Abstract
Dengue virus (DV) is the most rapidly spreading arbovirus in the world. Our previous studies indicated that Rac1, a kind of Rho GTPase, was related with the increased vascular permeability in DV infection. However, the molecular mechanisms that regulate the activity of the Rac1 pathway during DV infection is not fully understood yet. Recently, Rho-specific guanine nucleotide dissociated inhibitors (Rho GDIs), as a pivotal upstream regulator of Rho GTPase, attract our attention. To identify the role of GDI-1 in DV2 infection, the expression of GDI in Eahy926 cells was detected. Moreover, a GDI-1 down-regulated cell line was constructed to explore the correlation between GDI-1 and Rac1 and to further evaluate the function of GDI in DV life cycle. Our results indicated that DV2 infection could up-regulate GDI-1 expression, and down-regulation of GDI enhanced the activity of Rac1. In addition, down-regulated GDI-1 significantly inhibited all steps of DV2 replication cycle. GDI-1 plays an important role in DV2 infection via negatively regulating the activation of the Rac1-actin pathway. These results not only contribute to our further understanding of the pathogenesis of severe dengue but also provide further insight into the development of antiviral drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue is the most clinically common arbovirus infection in the world, and is caused by dengue virus (DV) from the genus Flavivirus in the family Flaviviridae. According to the recent epidemiologic studies, 390 million individuals are infected with DV globally per year, of which 96 million cases are symptomatic [1]. There are four related but distinct serotypes (DV 1–4). Infection with any of them can lead to the self-limited flu-like dengue fever (DF), and some DF patients might develop severe dengue, a potentially lethal complications previously known as dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS). Haemorrhage is one of typical manifestations for severe dengue, which is characterized with increased vascular permeability and plasma leakage. Usually, kinds of chemokines and cytokines released from dendritic cells and monocytes upon infection can activate the endothelium and then disrupt the endothelial barrier, which are contributed to the DV-induced vascular permeability. Additionally, recent studies found that DV can also infect endothelial cell and directly contribute to viremia and endothelial cell dysfunction [2,3,4]. However, except for the outcome, the mechanism that triggers DV entry and replicate in endothelial cells is as yet only poorly understood.
As we know, viruses depend on the host cellular machinery to replicate, and this process includes virus-cell interactions involving many cellular components [5,6,7,8,9]. Generally, Rho GTPases are master regulator which involve in a variety of cellular functions, including cytoskeletal dynamics and vesicle traffic [10] in a number of cell types, such as endothelial cells. In our previous studies, we mainly investigated the role of Rho GTPases, especially Rac1, in the process of dengue infection [11]. We found that the Rac1-microfilament signal pathway was involved in the life cycle of DV2 in an endothelium-like Eahy926 cells. Our results indicated that the activities of Rac1 decreased during the early phrase and gradually increased at the late phase of infection [11]. Accordingly, Rac1 activation likely serves as an important regulating factor in the DV-induced morphological changes in endothelial cells, which subsequently cause the vascular leakage observed in severe dengue. Thus, clarifying the molecular mechanisms that regulate the activity of the Rac1 pathway during DV infection is necessary.
Usually, Rho GTPases exert their function by a cycling between an active GTP-load state and an inactive GDP-load state, which are mainly regulated by three classes of proteins. Rho guanine nucleotide exchange factors (Rho GEFs) can facilitate the exchange of GDP for GTP, which convert Rho GTPase to their active state. Conversely, Rho GTPase activating proteins (Rho GAPs) catalyze the hydrolysis of GTP to GDP to inhibit Rho GTPase function. Besides GFFs and GAPs, a set of Rho specific guanine nucleotide dissociated inhibitors (Rho GDIs) act as a pivotal upstream regulator of Rho GTPase. Three species of Rho GDIs have been identified, namely, GDI-1, GDI-2 and GDI-3 [12, 13]. GDI-1 (also known as GDIα), the ubiquitously expressed form, is the best characterized GDI family member, while GDI-2 (also known as D4-GDI or Ly-GDI or GDIβ) is predominantly found in lymphocytic and hematopoietic tissues [14, 15], and GDI-3 (also known as GDIγ), the most divergent of the three, usually distributes in individual organs (such as brain and pancreas, etc.) [16]. The main function of GDIs is to block GDP dissociation from Rho GTPases, which maintains the Rho GTPases in inactive complex. Thus, as a primary modulator for limiting Rho GTPase activation [17], Rho-GDI interactions involve in many pathogenic process in vivo. For instance, the over-expression of GDIs induces the disruption of Rho-dependent cellular activities, including the organization of actin cytoskeleton and the loss of stress fibers and focal contact sites [18]. Because many studies have demonstrated that GDIs play a critical role in regulating cancer cell invasiveness by modulating the activities of Rho proteins and actin, GDIs have been considered as drug targets for cancer treatment [19,20,21,22]. In addition, a lymphoid-specific GDI-2 may also serve as a cytoskeletal-localized regulator at specific intracellular locations of platelets in a PKC-dependent manner [23]. However, only a few studies have demonstrated the involvement of GDIs in viral infections. Rho GDI-2 has been implicated in negatively regulating HIV-1 replication by weakening the activities of Rac1 and RhoA [24]. Kramer et al. reported that tobacco mosaic virus (TMV) infection could alter the localization of GDI-2 from the cytoplasm to ER-associated complexes. Additionally, partial silencing of GDI-2 significantly increases TMV infection [25]. However, little is known regarding whether GDI-1 plays a role in DV infection via regulating the activities of Rac1.
In the present study, the endothelium-like cell line, EAhy926, was used as a cell model to investigate the effect of GDI-1 on DV2 infection. We found that DV2 infection induced the up-regulation of GDI-1 and the down-regulation of active Rac1 during the early phase of infection, which promoted DV2 entry. GDI-1 knock-down inhibited all steps of DV2 replication cycle. Because increased Rac1 activities and disrupted actin filaments were detected in EAhy926 cells which GDI-1 level was down-regulated, we suggest that GDI-1 plays an important role in DV2 infection via negatively regulating the activation of the Rac1-actin pathway. Taken together, these results not only contribute to our further understanding of the pathogenesis of severe dengue but also provide further insight into the development of antiviral drugs.
Materials and methods
Cells and virus
The Aedes albopictus C6/36 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 28 °C. Vero cells were grown in minimal essential medium (MEM) supplemented with 5% FBS at 37 °C. Vascular endothelial cell-like EAhy926 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 15% FBS.
DV2 (Tr1751), isolated from a patient with dengue fever, was propagated in C6/36 cells and stored at − 80 °C until use. Virus titers were determined by plaque assay on Vero cells. In addition, no patient with dengue fever was involved in the study.
Antibodies and chemicals
Mouse anti-DV2 E monoclonal antibodies (mAbs) were kindly provided by Dr. XY Che (Zhujiang Hospital, Southern Medical University, Guangzhou, China). Rabbit anti-GAPDH pAbs were purchased by Bioworld Technology (China). Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (IgG) and HRP-conjugated goat anti-rabbit IgG were purchased from Dingguo Changsheng Biotechnology (China). Rabbit-anti-GDI mAb was purchased from Abcam (Britain). Lipofectamine was purchased from Invitrogen (USA). And the FBS was purchased from Gibico.
Detection of GDI expression levels after DV2 infection
EAhy926 cells were infected with DV2 (MOI = 10). At different time points after infection, total RNAs were obtained for the determination of GDI RNA levels by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) with specific primers (GDI-1–F: 5′-GGTATTGTCCTGCCTCTG-3′, GDI-1–R: 5′-CTTGGTCCCTTGTTTGTT-3′). Meanwhile, GDI expression levels in the lysates were determined by Western blotting. The blots were incubated with anti-GDI antibody (1:1000) at 4 °C overnight, followed by the addition of HRP-conjugated goat anti-rabbit IgG (1:2000). Subsequently, the protein levels were examined by ECL luminous intensities.
Generation of EAhy926 cells with down-regulated GDI expression by RNA interference (RNAi)
RNAi was used to target a GDI nucleotide sequence from 445 to 463 (5′-TGAAGGAGGGTGTGGAGTA-3′). The GDI-RNAi plasmid (pGDI-RNAi) was constructed using the EASY-shRNA eukaryotic expression vector pGV248 from GeneChem (China). And a negative control vector (pGDI-cont) was constructed similarly with an unrelated shRNA sequence (5′-TTCTCCGAACGTGTCACGT-3′). Then EAhy926 cells were transfected with 4 μg of pGDI-RNAi or pGDI-cont plasmid, respectively. At 48 h after transfection, the medium was replaced with fresh DMEM with 15% FBS. For the screening, the cells were cultured in medium containing 3 μg/ml of puromycin dihydrochloride at 37 °C for 14 days, and then single cell colonies carrying pGDI-RNAi or pGDI-cont were obtained and named EAhy-GDI-RNAi and EAhy-GDI-cont, respectively. After being identified by Western blotting and qRT-PCR, the cells were used for investigating the effect of GDI on DV2 infection.
Effect of GDI on DV2 infection
EAhy-GDI-RNAi and EAhy-GDI-cont cells were infected with DV2 (MOI = 10) at 37 °C for 1 h, and then the supernatants and cell lysates were collected at 1 h, 4 h and 8 h after infection. Plaque assay and Western blotting were used to detect the viral titers and E protein expressions, respectively, to analyze the effect of GDI on DV2 infection. The viral titer in EAhy-GDI-cont cells was considered 100%. Three independent experiments were performed for each group at each time point.
Analysis of Rac1 GTPase expression and of actin organization in EAhy-GDI-RNAi and EAhy-GDI-cont cells
The Rac1 GTPase expression were detected by immunohistochemistry as described previously [11, 26]. Activated Rac1 was identified by binding specifically to the GST-fused Rac/cdc42-binding domain (CRIB) of human p21 activated kinase 1 protein (PAK1). EAhy-GDI-RNAi and EAhy-GDI-cont cells grown on coverslips were fixed and blocked. Then, the cells were incubated with GST-CRIB (expressed in E.coli, 10 μg/mL) at 4 °C overnight. Subsequently, GST-CRIB was detected using anti-GST antibody (1:100) and HRP-conjugated secondary antibody. Meanwhile, total proteins of the cells were also collected for the detection of Rac1 GTPase expression by Western blotting.
Moreover, morphology of actin in EAhy-GDI-RNAi and EAhy-GDI-cont cells was revealed by incubation with TRITC-conjugated phalloidine (1:100, 37 °C, 1 h, Sigma) [26]. Three independent experiments were performed for each group.
Statistical analysis
A statistical analysis was performed using SPSS 16.0 software. The quantitative data between two groups were compared using Student’s t-test. Differences among the groups were considered significant at p < 0.05.
Results
DV2 infection induced the up-regulation of GDI-1 expression
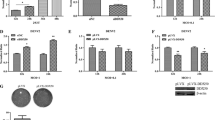
At different time points after DV2 infection, total RNA and proteins were extracted from EAhy926 cells to detect the levels of GDI-1 mRNA and protein. As shown in Fig. 1, the level of GDI-1 mRNA increased by 16.0-fold at 20 min and reached 18.5-fold at 1 h, which were significantly different from those in mock-infected cells (p < 0.01). Then, mRNA levels of GDI-1 reverted, but still maintained high levels at 8 h and 24 h, respectively (p < 0.05, Fig. 1a). Accordingly, the markedly elevated GDI-1 expression was also observed at 20 min and 1 h, and then it showed a gradually decreasing trend, maintaining high levels at 4 h, 8 h and 24 h (Fig. 1b). These results indicated that DV2 infection induced the up-regulation of GDI-1 expression.
Expression of GDI-1 in DV2-infected EAhy926 cells. a, b The lysates of DV2-infected EAhy926 cells were collected at different time points as indicated after infection, and the levels of GDI-1 mRNA and protein were analyzed by qRT-PCR and Western blotting, respectively. The data of qRT-PCR represent the mean ± SD from three independent experiments relative to mock-infected cells (**p < 0.01; *p < 0.05 vs. mock infection). GAPDH was used as loading control
Down-regulation of GDI disrupted Rac1-actin pathway
To further analyze the effects of GDI-1 on DV2 infection, we established a cell line with down-regulated GDI-1 expression using RNAi. The cell line was identified and named EAhy-GDI-RNAi. Significantly reduced levels of GDI-1 mRNA and protein were observed in EAhy-GDI-RNAi cells (Fig. 2a and b, p < 0.01), in which Rac-1 (GTP-Rac1) activity clearly enhanced (Fig. 2c and d, p < 0.05), and actin filament mainly distributed in the peri-nucleus compared with that in the control cell line, which was named EAhy-GDI-cont (Fig. 2e). These findings indicated that down-regulation of GDI-1 disrupted Rac1-actin pathway and that our cells could be used in the subsequent experiments.
Identification of EAhy-GDI-RNAi and EAhy-GDI-cont cells line, and analysis of Rac-1 (Rac1-GTP) activity and actin organization in those cells. a The mRNA expression levels of GDI-1 were analyzed by qRT-PCR in EAhy-GDI-RNAi and EAhy-GDI-cont cells (**p < 0.01 vs. EAhy-GDI-cont cells). The data represent the mean ± SD from three independent experiments relative to EAhy-GDI-cont cells. b Expression of GDI-1 protein was detected by Western blotting in EAhy-GDI-RNAi and EAhy-GDI-cont cells (**p < 0.01 vs. EAhy-GDI-cont cells). GAPDH was used as loading control. c Rac1 activity in EAhy-GDI-RNAi was detected by in situ detection with GST-CRIB, and activated Rac1 (GTP-Rac1) was mainly distributed in the peri-nucleus compared with EAhy-GDI-cont cells (× 200). d Elevated Rac-1 activity (GTP-Rac1) was detected in EAhy-GDI-RNAi cells by Western blotting compared with EAhy-GDI-cont cells (p < 0.05 vs. EAhy-GDI-cont cells). e Actin organization was detected by TRITC-conjugated phalloidine in EAhy-GDI-RNAi cells and EAhy-GDI-cont cells (Bar = 1 mm)
GDI-1 was involved in the DV2 life cycle
The lysates of EAhy-GDI-RNAi and EAhy-GDI-cont cells were collected at 1 h after infection to investigate the effect of GDI-1 on DV2 entry. The viral titer in EAhy-GDI-cont cells was considered 100%. As shown in Fig. 3a, the virus entry markedly decreased to 51% in EAhy-GDI-RNAi cells and significantly different from that in EAhy-GDI-cont cells (p < 0.01), indicating that GDI-1 is required for DV2 entry.
The effect of GDI-1 on DV2 infection. a The lysates of EAhy-GDI-RNAi and EAhy-GDI-cont cells were collected at 1 h after infection and the entry of DV2 was determined by plaque assay (n = 3). b The lysates of EAhy-GDI-RNAi and EAhy-GDI-cont cells were collected at 4 h and 8 h after infection and expression levels of E protein were detected by Western blotting. GAPDH was used as loading control. c, d The lysates or supernatants of EAhy-GDI-RNAi and EAhy-GDI-cont cells were collected at 4 h and 8 h after infection for determining the DV2 titers by plaque assay. The titers in control cells (EAhy-GDI-cont) were considered as 100% (n = 3). e The ratios of extra- to intracellular viral titer are shown as percentages. The data represent the mean ± SD from three independent experiments (**p < 0.01; *p < 0.05 vs. EAhy-GDI-cont cells)
Next, the lysates of EAhy-GDI-RNAi and EAhy-GDI-cont cells were collected for the detection of E protein expression. As shown in Fig. 3b, the down-regulation of GDI-1 led to reduced E protein expression levels at 4 h and 8 h. Moreover, the lysates and supernatants of EAhy-GDI-RNAi and EAhy-GDI-cont cells were also harvested to evaluate the intra- and extracellular viral titers at 4 h and 8 h after infection. The titers in control cells (EAhy-GDI-cont) were considered as 100%. The intracellular viral titers in EAhy-GDI-RNAi cells decreased to 21% of the control cells at 4 h and 28% at 8 h, respectively; while the extracellular viral titers in EAhy-GDI-RNAi cells decreased to 13% at 4 h and 10% at 8 h, respectively. The changes of intra- and extracellular viral titers showed significantly different with those in EAhy-GDI-cont cells (Fig. 3c and d, p < 0.01). The ratios of extra- to intracellular viral titers decreased to 62% at 4 h and 35% at 8 h (Fig. 3e, p < 0.01). These findings indicated that GDI-1 is required for the replication, assemble and release of DV2 and that GDI-1 down-regulation significantly inhibited all steps of the DV2 replication cycle.
Discussion
According to the reports of WHO, the incidence of dengue has grown dramatically around the world in recent decades [27]. Although, Dengvixia, the first commercial DV vaccine, was licensed in more than ten countries, it shows the risk of severe disease in non-immunes and those less than nine years old. In addition, there is no specific antiviral treatment against DV infection, and the pathogenesis of severe dengue, which is characterized with increased vascular permeability and hemorrhage, is not fully understood yet. Thus it is imperative to explore the mechanism that trigger the dysfunction of vascular endothelium in severe dengue.
As a kind of obligate intracellular parasite, viruses must commandeer the cellular machinery of host cells to complete their replication cycle and produce progeny viruses. Previously, we demonstrated that the reorganization of actin filaments and the altered activities of Rac1 were involved in DV2 infection in ECV304 cells and EAhy926 cells [11, 26]. Rac1, as a member of the Rho GTPase family, have also been proved to play important roles in many virus infections via the regulation of actin organization [28,29,30,31]. Despite the critical roles of Rho GTPases and their regulation in the rearrangement of cytoskeleton in the process of viral replication, many relevant Rho GTPase regulatory proteins remain uncharacterized for their effect in dengue infection. As mentioned above, three major regulators of the Rho GTPases cycle have been identified: GEFs, GAPs and Rho GDIs. Especially, the Rho GDIs, which operate in the background, might serve as an “invisible hand” to regulate the Rho-GTPase cycle through sequestering the inactivated GDP form of GTPases in the cytosol [13]. In addition, GDIs can also protect GTPase from degradation [32].
Generally, Rho GDIs have been proved to be associated with cell differentiation. Previous studies primarily focused on the relationship between RhoGDIs and the development of cancers [33]. Most of these reports demonstrated that Rho GDIs plays dual opposite roles as both promoters and metastasis suppressors in different tissues during tumor progression [19, 21, 34,35,36,37,38]. Thus, Rho GDIs may be useful as a diagnostic biomarker and/or a therapeutic target for tumor.
However, few reports have examined the role of Rho GDIs in virus infection. A study by Watanabe et al. showed [24] that the modest up-regulation of the Rho GDI-2, the hematopoietic cell-specific form, attenuated HIV-1 infection at the Env-dependent early phase of the viral life cycle in various T cell lines. However, this up-regulation appeared to have no effect on cell proliferation, indicating that Rho GDI might be a potential target for the treatment of HIV-1 infection. Furthermore, transfection with several HIV proteins, such as Tat or Nef, was observed to down-regulate the expression of the Rho GDIs [39, 40], which might be responsible for continuous HIV production. Another study reported that Rho GDI-2 was an essential component of the vesicle trafficking pathways of TMV [25].
In addition to these findings, in the present study, we mainly focused on the role of Rho GDI-1, the most ubiquitously expressed form, in DV2 infection. We found that DV2 infection significantly enhanced the expression levels of Rho GDI mRNA and protein in EAhy926 cells, which accompanied the viral replication cycle, suggesting a linkage between Rho GDI-1 levels and DV2 infection (Fig. 1). Then, we evaluated the variation in GDI-1 expression levels during the early phase of infection, and the down-regulation of GDI-1 caused an inhibitory effect on various steps of the DV2 life cycle (Fig. 3).
Additionally, we provided further evidence to demonstrate the molecular mechanisms that regulate the activity of the Rac1-actin pathway in the DV2 replication cycle. In parallel with our previous investigation [11], the dramatically enhanced GDI-1 level observed in present study (Fig. 1) is accompanied with the reduced activities of the Rac1, which indicated that both the up-regulation of GDI-1 expression and the down-regulation of Rac1 activities are necessary for the entry of DV2 into EAhy926 cells. Moreover, GDI-1 down-regulation enhanced the activities of Rac1 in EAhy-GDI-RNAi cells (Fig. 2c and d). Considering these results, we suggested that GDI-1 promoted DV2 entry by negatively regulating the activities of Rac1 during the early phase of infection. However, at 4 h and 8 h after infection, the GDI-1 levels in EAhy926 cells gradually decreased but remained high, accompanied by no obvious changes in the activities of Rac1 [11]. Simultaneously, significantly decreased E protein levels, intra- and extra-cellular viral titers, ratios of extra- to intracellular viral titers were also observed in DV2-infected EAhy-GDI-RNAi cells during this period (Fig. 3). These findings indicate that GDI-1 down-regulation inhibits the steps of the viral replication and release, and GDI-1 plays a more important role in DV2 infection than Rac1. Thus, taken together, GDI-1 may be a novel target for limiting DV infection.
According to our results and to other reports, we infer some possible regulatory roles of GDI-1 during DV2 infection. During the early phase of infection, interactions between DV2 and the receptors on the surface of host cells triggered the up-regulation of GDI-1 expression, and then Rac1 activity could be down-modulated by sequestering Rho GTPases in the GDI-bound form in the cytosol. The reduced Rac1 activity led to an enhanced viral entry into EAhy926 cells via regulating the reorganization of actin filaments, which is a key factor involved in the endocytosis and phagocytosis of several pathogens [41, 42]. Alternatively, the activated GDI-1 and Rac1 pathway may affect the normal function of the plasma membrane by gathering viral receptor proteins and actin filaments at local sites, facilitating virus entry. Our results revealed that DV2 creates a suitable microenvironment by manipulating cellular machines to establish effective infection during the early phase of infection. However, the changes in GDI-1 and active Rac1 were not coordinated at 4 h and 8 h. According to our previous results, the active Rac1 levels gradually returned to the control levels [11], while GDI-1 still maintained a higher expression levels than that in mock-infection at 4 h and 8 h. The continuously enhanced GDI-1 levels observed during DV2 infection lead to the reorganization of actin filaments and to the loss of cell to cell adhesion by regulating the activities of Rac1 [43], which further interfere with cellular junctions and then contribute to the pathogeneses of severe DF by increasing vascular permeability. Therefore, during the early phase of infection, down-regulating GDI-1, which is an up-stream regulator of the GDI-Rac1-actin pathway, may be a feasible strategy for limiting viral entry and DF progression.
In summary, the present study demonstrated that the GDI-Rac1-actin pathway has a complex role in the DV2 replication cycle. Increased GDI-1 expression and decreased Rac1 activity facilitates virus entry during the early phase of infection. These results suggested that GDI-1 is involved in DV2 entry and replication by regulating Rac1 activity and actin reorganization. Overall, this study revealed a mode by which the GDI-1–Rac1–actin pathway can be co-opted by DV2 to create a microenvironment for establishing successful infection. Insights into the DV2-host interaction, such as those provided in this study, not only aid in the design of novel anti-viral drugs but also contribute to our understanding of viral pathogenesis.
References
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496(7446):504–507. https://doi.org/10.1038/nature12060
Vervaeke P, Vermeire K, Liekens S (2015) Endothelial dysfunction in dengue virus pathology. Rev Med Virol 25(1):50–67. https://doi.org/10.1002/rmv.1818
Dalrymple NA, Mackow ER (2012) Roles for endothelial cells in dengue virus infection. Adv Virol 2012:840654. https://doi.org/10.1155/2012/840654
Srikiatkhachorn A, Kelley JF (2014) Endothelial cells in dengue hemorrhagic fever. Antiviral Res 109:160–170. https://doi.org/10.1016/j.antiviral.2014.07.005
Angelini MM, Akhlaghpour M, Neuman BW, Buchmeier MJ (2013) Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. doi: https://doi.org/10.1128/mBio.00524-13
Gondar V, Molina-Jimenez F, Hishiki T, Garcia-Buey L, Koutsoudakis G, Shimotohno K, Benedicto I, Majano PL (2015) Apolipoprotein E, but Not Apolipoprotein B, is essential for efficient cell-to-cell transmission of Hepatitis C virus. J Virol 89(19):9962–9973. https://doi.org/10.1128/JVI.00577-15
Stein KR, Gardner TJ, Hernandez RE, Kraus TA, Duty JA, Ubarretxena-Belandia I, Moran TM, Tortorella D (2019) CD46 facilitates entry and dissemination of human cytomegalovirus. Nat Commun 10(1):2699. https://doi.org/10.1038/s41467-019-10587-1
Mailler E, Waheed AA, Park SY, Gershlick DC, Freed EO, Bonifacino JS (2019) The autophagy protein ATG9A promotes HIV-1 infectivity. Retrovirology 16(1):18. https://doi.org/10.1186/s12977-019-0480-3
Baggen J, Thibaut HJ, Strating J, van Kuppeveld FJM (2018) The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol 16(6):368–381. https://doi.org/10.1038/s41579-018-0005-4
Moller LLV, Klip A, Sylow L (2019) Rho GTPases-emerging regulators of glucose homeostasis and metabolic health. Cells. https://doi.org/10.3390/cells8050434
Zhang J, Wu N, Gao N, Yan W, Sheng Z, Fan D, An J (2016) Small G Rac1 is involved in replication cycle of dengue serotype 2 virus in EAhy926 cells via the regulation of actin cytoskeleton. Sci China Life Sci 59(5):487–494. https://doi.org/10.1007/s11427-016-5042-5
Dransart E, Olofsson B, Cherfils J (2005) RhoGDIs revisited: novel roles in Rho regulation. Traffic 6(11):957–966. https://doi.org/10.1111/j.1600-0854.2005.00335.x
Garcia-Mata R, Boulter E, Burridge K (2011) The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12(8):493–504. https://doi.org/10.1038/nrm3153
Scherle P, Behrens T, Staudt LM (1993) Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci USA 90(16):7568–7572. https://doi.org/10.1073/pnas.90.16.7568
Lelias JM, Adra CN, Wulf GM, Guillemot JC, Khagad M, Caput D, Lim B (1993) cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc Natl Acad Sci USA 90(4):1479–1483. https://doi.org/10.1073/pnas.90.4.1479
Adra CN, Manor D, Ko JL, Zhu S, Horiuchi T, Van Aelst L, Cerione RA, Lim B (1997) RhoGDIgamma: a GDP-dissociation inhibitor for Rho proteins with preferential expression in brain and pancreas. Proc Natl Acad Sci USA 94(9):4279–4284. https://doi.org/10.1073/pnas.94.9.4279
Boulter E, Garcia-Mata R (2010) RhoGDI: A rheostat for the Rho switch. Small GTPases 1(1):65–68. https://doi.org/10.4161/sgtp.1.1.12990
Sasaki T, Takai Y (1998) The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun 245(3):641–645. https://doi.org/10.1006/bbrc.1998.8253
Zhang Y, Zhang B (2006) D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness. Can Res 66(11):5592–5598. https://doi.org/10.1158/0008-5472.CAN-05-4004
Harding MA, Theodorescu D (2010) RhoGDI signaling provides targets for cancer therapy. Eur J Cancer 46(7):1252–1259. https://doi.org/10.1016/j.ejca.2010.02.025
Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D (2012) RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Investig 122(4):1503–1518. https://doi.org/10.1172/JCI61392
Peng XC, Chen XX, Zhang YU, Wang HJ, Feng Y (2015) A novel inhibitor of Rho GDP-dissociation inhibitor alpha improves the therapeutic efficacy of paclitaxel in Lewis lung carcinoma. Biomed Rep 3(4):473–477. https://doi.org/10.3892/br.2015.475
Ngo AT, Thierheimer ML, Babur O, Rocheleau AD, Huang T, Pang J, Rigg RA, Mitrugno A, Theodorescu D, Burchard J, Nan X, Demir E, McCarty OJ, Aslan JE (2017) Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor Ly-GDI in platelet function: a spatial systems approach. Am J Physiol Cell Physiol 312(4):C527–C536. https://doi.org/10.1152/ajpcell.00274.2016
Watanabe T, Urano E, Miyauchi K, Ichikawa R, Hamatake M, Misawa N, Sato K, Ebina H, Koyanagi Y, Komano J (2012) The hematopoietic cell-specific Rho GTPase inhibitor ARHGDIB/D4GDI limits HIV type 1 replication. AIDS Res Hum Retroviruses 28(8):913–922. https://doi.org/10.1089/AID.2011.0180
Kramer SR, Goregaoker SP, Culver JN (2011) Association of the Tobacco mosaic virus 126kDa replication protein with a GDI protein affects host susceptibility. Virology 414(2):110–118. https://doi.org/10.1016/j.virol.2010.12.030
Wang JL, Zhang JL, Chen W, Xu XF, Gao N, Fan DY, An J (2010) Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0000809
(2019). https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
Trejo-Cerro O, Aguilar-Hernandez N, Silva-Ayala D, Lopez S, Arias CF (2019) The actin cytoskeleton is important for rotavirus internalization and RNA genome replication. Virus Res 263:27–33. https://doi.org/10.1016/j.virusres.2019.01.003
Lv X, Li Z, Guan J, Hu S, Zhang J, Lan Y, Zhao K, Lu H, Song D, He H, Gao F, He W (2019) Porcine hemagglutinating encephalomyelitis virus activation of the Integrin alpha5beta1-FAK-Cofilin pathway causes cytoskeletal rearrangement to promote its invasion of N2a cells. J Virol. https://doi.org/10.1128/JVI.01736-18
Ni B, Wen LB, Wang R, Hao HP, Huan CC, Wang X, Huang L, Miao JF, Fan HJ, Mao X (2015) The involvement of FAK-PI3K-AKT-Rac1 pathway in porcine reproductive and respiratory syndrome virus entry. Biochem Biophys Res Commun 458(2):392–398. https://doi.org/10.1016/j.bbrc.2015.01.126
Ospina Stella A, Turville S (2018) All-round manipulation of the actin cytoskeleton by HIV. Viruses. https://doi.org/10.3390/v10020063
Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K (2010) Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 12(5):477–483. https://doi.org/10.1038/ncb2049
Griner EM, Theodorescu D (2012) The faces and friends of RhoGDI2. Cancer Metastasis Rev 31(3–4):519–528. https://doi.org/10.1007/s10555-012-9376-6
Fiorillo M, Peiris-Pages M, Sanchez-Alvarez R, Bartella L, Di Donna L, Dolce V, Sindona G, Sotgia F, Cappello AR (1859) Lisanti MP (2018) Bergamot natural products eradicate cancer stem cells (CSCs) by targeting mevalonate, Rho-GDI-signalling and mitochondrial metabolism. Biochim Biophys Acta Bioenerg 9:984–996. https://doi.org/10.1016/j.bbabio.2018.03.018
Fang Y, Yi J, Lizhi L, Qiucheng C (2014) Rho GDP dissociation inhibitor beta promotes cell proliferation and invasion by modulating the AKT pathway in hepatocellular carcinoma. DNA Cell Biol 33(11):781–786. https://doi.org/10.1089/dna.2014.2545
Barone I, Brusco L, Gu G, Selever J, Beyer A, Covington KR, Tsimelzon A, Wang T, Hilsenbeck SG, Chamness GC, Ando S, Fuqua SA (2011) Loss of Rho GDIalpha and resistance to tamoxifen via effects on estrogen receptor alpha. J Natl Cancer Inst 103(7):538–552. https://doi.org/10.1093/jnci/djr058
Griner EM, Dancik GM, Costello JC, Owens C, Guin S, Edwards MG, Brautigan DL, Theodorescu D (2015) RhoC is an unexpected target of RhoGDI2 in prevention of lung colonization of bladder cancer. Mol Cancer Res 13(3):483–492. https://doi.org/10.1158/1541-7786.MCR-14-0420
Yamashita T, Okamura T, Nagano K, Imai S, Abe Y, Nabeshi H, Yoshikawa T, Yoshioka Y, Kamada H, Tsutsumi Y, Tsunoda S (2012) Rho GDP-dissociation inhibitor alpha is associated with cancer metastasis in colon and prostate cancer. Pharmazie 67(3):253–255
Coiras M, Camafeita E, Urena T, Lopez JA, Caballero F, Fernandez B, Lopez-Huertas MR, Perez-Olmeda M, Alcami J (2006) Modifications in the human T cell proteome induced by intracellular HIV-1 Tat protein expression. Proteomics 6(Suppl 1):S63-73. https://doi.org/10.1002/pmic.200500437
Saxena R, Gupta S, Singh K, Mitra K, Tripathi AK, Tripathi RK (2015) Proteomic profiling of SupT1 cells reveal modulation of host proteins by HIV-1 Nef variants. PLoS ONE 10(4):e0122994. https://doi.org/10.1371/journal.pone.0122994
Cossart P, Helenius A (2014) Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a016972
Stradal TEB, Schelhaas M (2018) Actin dynamics in host-pathogen interaction. FEBS Lett 592(22):3658–3669. https://doi.org/10.1002/1873-3468.13173
Leffers H, Nielsen MS, Andersen AH, Honore B, Madsen P, Vandekerckhove J, Celis JE (1993) Identification of two human Rho GDP dissociation inhibitor proteins whose overexpression leads to disruption of the actin cytoskeleton. Exp Cell Res 209(2):165–174. https://doi.org/10.1006/excr.1993.1298
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81671971, 81871641, 81972979, U1602223 and U1902210), Key Project of Beijing Natural Science Foundation B (KZ201810025035), the Scientific Research Plan of the Beijing Municipal Education Committee (KM201710025002) and the Foundation of Capital Medical University (PYZ19064).
Author information
Authors and Affiliations
Contributions
NG designed the study. DYF, NW, JZ and NG performed the experiments. ZYW analyzed the data. NG wrote the paper. PGW and JA reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, D., Wu, N., Zhang, J. et al. Effect of the Rho GTPase inhibitor-1 on the entry of dengue serotype 2 virus into EAhy926 cells. Mol Biol Rep 47, 9739–9747 (2020). https://doi.org/10.1007/s11033-020-05980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05980-9