Abstract
Changes in host immunity and parasite resistance to drugs are among the factors that contribute to decreased efficacy of antiparasitic drugs such as the antimonial compounds pentamidine, amphotericin (AMP B) and miltefosine. Bioactive natural products could be alternatives for the development of new drugs to treat neglected human diseases such as leishmaniasis. Natural coumarins and synthetic analogues have shown leishmanicidal activity, mainly in vitro. This study investigated the in vitro and in vivo leishmanicidal activity of synthetic coumarin compounds (C1–C5) in parasites Leishmania (L.) amazonensis and L. (L.) infantum chagasi. The cytotoxicity of these compounds in mammalian cells and their influence on production of reactive oxygen species was also investigated. In vitro assays showed that 8-methoxy-3-(4-nitrobenzoyl)-6-propyl-2H-chromen-2-one (C4) was as active as AMP B mainly in the amastigote form (p < 0.05); C4 presented a selectivity index (65.43) four times higher than C2 (15.4) in L. amazonensis and six times higher (33.94) than C1 (5.46) in L. infantum chagasi. Additionally, coumarin C4 reduced the H2O2 concentration 32.5% more than the control group in L. amazonensis promastigotes during the lag phase of proliferation. No interference of C4 was observed on the mitochondrial membrane potential of the parasites. In vivo, coumarin C4 in corn oil (oral route) led to a reduction in the number of amastigotes from L. infantum chagasi to 1.31 × 106 and 4.09 × 104 in the spleen and liver, respectively (p < 0.05). Thus, C4 represents a candidate for further studies aiming at new treatments of leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a neglected tropical disease that still requires a great deal of effort from control and elimination programmes [1]. Leishmaniasis is endemic to 98 countries and affects mainly the vulnerable groups from a social, environmental and economic point of view, living in Africa, Asia and Latin America [2]. Meanwhile, the clinical presentations of the disease can be determined by the parasite features and the host's immune response [3]. The disease outcome can show both visceral and cutaneous forms [4]. Leishmaniasis is a parasitic disease transmitted to the vertebrate host by an insect (phlebotomine sandfly) that can inoculate the protozoan Leishmania spp.[5].
The first line of drugs for the leishmaniasis treatment have been the antimonial compounds, used for more than 60 years [6]. Pentamidine and AMP B are included in the second line of this treatment [7]. Miltefosine has been used as an oral drug for visceral leishmaniasis in some countries, such as India, Nepal and Bangladesh [8]. However, many factors have contributed to decrease the efficacy of these drugs, such as changes in the host immunity and parasite resistance by different mechanisms [9]. There are also other difficulties inherent to chemotherapy of leishmaniasis in relation to diagnosis, drug toxicity, patient compliance to treatment, healing criterion and patient follow-up.
Natural products have been a source of underexplored bioactive molecules that could be used as models in the discovery and development of new drugs for the treatment of neglected tropical diseases. The uses of natural compounds for the treatment of various parasitic diseases such as trypanosomiasis, leishmaniasis, schistosomiasis and filariasis has encouraged the survey for novel molecules [10]. The classes of natural compounds such as alkaloids, chalcones, lactones, saponins and coumarins have been studied as antiparasitic agents [11,12,13]. Natural and synthetic coumarins present a wide range of biological activity [14, 15]. Tiuman et al. [16] reported that the coumarin obtained from Calophyllum brasiliense showed in vivo leishmanicidal activity and could be used to control the development of cutaneous lesions caused by L. amazonensis. Prenylated coumarins auraptene, umbelliprenin and galbanic acid isolated from Ferula szowitsiana (Apiaceae) [17] have shown activity against L. major. In the New World, the L. panamensis was inhibited by isopropenyl coumarins from the extracts of Galipea panamensis leaves [18]. In L. amazonensis, there are reports of leishmanicidal activity of the coumarin (-) mammea A/BB isolated from C. brasiliense leaves [19]. Rosa et al. [10] showed the in vitro leishmanicidal potential of two 4-phenylcoumarins (HPC4 and HPC7, Fig. 1) against the promastigote and amastigote forms of L. amazonensis; however, the in vivo leishmanicidal activity of these coumarins was not evaluated.
In this work, it was described the evaluation of the coumarins HPC4 and HPC7 on parasite burden in an in vivo model of leishmaniasis by L. (L.) infantum chagasi. In addition, we report herein for the first time the in vitro and in vivo leishmanicidal activities of a synthetic nitro coumarin (C4, Fig. 1), previously described as an anti-Trypanosoma cruzi agent [20]. We also investigate its influence on the parasite membrane potential and production of reactive oxygen species by the parasite cells.
Methods
Chemistry
Generalities
Reagents and solvents were obtained from Sigma-Aldrich (Saint Louis, MO, USA) and were used without further purification. For the evaluation of purity and progress of the reaction, it was used thin-layer chromatography (TLC) on silica gel TLC plates (ALUGRAM® Xtra Sil G/UV254) and detection by exposure to UV light at 254 nm. For the determination of melting point values, a PFM-II melting-point apparatus was employed (MS Tecnopon, Piracicaba, Brazil) and the obtained results were not corrected. Infrared (IR) spectra were obtained on an FT-IR-Affinity−1 equipment operating with an ATR dispositive (Shimadzu®, Kyoto, Japan). For the obtaining of Nuclear Magnetic Resonance (NMR) spectra it was used an AC-300 spectrometer operating at 300 MHz for 1H-NMR and 75 MHz for 13C-NMR spectra (Bruker®, Billerica, USA). TMS was used as the internal standard in NMR analysis and the coupling constants (J) were registered in Hertz. NMR signals were assigned as: s (singlet), d (doublet), dd (doublet of doublets), t (triplet), tt (triplet of triplets), q (quartet), sex (sextet) and m (multiplet). ClogP values of synthesized compounds were calculated using ChemDraw® Ultra 11.0.
General procedure for the synthesis of the coumarins C1-C5
The respective aromatic aldehyde (1 eq), a beta-ketoester (1 eq) and piperidine as a catalyst (3 drops) were added to a 50-mL round bottom flask containing ethanol (15 mL). This reaction mixture was stirred under heating at 85 °C with a reflux apparatus for two hours (Fig. 2). The progress of the reaction was checked by TLC (hexane:ethyl acetate, 7:3, v/v). The mixture was cooled to 25 °C; the solid product was collected by vacuum filtration, washed throughout with cold ethanol and dried in a desiccator. All products were sufficiently pure for further evaluations.
Characterization data
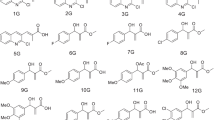
6-allyl-3-benzoyl-8-methoxy-2H-chromen-2-one (C1)
White solid; Yield 83%; CLogP: 3.86; M.p.: 159–160 °C; F.M.: C20H16O4; IR (νmax/cm–1) 3048 (aromatic C–H), 2962 (sp3 C–H), 1713 (ester C=O), 1659 (ketone C=O), 1578; 1476 (aromatic C=C); 1H NMR (CDCl3) 3.43 (d, J H13,H14 = 6.63, 2H), 3.96 (s, 3H), 5.09–5.16 (m, 2H), 5.89–6.02 (m, 1H), 6.96 (s, 1H), 7.00 (s, 1H), 7.42–7.47 (m, 2H), 7.59 (tt, JH4′,H3′ = 7.41, JH4′,H2′ = 2.61, 1H), 7.84–7.87 (m, 2H), 8.00 (s, 1H); 13C NMR (CDCl3) 39.82, 56.46, 116.14, 117.11, 118.67, 119.80, 127.28, 128.66, 129.70, 133.86, 136.35, 137.25, 143.14, 145.76, 147.19, 158.12, 191.89.
3-benzoyl-8-methoxy-6-propyl-2H-chromen-2-one (C2)
Light yellow solid; Yield 65%; CLogP: 4.36; M.p.:140–141 °C; F.M.: C20H18O4; IR (νmax/cm–1) 3051 (aromatic C–H), 2953 (sp3 C–H), 1716 (ester C=O), 1676 (ketone C=O), 1579, 1466 (aromatic C=C); 1H NMR (DMSO-d6) 0.97 (t, JH15.H14 = 7.35, 3H), 1.68 (sex, JH14.H13 = 7.58, JH14.H15 = 7.35, 2H), 2.65 (t, JH13.H14 = 7.58, 2H), 3.98 (s, 3H), 6.95 (s, 1H), 7.00 (s, 1H), 7.44–7.49 (m, 2H), 7.60 (tt, JH4′.H3′ = 7.39, JH4′.H2′ = 2.65, 1H), 7.85–7.89 (m, 2H), 8.02 (s, 1H); 13C NMR (DMSO-d6) 13.68, 24.46, 37.70, 56.36, 116.06, 118.50, 119.54, 127.16, 128.56, 129.62, 133.74, 136.31, 139.76, 142.85, 145.82, 146.98, 191.92.
3-benzoyl-8-methoxy-2H-cromen-2-one (C3)
Light Orange solid; Yield 80%; CLogP: 2.84; M.p.: 130–131 °C; F.M.: C17H12O4; IR (νmax/cm–1) 3052 (aromatic C–H), 3008 (sp3 C–H), 1712 (ester C = O), 1656 (ketone C = O), 1572; 1471 (aromatic C = C); 1H NMR (CDCl3) 3.94 (s, 3H), 7.10–7.15 (m, 2H), 7.20–7.23 (m, 1H), 7.42 (t, JH3′,H2′ 7.36; JH3′,H4′ = 7.66, 2H), 7.56 (t, JH4′,H3′ = 7.66, 1H), 7.83 (d, JH2′,H3′ = 7.36, 2H), 8.00 (s, 1H); 13C NMR (CDCl3) 56.47, 115.38, 118.90, 120.49, 124.96, 127.37, 128.68, 129.71, 133.91, 136.29, 144.56, 145.70, 147.35, 158.01, 191.82.
8-methoxy-3-(4-nitrobenzoyl)-6-propyl-2H-chromen-2-one (C4)
Light yellowish solid; Yield 91%; CLogP: 4.32; M.p.: 143–144 °C; F.M.: C20H17NO6; IR (νmax/cm–1) 3069 (aromatic C–H), 3049 (sp2 C–H), 1582 (aromatic C=C), 1705 (ester C=O), 1682 (ketone C=O), 1513 (NO2), 1347 (NO2); 1H NMR (DMSO-d6) 0.98 (t, JH15.H14 = 7.37, 3H), 1.69 (sex, JH14,H13 = 7.5; JH14.H15 = 7.37, 2H), 2.67 (t, JH13,H14 = 7.5, 2H), 3.99 (s, 3H), 7.01 (d, JH7,H5 = 1.71, 1H), 7.05 (d, JH5,H7 = 1.71, 1H), 7.99–7.95 (m, 2H), 8.23 (s, 1H), 8.28–8.33 (m, 2H); 13C NMR (DMSO-d6) 13.67, 24.43, 37.8, 56.40, 116.86, 118.40, 119.91, 123.68, 125.58, 130.18, 140.20, 141.52 (2C), 143.24, 147.05, 148.12, 150.39, 190.74.
8-methoxy-3-(4-nitrobenzoyl)-2H-chromen-2-one (C5):
Light greenish solid; Yield: 89%; CLogP: 2.80; M.p.: 260–261 °C; F.M.: C17H11NO6; IR (νmax/cm–1) 3105 (sp2 C–H), 3075 (aromatic C–H), 1574 (aromatic C=C), 1698 (ester C=O), 1660 (ketone C=O), 1514 (NO2), 1345 (NO2); 1H NMR (DMSO-d6) 3.95 (s, 3H), 7.37 (t, JH6,H5=7.65; JH6,H7=7.92, 1H), 7.43–7.48 (mm, 2H), 8.15 (d, JH3′,H2′=9.00, 2H), 8.33 (d, JH2′,H3′=9.00, 2H), 8.56 (s, 1H); 13C NMR (DMSO-d6) 11.31, 56.28, 118.81, 121.20, 123.70, 124.97, 125.45, 130.64, 141.41, 143.86, 146.48, 147.65, 150.09, 157.81, 190.95.
Biological assays
Leishmanicidal activity against promastigotes and amastigotes; cytotoxicity assay
Promastigotes of L. (L.) amazonensis (strain MHOM/BR/71973/M2269) and L. (L.) infantum chagasi (strain MHOM/BR/1972/BH46) assays were performed using LIT medium with foetal bovine serum (10%; Gibco®, USA) and antibiotics. The compounds C1–C5 were dissolved in dimethyl sulfoxide (DMSO 0.6%, v/v in all wells), added to promastigote cultures (1 × 106 cells/mL) in the range of 0.10–40.00 µg/mL, and incubated at 25 °C. The tests were performed in triplicate and amphotericin B (AMP B; Sigma) was used as the reference drug. The maintenance of murine peritoneal macrophages was in RPMI 1640 medium (Sigma, USA) with 10.0% heat-inactivated foetal bovine serum at 37 °C in a 5.0% CO2 incubator. The cells were infected with late log-phase promastigotes at a 10:1 parasite:macrophage ratio and incubated at the same conditions. The compounds C1–C5 (from 0.10 to 40.00 µg/mL in DMSO) were added after the removal of non-phagocytosed promastigotes. The calculation of inhibition ratio (IC50 value) was did in comparison to DMSO alone. All assays were performed in triplicate on independent occasions using AMP B (Sigma) as the reference drugs. For the cytotoxicity evaluation, 8 × 105 murine peritoneal macrophages in RPMI 1640 suspension with 10.0% heat-inactivated foetal bovine serum and antibiotics were added to each well in 96-well plates. The compounds C1–C5 and reference drug ranging from 3.91 to 500.00 µg/mL in DMSO were added to the wells containing the cells. The leishmanicidal activity was evaluated by the MTT method [21]. The best coumarin derivative for the in vitro leishmanicidal tests was selected to carry out the inhibition of reactive oxygen species production and in vivo leishmanicidal assays. The complete protocols were previously described [21]. All statistical analysis was performed using Prism software (version 5.0; GraphPad Software, Inc., La Jolla, California, USA), by nonlinear regression to obtain the values of IC50 and CC50, followed by variance analyses and Tukey’s test. Differences of IC50 among standard drug and coumarin derivatives were considered significant when the p-value was less than 0.05.
Determination of hydrogen peroxide release and mitochondrial membrane potential (ΔΨ)
Promastigotes (108 cells/mL) of L. (L.) amazonensis at lag (2 days) and log (4 days) phases of the proliferation curve were treated or not (control). The coumarin derivative was selected among C1–C5 (according to in vitro activity); they were incubated in reaction buffer 1X from Amplex Red® kit in the presence of 40 μM digitonin, 5 mM succinate, 0.1 U/mL horseradish peroxidase and 25 μM Amplex Red (Molecular Probes®). The fluorescence was monitored at the emission and excitation wavelengths of 585 nm and 571 nm, respectively, in a 96-well plate using a Varian Cary Eclipse Fluorescence Spectrophotometer. The quantitative correlation between the fluorescence and the H2O2 released by the cells was determined as previously described [22]. The ΔΨ was performed as previously described [23]. Briefly, L. amazonensis promastigotes (1 × 107 cells/mL) in the lag phase of the proliferation curve were treated or not (control) with 30 μM of a coumarin derivative (selected among C1–C5, according to their in vitro activity) for 48 h. After incubation, the cells were washed and resuspended in phosphate buffer (pH 7.4); cells were incubated for thirty minutes with a JC-10 probe at a concentration of 10 mg/mL at 25 ºC. After this period, the promastigotes were washed twice and resuspended in phosphate buffer (pH 7.4); ΔΨ was determined at emission wavelengths of 530 nm and 590 nm and excitation wavelength of 480 nm using a spectrofluorimeter (Spectrophotometer of Varian Cary Eclipse Fluorescence®). As a positive control of uncoupling, the parasites were incubated with 20 μM CCCP (Carbonyl cyanide m-chlorophenyl hydrazone). The data obtained correspond to the mean ± standard deviation of at least three independent experiments carried out in triplicate. The comparison was determined using Student’s t-test with Origin 6.0 software, and p < 0.05 was considered statistically significant.
In vivo leishmanicidal assay
Parasites and animals
All experimental procedures involving animals were approved by the Research Ethics Commission of the Federal University of Alfenas (CEUA/UNIFAL-MG 566/2014) and were performed according to the Guide for the Care and Use of Laboratory Animals. Leishmania (L.) infantum chagasi (strain MHOM/BR/1972/BH46) promastigotes were maintained in M-199 medium and amastigotes were maintained by passaging in golden hamsters (Mesocricetus auratus). The organs (spleens and liver) of infected animals were removed and macerated using a tissue grinder; and the number of amastigotes was determined as described previously [24].
In vivo testing of experimental compounds against Leishmania
For the in vivo assay were used female golden hamsters that had been recently weaned (120 g) and were infected intraperitoneally with 1 × 107 amastigotes of L. (L.) infantum chagasi (MHOM/BR/1972/BH46). After 50 days of infection the animals were divided into 5 groups (5 per group) and subjected for 10 consecutive days to one of the following treatments: 0.5% of carboxymethyl cellulose (CMC) suspension, administered orally (untreated, or UTG group); 50 mg/kg/day of Glucantime (GLU), by intraperitoneal injection (GLU group); 20 mg/kg/day of compounds HPC4, HPC7 and the best coumarin derivative selected among C1–C5 (according to their in vitro activity), administered orally as suspensions in 0.5% of CMC. In addition, a choice coumarin derivative (according logP and in vitro activity) was assayed via the oral route with corn oil in a final solution undiluted of 137.0 μL each animal/day, using 20 mg/kg/day. After 10 days of treatment, animals were sacrificed in a CO2 chamber, and a sample of the spleen and the liver (approximately 50 mg) was removed, weighed and used for total RNA extraction, as previously described [25]. Were used standard curves assays by quantitative real-time PCR (qPCR) of parasite DNA as described previously [26]. A parasite load estimation by qPCR was performed using the TaqMan® probe double-labelled with FAM and the primers LinJ31. From the linear regression data from the standard curve performed with promastigote DNA were calculated the number of parasites per gram of organs tissues and the statistical analysis was performed by Student’s t-test with Mann–Whitney (unpaired, two-tailed) for the significance test (p < 0.05), as described elsewhere [27].
Results
Chemistry
The reactions took an average of 2 h to complete, and their equilibrium was favoured for the product formation since they are insoluble in ethanol. This also helped in the reaction workup by filtration and there was no need for additional treatments. Coumarin formation was certified by the analysis of their IR, 1H and 13C NMR spectra and comparison to previously reported data, with which they had total agreement. The main findings in IR spectra were the two carbonyl stretching bands of ketone and lactone groups typical for all these coumarins. In coumarins C4–C5, the asymmetric and symmetric stretching bands were observed for the nitro group.
By the analysis of 1H-NMR spectra, a singlet was observed in the range 8.56–8.0 ppm attributed to H-4, which is shared by all coumarins. Ketone and lactone carbonyl signals were observed near 190 and 158 ppm, respectively. Coumarins C4 and C5 exhibited the expected pattern for para-substituted phenyl rings, and a deshielding effect was evident on the hydrogens ortho to the nitro group. TLC and NMR were used to infer about the purities of products, which were sufficiently pure for the biological evaluations.
In vitro leishmanicidal activity
The data obtained in the in vitro leishmanicidal assays are shown in Table 1. The coumarin C4 showed a similar leishmanicidal activity to AMP B against promastigote and amastigote forms of L. amazonensis with IC50 values of 12.0 and 3.53 μM, respectively (p < 0.05). In L. infantum chagasi promastigotes, the IC50 for AMP B was the lowest, but most compounds had IC50 values less than 27.0 μM.
The coumarin C4 had an activity against L. infantum chagasi amastigote similar to AMP B. The coumarin nitro compound C4 was as active as AMP B, mainly in amastigote forms (p < 0.05), and presented a selectivity index (65.43) four times higher than C2 (15.4) in L. amazonensis and six times higher (33.94) than C1 (5.46) in L. infantum chagasi.
Determination of hydrogen peroxide (H2O2) release
The influence of the coumarin C4 (choice compound) on the production of H2O2 in L. amazonensis promastigotes in the lag and log phases of the proliferation curve was evaluated. Our results demonstrated under our experimental conditions that treatment with the coumarin C4 reduced the concentration of H2O2 by approximately 32.5% compared to the control group (untreated) in the lag phase (Fig. 3).
When analysing the effect of this treatment on log-phase promastigotes, there was no significant difference between the H2O2 concentration in parasites treated and those not treated (Fig. 3). We observed that treatment with the coumarin C4 did not influence ΔΨ, because there was no significant difference between treated parasites and the control (Fig. 4).
Evaluation of the mitochondrial membrane potential (ΔΨ). Leishmania amazonensis promastigotes (1 × 107 cells/mL) in the lag phase of the proliferation curve were treated or not (Ctrl) with the coumarin C4. The values are expressed as the ratio of the fluorescence measurements at 530 nm (for J-monomer) versus 590 nm (J-aggregate). The experiments were carried out in triplicate on independent occasions. *No statistical difference
In vivo leishmanicidal activity evaluation
The coumarin C4, HPC4 and HPC7 were chosen to the in vivo assay. In the untreated group (UTG; vehicle-treated), the average number of parasites was 1.53 × 108 and 3.47 × 106 per gram of tissue in the spleen and liver, respectively, confirming that these animals had an established infection (clinical symptoms such as ascites and alopecia) with L. (L.) infantum chagasi.
Treatment with compound HPC4 did not decrease the number of amastigotes in the spleen and liver of infected hamsters (1.67 × 107 and 1.25 × 107) when compared to UTG. However, the treatment with compounds C4 and HPC7 showed a parasite burden of 5.3 × 106 and 7.16 × 106 to spleen and 2.23 × 106 and 1.23 × 107 to the liver, respectively, both in carboxymethyl cellulose (C4 CMC) by the oral route; these values are comparable with UTG results.
Glucantime (50 mg/kg/day, intraperitoneal route) and C4 Corn Oil (20 mg/kg/day, oral route) were the most effective compounds at reducing the parasite burden in infected animals, since treatment with these drugs led to a reduction in the number of amastigotes to 1.85 × 104 and 1.31 × 106 in the spleen, respectively; the drugs reduced amastigotes to 6.02 × 103 and 4.09 × 104 in the liver, respectively, when compared to UTG (Fig. 5; p < 0.05).
Quantification of parasite burden in the spleen and liver from hamsters infected with Leishmania (L.) infantum chagasi, as quantified by quantitative reverse transcription PCR (qRT-PCR) for the detection of the Linj31 marker. The numbers of parasites (amastigotes) per gram of tissue were estimated based on a qPCR standard curve using promastigotes. UTG untreated group (vehicle-treated), GLU animals treated with Glucantime (50 mg/kg/day), coumarin derivatives HPC4, C4 CMC, C4 Corn Oil and HPC7 (20 mg/kg/day). Data are represented as group mean and individual values for each animal. Statistical difference *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The coumarins C1–C5 have already been synthesized and described elsewhere for other biological purposes [20, 28]. In this work, all coumarin derivatives were obtained in yields mostly above 80% by a Knoevenagel condensation methodology as described by Vazquez-Rodriguez et al. [29] for other coumarins. In the biological tests the compounds C2, C3 and C4 were more potent against the amastigote form of L. amazonensis (IC50 was 17.7, 43.8 and 3.53, respectively) compared to their action against the promastigote form (IC50 was 82.0, 122.7 and 12.0, respectively); this difference in intracellular activity may be derived from the formation of a more active metabolite in the infected cell [21].
Only the coumarin C4 showed significant activity against the promastigote and amastigote forms of L. amazonensis and L. infantum chagasi. This coumarin derivative is the only one tested that bears nitro and propyl groups in its structure. Brancaglion et al. [20] evaluated the coumarin C4 and congeners against Trypanosoma cruzi and suggested that the presence of a propylic side chain on the coumarin nucleus next to a nitro group positively affects the trypanocidal activity. Hydrophobic interactions between this flexible propyl side chain and a molecular target from trypasonosomatid could be essential for the trypanocidal activity. In their review, Patterson and Wyllie [30] highlighted a repurposing of nitroaromatic drugs, noting the requirements for new drugs for trypanosomatid diseases.
In this sense, the approach would be through reducing the toxicity of nitro compounds. However, the cytotoxicity of the nitro coumarin C4 was very low in our evaluation. According to Lehnhardt Pires et al. [31], a selectivity index higher than 10 is required to ensure safety for a drug. In the present work, the coumarin nitro compound C4 showed the best SI, which value indicated the high selective toxicity for the parasite. Rosa et al. [10] also reported that 7-hydroxy-4-phenylcoumarin derivatives did not display toxicity in mammalian cells.
The metabolism of different unicellular or multicellular organisms is dependent on the synthesis of energetic molecules such as ATP. Specifically, protozoan Leishmania has ATP synthesis, mainly in their single mitochondrion [32], which, in addition to this function, are involved in other processes such as calcium homeostasis and apoptosis [33]. At the same time, the mitochondrion is the main site for the generation of reactive oxygen species (ROS) [33], molecules that must be kept in adequate concentrations to prevent the oxidation of cellular components such as DNA, lipids, proteins and carbohydrates due to oxidative stress. This peculiarity of trypanosomatids in having only one mitochondrion makes this organelle an interesting therapeutic target since mitochondrial integrity must be maintained so that trypanosomatids, such as Leishmania, can survive.
It has also been reported that coumarins have a wide range of biological activities, including antioxidant [34]. Feriani et al. [35] reported that the coumarin-derived compound (E)-N′-(1-(7-hydroxy-2-oxo-2H-chromen-3-yl)ethylidene)-benzohydrazide is a scavenger of free radicals, which led to a reduction in oxidative stress, inflammation and apoptosis. Thus, it was possible to notice the influence of the coumarin C4 on the production of H2O2 in L. amazonensis promastigotes. This result corroborates with the role of coumarin derivatives as a scavenger of free radicals [35], thereby reducing the concentration of oxidising agents such as H2O2.
On the other hand, it was not possible to note the same effect on log-phase promastigotes. This could be related to the differential expression of antioxidant enzymes from trypanosomatids along the proliferation curve, as in Trypanosoma cruzi [22]. In other words, less activity of the cytosolic and mitochondrial triparedoxins peroxidases of Leishmania in the log phase would allow a higher concentration of H2O2, so that when incubating this protozoan with an ROS scavenger compound, the reduction in the concentration of H2O2 would not be detectable. However, since the H2O2 concentration is the same in the lag and log phase control groups, this hypothesis could not support this result.
The biological role of H2O2 in trypanosomatids has been widely explored. In this family of protozoa, through the activities of antioxidant enzymes, the intracellular environment is maintained in a reduced form, balancing, for example, the concentration of reactive species such as H2O2 [36]. At the same time, Peloso et al. [22] demonstrated that the increase in H2O2 production signals an increase in the expression of cytosolic and mitochondrial peroxidases with differential expression of these antioxidant enzymes along the proliferation curve. Additionally, it has been reported that a low concentration of H2O2 stimulates cell proliferation [37]. Finzi et al. [37] demonstrated that 20 μM of H2O2 stimulated cell proliferation in T. cruzi while increasing the resistance of this parasite to sublethal doses of H2O2 (100 μM).
Another hypothesis for the reduction in H2O2 concentration observed in the lag phase of the proliferation curve when the parasites were treated with the coumarin C4 would be the difference in biochemical aspects between the lag, log and stationary phases of the parasite. Along the proliferation curve, there is a difference in the synthesis of various compounds, such as polyamines, which are essential for trypanosomatid metabolism [38]. Proteome studies, such as the one reported by Alcolea et al. [39], have also showed a high number of proteins with varied functions, differentially expressed between the phases of the proliferation curve. Thus, these biochemical aspects could exert a strong influence on the activity of the coumarin C4 due to several possibilities of chemical interaction, thus facilitating its role as an antioxidant in the lag phase, and, consequently, reduce the concentration of H2O2.
Additionally, the production of ROS such as H2O2 may directly influence mild dysfunctions in the mitochondrial membrane potential (ΔΨ) [40]. The maintenance of ΔΨ is essential for the functionality of mitochondria since it is directly involved with oxidative phosphorylation and ATP synthesis, control of ROS production levels and intracellular Ca+2 homeostasis [41]. Mitochondrial changes such as swelling, alteration of ΔΨ and release of cytochrome c, represent the main signs of the apoptosis-like processes in Leishmania [42]. Additionally, the ΔΨ reduction is characteristic of a cell undergoing programmed cell death [43]. In promastigotes from Leishmania, both hyperpolarisation and depolarisation can result in cell death from apoptosis [44]. Mandlik et al. [45] reported that the coumarin derivate C2 induces ΔΨ reduction in L. major promastigotes. In this sense, we evaluated ΔΨ in parasites in the lag phase, treated with the coumarin C4, to clarify whether the reduction in H2O2 production was the result of a mitochondrial alteration caused by this compound. However, the coumarin C4 does not alter the energetic functionality of the mitochondrion since ΔΨ remained unchanged.
Colombo et al. [46] described a quantitative reverse transcription PCR (qRT-PCR) assay that can be used for sensitive and accurate quantification of parasites in infected tissues. For this purpose, we used the detection of the LinJ31 marker (Linj31-qPCR) for the quantification of live amastigotes of L. (L.) infantum chagasi in the spleen and liver of infected hamsters after experimental treatment using benzophenone derivatives.
In this way, the compound C4 was chosen to assay using corn oil as a vehicle because it showed the best in vitro activity, particularly against the L. amazonensis amastigote form. In addition, coumarin C4 has a theoretical logP value that is suitable for oral route evaluations; it has a good gastrointestinal absorption profile, as predicted by the SwissADME platform. Besides a higher dosage, Glucantime was administered intraperitoneally, while the assayed compounds HPC4, HPC7, C4 CMC and C4 Corn Oil were administered orally. Theoretically, soluble and neutral compounds are well absorbed after oral treatment. The absorbed dose may therefore be different from the administered dose because of the nature of the compounds, which can lead to different absorption rates being faster for compounds in solution than for those in suspension.
The level of hydrophobicity can indicate the choice of the suitable vehicle for administering the test compounds [47]. The coumarin C4 has a logP around 4.32 which makes it suitable to be administered as a suspension using CM/CMC or as a solution in corn oil. However, vehicles such as CMC can retard the passive diffusion of the drug and, consequently, the absorption from the gastrointestinal tract, but the administration with corn oil can improve this absorption according to the chemical nature of the compounds [48]. The difference observed in the in vivo results with C4 CMC and C4 Corn Oil can be explained by the distinct formulations used our study and can represent an alternative to overcome the difficulties in the oral administration of compounds from natural origin [49].
Solubility issues of coumarins were observed in other studies considering an in vivo model of cutaneous leishmaniasis [45, 50]. Kermani et al. [50] assayed osthole, a prenylated coumarin extracted from Prangos asperula Boiss, against L. major. These authors reported that osthole could decline the progression of lesions compared to untreated mice, and its effect was restricted because it is a poorly soluble coumarin. In another study, Mandlik et al. [45] developed a nanoliposomal formulation to circumvent the problem with an insoluble coumarin derivative. They observed a healing effect in the footpads of BALB/c mice infected with L. major when treated with this nano formulated compound. This approach is under investigation in our group to enhance the in vivo activity of the presented coumarins.
Conclusion
In our study, coumarin derivatives showed leishmanicidal activity in vitro and in vivo, especially C4 when compared to reference drugs. The coumarin C4 could reduce the concentration of H2O2 produced in L. amazonensis promastigotes and the parasite burden in the liver and spleen of infected animals with L. infantum chagasi when given orally in a corn oil solution. These results suggest that the presence of a propylic side chain on the coumarin nucleus, as seen in coumarin C4, can improve the leishmanicidal activity probably by influencing solubility issues and hydrophobic interactions with molecular targets from trypasonosomatid. Probably, this feature enables the access to the macrophage and to the intracellular parasite. Thus, this series of coumarin derivatives, and especially the coumarin C4, could be explored as candidates for the development of new drug prototypes against leishmaniasis.
References
Antinori S, Schifanella L, Corbellino M (2012) Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis 31:109–118. https://doi.org/10.1007/s10096-011-1276-0
WHO (2020) WHO|Leishmaniasis. https://www.who.int/gho/neglected_diseases/leishmaniasis/en/. Accessed 15 Mar 2015
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Research 6:750. https://doi.org/10.12688/f1000research.11120.1
Handler MZ, Patel PA, Kapila R et al (2015) Cutaneous and mucocutaneous leishmaniasis. J Am Acad Dermatol 73:897–908. https://doi.org/10.1016/j.jaad.2014.08.051
Lestinova T, Rohousova I, Sima M et al (2017) Insights into the sand fly saliva: blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis 11:e0005600. https://doi.org/10.1371/journal.pntd.0005600
Haldar AK, Sen P, Roy S (2011) Use of Antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011:1–23. https://doi.org/10.4061/2011/571242
Mishra M, Biswas UK, Jha DN, Khan AB (1992) Amphotericin versus pentamidine in antimony-unresponsive kala-azar. Lancet 340:1256–1257. https://doi.org/10.1016/0140-6736(92)92952-C
Sundar S, Olliaro PL (2007) Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther Clin Risk Manag 3:733–740
Ponte-Sucre A, Gamarro F, Dujardin JC et al (2017) Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052
Rosa IA, de Almeida L, Alves KF et al (2017) Synthesis and in vitro evaluation of leishmanicidal activity of 7-hydroxy-4-phenylcoumarin derivatives. Med Chem Res 26:131–139. https://doi.org/10.1007/s00044-016-1729-1
Sobarzo-Sánchez E, Bilbao-Ramos P, Dea-Ayuela M et al (2013) Synthetic oxoisoaporphine alkaloids: in vitro, in vivo and in silico assessment of antileishmanial activities. PLoS ONE. https://doi.org/10.1371/journal.pone.0077560
Jaiswal AK, Rao KB, Kushwaha P et al (2016) Development of Leishmania donovani stably expressing DsRed for flow cytometry-based drug screening using chalcone thiazolyl-hydrazone as a new antileishmanial target. Int J Antimicrob Agents 48:695–702. https://doi.org/10.1016/j.ijantimicag.2016.09.018
Ulloa JL, Spina R, Casasco A et al (2017) Germacranolide-type sesquiterpene lactones from Smallanthus sonchifolius with promising activity against Leishmania mexicana and Trypanosoma cruzi. Parasites Vectors 10:567. https://doi.org/10.1186/s13071-017-2509-6
Ghate M, Manohar D, Kulkarni V et al (2003) Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem 38:297–302. https://doi.org/10.1016/S0223-5234(03)00016-3
Kontogiorgis C, Nicolotti O, Mangiatordi GF et al (2015) Studies on the antiplatelet and antithrombotic profile of anti-inflammatory coumarin derivatives. J Enzyme Inhib Med Chem 30:925–933. https://doi.org/10.3109/14756366.2014.995180
Tiuman TS, Brenzan MA, Ueda-Nakamura T et al (2012) Intramuscular and topical treatment of cutaneous leishmaniasis lesions in mice infected with Leishmania amazonensis using coumarin (-) mammea A/BB. Phytomedicine 19:1196–1199. https://doi.org/10.1016/j.phymed.2012.08.001
Iranshahi M, Arfa P, Ramezani M et al (2007) Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 68:554–561. https://doi.org/10.1016/j.phytochem.2006.11.002
Arango V, Robledo S, Séon-Méniel B et al (2010) Coumarins from Galipea panamensis and their activity against Leishmania panamensis. J Nat Prod 73:1012–1014. https://doi.org/10.1021/np100146y
Brenzan MA, Nakamura CV, Dias Filho BP et al (2008) Structure-activity relationship of (-) mammea A/BB derivatives against Leishmania amazonensis. Biomed Pharmacother 62:651–658. https://doi.org/10.1016/j.biopha.2008.08.024
Brancaglion GA, Toyota AE, Cardoso Machado JV et al (2018) In vitro and in vivo trypanocidal activities of 8-methoxy-3-(4-nitrobenzoyl)-6-propyl-2H-cromen-2-one, a new synthetic coumarin of low cytotoxicity against mammalian cells. Chem Biol Drug Des 92:1888–1898. https://doi.org/10.1111/cbdd.13362
Espuri PF, dos Reis LL, de Figueiredo Peloso E et al (2019) Synthesis and evaluation of the antileishmanial activity of silver compounds containing imidazolidine-2-thione. J Biol Inorg Chem 24:419–432. https://doi.org/10.1007/s00775-019-01657-2
Peloso EF, Gonalves CC, Silva TM et al (2012) Tryparedoxin peroxidases and superoxide dismutases expression as well as ROS release are related to Trypanosoma cruzi epimastigotes growth phases. Arch Biochem Biophys 520:117–122. https://doi.org/10.1016/j.abb.2012.02.020
Inacio JDF, Gervazoni L, Canto-Cavalheiro MM, Almeida-Amaral EE (2014) The effect of (-)-epigallocatechin 3-O - gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action. PLoS Negl Trop Dis 8:e3093. https://doi.org/10.1371/journal.pntd.0003093
Stauber LA, Franchino EM, Grun J (1958) An eight-day method for screening compounds against Leishmania donovani in the golden hamster. J Protozool 5:269–273. https://doi.org/10.1111/j.1550-7408.1958.tb02565.x
Reimão JQ, Colombo FA, Pereira-Chioccola VL, Tempone AG (2011) In vitro and experimental therapeutic studies of the calcium channel blocker Bepridil: detection of viable Leishmania (L.) chagasi by real-time PCR. Exp Parasitol 128:111–115. https://doi.org/10.1016/j.exppara.2011.02.021
Colombo FA, Pereira-Chioccola VL, da Meira CS, et al (2015) Performance of a real time PCR for leishmaniasis diagnosis using a L. (L.) infantum hypothetical protein as target in canine samples. Exp Parasitol 157:156–162. https://doi.org/10.1016/j.exppara.2015.08.014
Colombo FA, Odorizzi RMFN, Laurenti MD et al (2011) Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res 109:267–274. https://doi.org/10.1007/s00436-010-2247-6
Horning EC, Horning MG (1947) Coumarins from 2-Hydroxy-3-Methoxybenzaldehyde. J Am Chem Soc 69:968–969. https://doi.org/10.1021/ja01196a506
Vazquez-Rodriguez S, Figueroa-Guíñez R, Matos MJ et al (2013) Synthesis of coumarin-chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. Medchemcomm 4:993–1000. https://doi.org/10.1039/c3md00025g
Patterson S, Wyllie S (2014) Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol 30:289–298
Lehnhardt Pires C, Rodrigues SD, Bristot D et al (2013) Evaluation of macroalgae sulfated polysaccharides on the Leishmania (L.) amazonensis promastigote. Mar Drugs 11:934–943. https://doi.org/10.3390/md11030934
Aparicio IM, Scharfstein J, Lima APCA (2004) A new cruzipain-mediated pathway of human cell invasion by Trypanosoma cruzi requires trypomastigote membranes. Infect Immun 72:5892–5902. https://doi.org/10.1128/IAI.72.10.5892-5902.2004
Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529:57–68
Pérez-Cruz K, Moncada-Basualto M, Morales-Valenzuela J et al (2018) Synthesis and antioxidant study of new polyphenolic hybrid-coumarins. Arab J Chem 11:525–537. https://doi.org/10.1016/j.arabjc.2017.05.007
Feriani A, Khdhiri E, Tir M et al (2020) (E)-N ′-(1-(7-Hydroxy-2-Oxo-2H-Chromen-3-Yl) Ethylidene) benzohydrazide, a novel synthesized coumarin, ameliorates isoproterenol-induced myocardial infarction in rats through attenuating oxidative stress, inflammation, and apoptosis. Oxid Med Cell Longev 2020:1–15. https://doi.org/10.1155/2020/2432918
Wilkinson SR, Temperton NJ, Mondragon A, Kelly JM (2000) Distinct mitochondrial and cytosolic enzymes mediate trypanothione- dependent peroxide metabolism in Trypanosoma cruzi. J Biol Chem 275:8220–8225. https://doi.org/10.1074/jbc.275.11.8220
Finzi JK, Chiavegatto CWM, Corat KF et al (2004) Trypanosoma cruzi response to the oxidative stress generated by hydrogen peroxide. Mol Biochem Parasitol 133:37–43. https://doi.org/10.1016/j.molbiopara.2003.08.011
Balana-Fouce R, MarÍa E, Alunda JM (1991) Leishmania infantu: polyamine biosynthesis and levels during the growth of promastigotes. Int J Biochem 23:1213–1217. https://doi.org/10.1016/0020-711X(91)90218-C
Alcolea PJ, Alonso A, García-Tabares F et al (2016) Proteome profiling of the growth phases of Leishmania pifanoi promastigotes in axenic culture reveals differential abundance of immunostimulatory proteins. Acta Trop 158:240–247. https://doi.org/10.1016/j.actatropica.2016.03.015
Brookes PS (2005) Mitochondrial H + leak and ROS generation: an odd couple. Free Radic Biol Med 38:12–23
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312
Mehta A, Shaha C (2004) Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J Biol Chem 279:11798–11813. https://doi.org/10.1074/jbc.M309341200
Saudagar P, Saha P, Saikia AK, Dubey VK (2013) Molecular mechanism underlying antileishmanial effect of oxabicyclo[3.3.1]nonanones: inhibition of key redox enzymes of the pathogen. Eur J Pharm Biopharm 85:569–577. https://doi.org/10.1016/j.ejpb.2013.08.014
Dagnino APA, Mesquita CS, Dorneles GP et al (2018) Phloroglucinol derivatives from hypericum species trigger mitochondrial dysfunction in Leishmania amazonensis. Camb Law J 145:1199–1209. https://doi.org/10.1017/S0031182018000203
Mandlik V, Patil S, Bopanna R et al (2016) Biological activity of coumarin derivatives as anti-leishmanial agents. PLoS ONE 11:e0164585. https://doi.org/10.1371/journal.pone.0164585
Colombo FA, Azara Reis R, Barbosa Nunes J et al (2017) In vivo evaluation of leishmanicidal activity of benzophenone derivatives by qPCR. Med Chem (Los Angeles) 07:890–893. https://doi.org/10.4172/2161-0444.1000448
Singh S, Dwivedi R, Chaturvedi V (2012) Influence of vehicles used for oral dosing of test molecules on the progression of Mycobacterium tuberculosis infection in mice. Antimicrob Agents Chemother 56:6026–6028. https://doi.org/10.1128/AAC.01702-12
Boroujerdi M (2015) Pharmacokinetics and toxicokinetics, 1st edn. Taylor & Francis Group, Boca Raton
Charlton RL, Rossi-Bergmann B, Denny PW, Steel PG (2018) Repurposing as a strategy for the discovery of new anti-leishmanials: the-state-of-the-art. Parasitology 145:219–236
Kermani EK, Sajjadi SE, Hejazi SH et al (2016) Anti-leishmania activity of osthole. Pharmacogn Res 8:S1–S4. https://doi.org/10.4103/0974-8490.178650
Funding
This work was supported by PPM-00482-16 from FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), INCT-INOFAR from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FINEP (Financiadora de Estudos e Projetos), Núcleo de Estudos, Pesquisa e Assessoria à Saúde.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Figueiredo Peloso, E., Merli, R.J., Espuri, P.F. et al. Investigation of 8-methoxy-3-(4-nitrobenzoyl)-6-propyl-2H-chromen-2-one as a promising coumarin compound for the development of a new and orally effective antileishmanial agent. Mol Biol Rep 47, 8465–8474 (2020). https://doi.org/10.1007/s11033-020-05887-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05887-5