways conducing to clonorchiasis.
Hepatic fibrosis is a wound-healing response to chronic liver injury. In normal liver, HSC is the major storage of vitamin A and situated in the space of Disse. Activation of HSCs is the central event of fibrogenesis, leading to accumulation of extracellular matrix (ECM). In response to liver injury, HSCs proliferate, lose lipid droplets, acquire a myofibroblastic phenotype with α-SMA as its marker and synthesize large amounts of ECM components, MMPs and TIMPs (Friedman 2004). Evidence for the role of HSC in parasitic diseases has been shown lately. In human and murine S. japonicum infection, HSCs have been identified in the periphery of egg granulomas, where myofibroblasts were found to be present in fibrotic patients with end-stage disease (Bartley et al. 2006). In a separate study, the existence of activated HSCs in human Schistosoma mansoni infection was also reported (Chang et al. 2006). In those studies identifying HSCs as key in granuloma formation, it is noteworthy that activated HSCs were found at the periphery of the granulomas and not in the immediate vicinity of the eggs. This is suggestive of a concentration gradient of egg-secreted antigens, descending toward the periphery of the granuloma. As a result, HSC activation occurs in areas of lower concentrations of egg antigens. Recently, the direct effects of egg-secreted antigens on the trans-differentiation status of HSC were investigated (Anthony et al. 2010). Similarly, C. sinensis might also secret antigens to mediate interplay with HSC. In the current study, the impact of rCslysoPLA on human hepatic stellate cell line LX-2 was investigated. As endotoxin can affect cell proliferation, triton X-114 was utilized to remove endotoxin from the recombinant protein. Endotoxin-eliminated rCslysoPLA showed proliferative effect on LX-2 at lower concentrations, while the protein induced apoptosis at higher concentrations. Such double effects on cells is corresponding to the previous research (Mora et al. 2005) and is also consistent with the impact of lower concentrations of parasite antigens on HSC activation in S. japonicum and S. mansoni infection as aforementioned.
What is more, quantitative RT-PCR analysis showed that α-SMA was upregulated in LX-2 treated with rCslysoPLA, collectively revealing that these cells underwent activation into proliferative and fibrogenic myofibroblast-like cells. In normal liver, the subendothelial space of Disse separates the epithelium (hepatocyte) from the sinusoidal endothelium. This space is composed of a basement membrane-like matrix, which contains non-fibril-forming collagens including types IV, VI and XIV. The so-called interstitial ECM in normal liver is confined to the capsule and around large vessels as well as in the portal areas, which comprises fibril-forming collagens (e.g. types III and I). When liver turns fibrotic, significant changes take place both quantitatively and qualitatively. The fibril-forming interstitial collagens III and I increase three- to eightfold (Rojkind et al. 1979), together with a conversion of the type of ECM in the space of Disse from the normal basement membrane-like matrix to interstitial type matrix. In this study, the expression level of collagen III in LX-2 became significantly higher after stimulation with rCslysoPLA. While the quantitative aspect may be conceivable in the light of increased de novo synthesis of almost all ECM components, the qualitative changes of ECM require additional mechanisms such as fibrolytic activities of MMPs. MMPs are a family of neutral proteinases with variable substrate spectra. In human hepatic diseases, there is a downregulation of MMP 1 (interstitial collagenase) and an upregulation of MMP 2 (gelatinase A) (Milani et al. 1994); thus, they can modulate ECM remodelling and might explain increased degradation of basement membrane collagens and decreased degradation of interstitial collagens. The activated MMPs are strictly regulated by TIMPs (TIMP I and TIMP II), which are relevant to progressive fibrosis. TIMPs are upregulated relative to MMP 1, giving rise to decreased degradation of interstitial collagens and formation of hepatic fibrosis (Herbst et al. 1997). In the present study, the upregulation of MMP 2 and TIMP II in LX-2 indicated that the cells were activated by rCslysoPLA. However, CslysoPLA alone did not enhance TIMP I, implying that either there are other parasite antigens stimulating TIMP I or, alternatively, there is only one kind of TIMP (either TIMP I or TIMP II) predominating over a certain period of time.
Meanwhile, we showed that CslysoPLA is probably contributory in promoting proliferation of human biliary epithelial cell line 5100. This agrees with previous study, which showed that C. sinensis ESPs stimulated proliferation of human epithelial cell line HEK293 by inducing E2F1 expression, and may play an important part in the development of cholangiocarcinoma via the overgrowth of the bile duct epithelia (Kim et al. 2008). We also demonstrated that CslysoPLA was localized in the adenomatoid hyperplastic tissue in the intrahepatic bile duct of the infected rat. The adenomatoid hyperplastic tissues are close to fibrosis regions, which display along intrahepatic bile ducts and initiate at the hepatic sinusoids. This is the first report of a secreted lysoPLA from a parasitic worm that was present in adenomatoid hyperplastic tissue. Adenomatoid hyperplasia is formed by malignant transformation of biliary cells, and the biliary cells might have internalized CslysoPLA, which may explain the existence of CslysoPLA in the adenomatoid hyperplastic tissue. Proliferative responses, including epithelial hyperplasia and adenomatoid hyperplasia, may represent predisposing lesions that enhance susceptibility of DNA to carcinogens. DNA damage in biliary epithelial cells results in genetic alterations which may become fixed and lead to malignant transformation (Thamavit et al. 1978). Taken together, epithelial hyperplasia and adenomatoid hyperplasia may conduce to the cell proliferation-mediated cholangiocarcinogenesis. Further studies are required to pay attention to the precise mechanism of CslysoPLA in C. sinensis-associated cholangiocarcinogenesis.
Overall, we demonstrated that CslysoPLA was possibly responsible for the pathogenesis of hepatic fibrosis caused by C. sinensis. In the infected cat’s liver, CslysoPLA was not only recognized in the lumen between adult worms and surrounding biliary epithelial cells but also internalized by host cells. By promoting proliferation and activation of HSCs, CslysoPLA might be a pathogenic factor of C. sinensis-related human hepatic fibrosis. Moreover, CslysoPLA served as one of the mitogens on human biliary epithelia and was discovered in the adenomatoid hyperplastic tissue within the intrahepatic bile duct of infected rat’s liver, laying the foundation for further studies on the association between CslysoPLA and cholangiocarcinoma. This is a study to characterize the parasite antigen in a well-validated human hepatic stellate cell line LX-2 and human biliary epithelial cell line 5100, which may provide new insights in understanding the pathogenesis of a common parasitic disease that afflicts people in a number of poor countries and facilitate targeted chemotherapy and drug design in the clinical aspects.
References
Anthony B, Mathieson W, de Castro-Borges W, Allen J (2010) Schistosoma mansoni: egg-induced downregulation of hepatic stellate cell activation and fibrogenesis. Exp Parasitol 124:409–420
Bartley PB, Ramm GA, Jones MK, Ruddell RG, Li Y, McManus DP (2006) A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol 36:993–1001
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, EI Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens—part B: biological agents. Lancet Oncol 10:321–322
Cai GB, Bae YA, Kim SH, Sohn WM, Lee YS, Jiang MS, Kim TS, Kong Y (2008) Vitellocyte-specific expression of phospholipid hydroperoxide glutathione peroxidases in Clonorchis sinensis. Int J Parasitol 38:1613–1623
Chang D, Ramalho LN, Ramalho FS, Martinelli AL, Zucoloto S (2006) Hepatic stellate cells in human schistosomiasis mansoni: a comparative immunohistochemical study with liver cirrhosis. Acta Trop 97:318–323
de Oliveira D, de Souza TA, Murate LS, Jankevicius JV, Gaziri LC, Jankevicius SI (2004) Protease and phospholipase inhibition protect Veneza zonata (Hemiptera Coreidae) against septicemia caused by parasite trypanosomatid 563DT. J Invertebr Pathol 85:9–17
Dong Q, Patel M, Scott KF, Graham GG, Russell PJ, Sved P (2006) Oncogenic action of phospholipase A2 in prostate cancer. Cancer Lett 240:9–16
Friedman SL (2004) Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1:98–105
Gao GH, Wang YZ, Wu F, Jin XX, Yuan T (1994) Study on experimental pathology of Clonorchis sinensis. Chin J Parasitic Dis Control 7:197–200
Ghannoum MA (2000) Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 13:122–143
Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D (1997) Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol 150:1647–1659
Hu F, Hu X, Ma C, Zhao J, Xu J, Yu X (2009) Molecular characterization of a novel Clonorchis sinensis secretory phospholipase A(2) and investigation of its potential contribution to hepatic fibrosis. Mol Biochem Parasitol 167:127–134
Hu Y, Huang L, Huang Y, He L, Zhang F, Li W, Liang P, Li R, Sun J, Wang X, Liang C, Li X, Yu X (2012) Molecular cloning, expression, and immunolocalization of protein disulfide isomerase in excretory–secretory products from Clonorchis sinensis. Parasitol Res 111:983–989
Huang L, Hu Y, Huang Y, Fang H, Li R, Hu D, Li W, Li X, Liang C, Yu X (2011) Gene/protein expression level, immunolocalization and binding characteristics of fatty acid binding protein from Clonorchis sinensis (CsFABP). Mol Cell Biochem 363:367–376
Ishii M, Vroman B, LaRusso NF (1990) Fluid-phase endocytosis by intrahepatic bile duct epithelial cells isolated from a normal rat liver. J Histochem Cytochem 38:515–524
Kim YJ, Choi MH, Hong ST, Bae YM (2008) Proliferative effects of excretory/secretory products from Clonorchis sinensis on the human epithelial cell line HEK293 via regulation of the transcription factor E2F1. Parasitol Res 102:411–417
Kim YJ, Choi MH, Hong ST, Bae YM (2009) Resistance of cholangiocarcinoma cells to parthenolide-induced apoptosis by the excretory-secretory products of Clonorchis sinensis. Parasitol Res 104:1011–1016
Liang C, Hu XC, Lv ZY, Wu ZD, Yu XB, Xu J, Zheng HQ (2009) Experimental establishment of life cycle of Clonorchis sinensis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 27:148–150
Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY (2005) Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis 5:31–41
Lv X, Chen W, Wang X, Li X, Sun J, Deng C, Men J, Tian Y, Zhou C, Lei H, Liang C, Yu X (2011) Molecular characterization and expression of a cysteine protease from Clonorchis sinensis and its application for serodiagnosis of clonorchiasis. Parasitol Res 110:2211–2219
Ma C, Hu X, Hu F, Li Y, Chen X, Zhou Z, Lu F, Xu J, Wu Z, Yu X (2007) Molecular characterization and serodiagnosis analysis of a novel lysophospholipase from Clonorchis sinensis. Parasitol Res 101:419–425
McManus DP, Bryant C (1986) Biochemistry and physiology of Echinococcus. In: Thompson RCA (ed) The biology of Echinococcus and hydatid disease, 1st edn. George Allen and Unwin, London
Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabro A, Ciancio G, Stefanini F, Burroughs AK, Surrenti C (1994) Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol 144:528–537
Moolenaar WH (1995) Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem 270:12949–12952
Mora R, Valverde B, Díaz C, Lomonte B, Gutiérrez JM (2005) A Lys49 phospholipase A2 homologue from Bothrops asper snake venom induces proliferation, apoptosis and necrosis in a lymphoblastoid cell line. Toxicon 45:651–660
Morris MJ, Craiq SJ, Sutherland TM, Board PG, Casarotto MG (2009) Transport of glutathione transferase-fold structured proteins into living cells. Biochim Biophys Acta 1788:676–685
Mulvenna J, Moertel L, Jones MK, Nawaratna S, Lovas EM, Gobert GN, Colgrave M, Jones A, Loukas A, McManus DP (2010) Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol 40:543–554
Pak JH, Kim DW, Moon JH, Nam JH, Kim JH, Ju JW, Kim TS, Seo SB (2009) Differential gene expression profiling in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory–secretory products. Parasitol Res 104:1035–1046
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Rim HJ (2005) Clonorchiasis: an update. J Helminthol 79:269–281
Rojkind M, Giambrone MA, Biempica L (1979) Collagen types in normal and cirrhotic liver. Gastroenterology 76:710–719
Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M (2007) Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447:330–333
Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI (2002) Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis 22:303–313
Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, Loukas A (2009) A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog 5:e1000611
Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ (2007) Liver fluke induces cholangiocarcinoma. PloS Med 4:e201
Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S (1978) Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res 38:4634–4639
Wang A, Dennis EA (1999) Mammalian lysophospholipases. Biochim Biophys Acta 1439:1–16
Wang X, Chen W, Huang Y, Sun J, Men J, Liu H, Luo F, Guo L, Lv X, Deng C, Zhou C, Fan Y, Li X, Huang L, Hu Y, Liang C, Hu X, Xu J, Yu X (2011) The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol 12:R107
Wang X, Chen W, Lv X, Tian Y, Men J, Zhang X, Lei H, Zhou C, Lu F, Liang C, Hu X, Xu J, Wu Z, Li X, Yu X (2012) Identification and characterization of paramyosin from cyst wall of metacercariae implicated protective efficacy against Clonorchis sinensis infection. PLoS One 7:e33703
Wright LC, Payne J, Santangelo RT, Simpanya MF, Chen SC, Widmer F, Sorrell TC (2004) Cryptococcal phospholipases: a novel lysophospholipase discovered in the pathogenic fungus Cryptococcus gattii. Biochem J 384:377–384
Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ (2005) Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54:142–151
Yoo WG, Kim TI, Li S, Kwon OS, Cho PY, Kim TS, Kim K, Hong SJ (2009) Reference genes for quantitative analysis on Clonorchis sinensis gene expression by real-time PCR. Parasitol Res 104:321–328
Zheng M, Hu K, Liu W, Hu X, Hu F, Huang L, Wang P, Hu Y, Huang Y, Li W, Liang C, Yin X, He Q, Yu X (2011) Proteomic analysis of excretory secretory products from Clonorchis sinensis adult worms: molecular characterization and serological reactivity of a excretory–secretory antigen-fructose-1,6-bisphosphatase. Parasitol Res 109:737–744
Acknowledgments
This work was supported by the National Key Basic Research and Development Project of China (973 project; no. 2010CB530000), National Natural Science Foundation of China (no. 81101270 and no. 81171602), China Postdoctoral Science Foundation (no. 20110490952), Fundamental Research Funds for the Central Universities (no. 3164015) and the National Important Sci-tech Special Projects (no. 2012ZX10004220).
Author information
Authors and Affiliations
Corresponding author
Additional information
Fan Zhang and Pei Liang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, F., Liang, P., Chen, W. et al. Stage-specific expression, immunolocalization of Clonorchis sinensis lysophospholipase and its potential role in hepatic fibrosis. Parasitol Res 112, 737–749 (2013). https://doi.org/10.1007/s00436-012-3194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3194-1