Abstract
The occurrence of the insect vector (sand flies) with low rates of Leishmania infection, as well as autochthonous transmission in the absence of the natural vector in dogs, have been reported. These unexpected data suggest a hypothesis of other arthropods as a possible way of Leishmania transmission. The prevalence of Leishmania (Leishmania) infantum in fleas and ticks collected from dogs with canine visceral leishmaniasis (CVL), as well as parasite viability, were evaluated herein. The presence of L. (L.) infantum was assayed by PCR and ELISA in ectoparasites and biological samples from 73 dogs living in a Brazilian endemic area. As the occurrence of Leishmania DNA in ticks and fleas is expected given their blood-feeding habits, we next investigated whether parasites can remain viable inside ticks. PCR and ELISA confirmed that 83% of the dogs had CVL. Fleas and ticks (nymphs, male and female adults) were collected in 55% and 63% of the 73 dogs, respectively. Out of the 60 dogs with CVL, 80% harbored ectoparasites infected with L. (L.) infantum. The infection rates of the ectoparasites were 23% and 50% for fleas and ticks, respectively. The RNA analysis of the extract from ticks left in laboratory conditions during 7 to 10 days after removal from CVL dogs showed that parasites were alive. In addition, live parasites were also detected inside adult ticks recently molted in laboratory conditions. These findings indicate a higher infection rate of L. (L.) infantum in ticks and fleas, but they do not conclusively demonstrate whether these ticks can act as vectors of CVL, despite the fact that their rates were higher than those previously described in Lutzomyia longipalpis. The presence of viable L. (L.) infantum in ticks suggests the possible importance of dog ectoparasites in CVL dissemination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmania causes a wide spectrum of human diseases, ranging from self-limited cutaneous forms to the more severe diffuse cutaneous and visceral forms, as a consequence of the complex host immunological response and the species of parasite involved. Particularly, visceral leishmaniasis is an important anthropozoonosis in which men and dogs may act as hosts. The disease has spread worldwide and is found in the Americas, Africa, Southern Europe, and Asia. In the Americas, it is prevalent from Mexico to Argentina (Grimaldi and Tesh 1993; Lainson and Shaw 1998).
The worldwide prevalence of visceral leishmaniasis is estimated at 400,000 to 600,000 new cases per year (Stuart et al. 2008; WHO 2010). In Brazil, between 2,500 and 5,000 cases are reported each year, with 600 in Sao Paulo state (MSB 2010). Another considerable problem is the urbanization of the infection, with a growing incidence in periurban and urban cases. Approximately 10% of the populations who live in endemic areas are at risk for acquiring the infection (CVE 2010).
Viscerotropic leishmaniasis in Latin America is caused by Leishmania (Leishmania) infantum (Degrave et al. 1994; Lainson and Shaw 1998). Amastigote forms live and multiply by binary division within cells of the mononuclear phagocytic system of their mammalian hosts (Grimaldi and Tesh 1993). The extracellular promastigote forms of the protozoan occur in the gut of phlebotomine sand flies. The main vector is Lutzomyia longipalpis; however, other phlebotomine species may also be potential vectors (Gaskin et al. 2002; Lainson and Rangel 2005). Natural transmission occurs principally by the bite of infected sand flies, although other means of transmission may be involved, since in some places of South America, these insects usually show low rates of natural infection. Alternative transmission has been discussed, particularly in some areas where cases of canine visceral leishmaniasis (CVL) have been reported, but the presence of proven vectors has not yet been demonstrated (Gaskin et al. 2002; Dantas-Torres et al. 2005; Schantz et al. 2005; Duprey et al. 2006). In Sao Paulo state, a high incidence of CVL has been observed despite low or no occurrence of its natural vector L. longipalpis in some counties (Costa et al. 2001; Camargo-Neves 2004). Alternative means of transmission might include dog bites; transplacental, sexual, and blood transfusion; and arthropods such as fleas and ticks (Coutinho et al. 2005; Rosypal et al. 2005; Duprey et al. 2006; Freitas et al. 2006; Coutinho and Linardi 2007; Silva et al. 2009a, b).
The hypothesis of the transmission of L. (L.) infantum by fleas (Ctenocephalides felis felis) and ticks (Rhipicephalus sanguineus) has long been discussed (Blanc and Caminopetros 1930; Giraud et al. 1954; Sherlock 1964) and has already been demonstrated experimentally (Coutinho et al. 2005; Coutinho and Linardi 2007; Dantas-Torres et al. 2010a, b; Paz et al. 2010). Ticks and fleas are widely distributed and abundant among high-density canine populations. In addition, they have high reproductive rates. Ticks fed on blood may undergo long periods of fasting. If fed, R. sanguineus can live between 160 and 170 days until reaching adulthood. Unfed adults can survive for 12 months or more under standardized laboratory conditions (Troughton and Levin 2007). The dietary habit associated with high longevity may contribute to the species being a potential vector of leishmaniasis in endemic areas.
These data led us to investigate two subjects. The first was the prevalence of L. (L.) infantum in fleas and ticks (nymphs, female and male adults), by PCR, collected from a group of dogs naturally infected in an endemic area of CLV in Sao Paulo, Brazil. The second was whether ticks were infected with live parasites, using gene expression.
Materials and methods
Canine samples, ectoparasites, and parasite strain
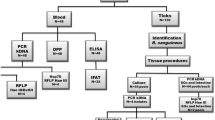
From August 2008 to May 2009, we collected ectoparasites and biological samples from 73 dogs living in Mirandópolis, a small city located in northwest Sao Paulo state, Brazil. The dogs were brought to the “Zoonosis Control Center,” as part of the standard CVL control policy in Brazil. The majority of the animals were stray dogs or were sent by the owners due to the onset of clinical signs of disease. The animals were selected considering epidemiological risk factors, such as proximity to other infected dogs as well as serological diagnosis, signs, or symptoms of the disease. All dogs showed clinical signs of the disease such as onychogryphosis, alopecia, lymphadenopathy, ulcerative lesions, intense itching, opaque peltry, seborrheic dermatitis, hyporexia, and cachexia. After immobilization and clinical examination, each dog was sedated for the removal of all ticks and/or fleas on the entire body, using forceps and/or by picking off with the fingers. After being removed from the dogs, the ectoparasites were stored in plastic tubes and individually identified. Ticks were identified and separated into groups of nymphs and female and male adults according to the classification previously described (Aragão 1936). Fleas were identified according to the classification described in Linari and Guimarães (2000),
The specimen number (ticks or fleas) varied among dogs (10 to 50 per animal). The tubes containing the ectoparasites were transported to the Instituto Adolfo Lutz (around 600 km) within 7 to 10 days and stored at −20°C until use. For parasite viability experiments, 25 nymphs collected from ten dogs were used; these were maintained in plastic petri dishes at 22°C until reaching adulthood. Collected canine sera and lymph node necropsy fragments were stored at −20°C until use.
DNA obtained from L. (L.) infantum (MHOM/BR/72/LD/strain 46; WHO reference strain) was used as a positive control in molecular techniques and as antigen in ELISA. Promastigotes were grown at 25°C in Eagle's medium, supplemented with 292 mg/L l-glutamine, 110 mg/L sodium pyruvate, 2.2 g/L sodium bicarbonate, 0.02% hemin, 10% heat-inactivated fetal calf serum, and 200 μg/ml gentamicin (Armstrong and Patterson 1994; Gomes et al. 2007). In the log curve phase, parasites were harvested and washed three times in phosphate-buffered saline (pH 7.2) at 1,000 g for 10 min. This study was performed according to the recommendations of the “Colegio Brasileiro de Experimentação Animal” (COBEA) and approved by ethics committees of the institutions involved.
Serological diagnosis in dogs
The assays were made by ELISA using a crude soluble antigen of L. (L.) infantum promastigotes. The parasite pellets, dissolved in phosphate-buffered saline, were lysed by freeze–thawing, followed by sonication, and centrifuged at 10,000×g for 20 min at 4°C. The supernatant was used as antigen in a concentration of 10 μg/ml using high-binding microplates. Canine serum samples were diluted 1:400 in PBS-T, and bound antibodies were detected using canine anti-IgG alkaline-phosphatase conjugant followed by cromogen solution (10 mg/ml of pNPP). The optical density reactions were read at 405 nm. Positive and negative control sera were run in each plate to standardize the readings and plate variations. The cutoff point between negative and positive results was calculated as the mean of the negative controls plus three standard deviations.
Molecular experiments
DNA and RNA extractions
Canine DNA samples were obtained from lymph node necropsy fragments. Ectoparasite DNA samples were taken in groups per dog. The number of the ectoparasites used in DNA or RNA extraction depended on the infestation of each animal. Normally, groups of female and male ticks, nymphs, or fleas for each dog were composed of two to five specimens per extraction. The whole bodies of the ectoparasites were re-frozen with liquid nitrogen and homogenized into a mash with the aid of a disposable plastic pestle. For DNA extraction, lymph node fragments and ectoparasites were dissolved in 10 mM tris–HCl (pH 8.0), 10 mM EDTA, 0.5% SDS, 0.01% N-laurilsarcozyl, and 100 μg/ml proteinase K, vortex mixed, and incubated at 56°C until complete cell lysis (around 18 h). DNA was extracted using PureLink Genomic DNA Kit (Invitrogen) according to the manufacturer's instructions. The positive samples in PCR were selected for RNA extraction using two to five specimens per dog. RNA molecules were extracted and purified using the Spin Tissue RNA Mini Kit (Invisorb) according to the manufacturer's instructions. DNA and RNA purities were determined at 260 and 280 nm, respectively, in a NanoDrop ND100 (Thermo Scientific).
Primer selection
The experiments for PCR were made using the following primer sets: The presence of L. (L.) infantum in canine and ectoparasite samples was determined by RV1-RV2 marker (5′CTTTTCTGGTCCCGCGGGTAGG3′ and 5′CCACCTGGCCTATTTTACACCA3′), which amplified a 145-bp product from LT1 fragment of kDNA minicircles of the Leishmania. donovani complex (Ravel et al. 1995; Le Fichoux et al. 1999; Gomes et al. 2007). The determination of live parasites in the ectoparasites was made by reverse transcription PCR (RT PCR) using RV1-RV2 and PRP (5′CGCGCAAGCAGAAGATCCACAACC3′ and 5′TCCATGAACTGCGGCTTGTCC 3′) markers. PRP amplified a 272-bp sequence from L. (L.) infantum JPCM5 paraflagellar rod protein (partial mRNA-GeneBank accession number XM_0014645931). To control the course of extraction and check for PCR inhibitors, all canine samples were assayed using the marker GAPDH 4 (5′AGGCTGAGAACGGGAAACTT3′ and 5′ATTAAGTTGGGGCAGGGACT3′) (Kullberg et al. 2006) that amplified a 911-bp fragment of a canine glyceraldehyde-3-phosphate dehydrogenase. The reactions using the canine primer pair were run simultaneously with the same temperature protocol for the RV1-RV2 marker and in the same PCR machine. All positive PCRs for canine GAPDH confirm the good quality of dog DNA extractions. DNA sequencing was made using the HPLJ31 marker (5′ATGCAGCTGCTGATTGTCG3′ and 5′CAGGTGCAGCTCATCAGG3′), which amplified 140 bp from L. (L.) infantum hypothetical protein, partial mRNA (GeneBank accession number XM_001467352). The LINJ31 primer set for a real-time PCR was designed in Primer Express software by Applied Biosystems and was also based on the same L. (L.) infantum hypothetical protein (partial mRNA; GeneBank accession number LinJ31.1310). The LINJ31 designer is 5′CCGCGTGCCTGTCG3′ and 5′CCCACACAAGCGGGAACT3′, and the TaqMan probe, FAM dye-labeled, is 5′CCTCCTTGGACTTTGC3′. The primer set had NFQ as reporter quencher.
Conventional PCR and reverse transcriptase PCR (RT-PCR)
After RNA extraction, the samples were immediately processed for cDNA syntheses. Each RT-PCR reaction was performed according to the manufacturer's instructions (Invitrogen). The RNA samples (11 μl) were mixed with 1 mM dNTP, 3 μg random primers, and 10 mM DTT in 250 mM tris–HCl ( pH 8.3) in a final volume of 20 μl. cDNA samples were stored at −20°C until use. Each PCR was performed by adding 5 μl of DNA template (or cDNA) and 25 pmol of each primer, using a kit purchased from Promega (Go Taq Green Master Mix), in a final volume of 25 μl. The PCR mix (12.5 μl) was composed of 1 unit of Taq DNA polymerase, 10 mM tris–HCl (pH 8.5), 50 mM KCl, 1.5 mM MgCl2, and 200 mM of each dNTP. Each amplification run contained two negative controls (ultra-pure water and a canine DNA sample negative for Leishmania) and one positive control (DNA extract of L. (L.) infantum promastigotes). After the thermal cycles, PCR products were electrophoresed in 2% agarose gel and stained with ethidium bromide. DNA fragments were visualized under UV illumination. The images were analyzed by a Mini Bis Gel Imager and Documentation (BioSystematica). The fragment sizes were estimated based on comparisons with a 100-bp ladder.
Real-time PCR
The reactions were performed with Applied Biosystems (ABI 7300 Real Time PCR System) in a final volume of 20 μl. The samples or control DNA was added to a reaction mixture containing 10 μl of 2× TaqMan Universal PCR Master Mix (NoAmpliErase UNG) and 1 μl of the “Assay Mix” that included: (i) the forward primer, 18 μM; (ii) the reverse primer, 18 μM; (iii) the TaqMan probe, FAM dye-labeled, 5 μM and reporter quencher NFQ. Amplification runs contained two negative controls and one positive control, as used in PCR. The thermal profile was performed at 50°C for 2 min for optimal AmpliErase UNG activity and at 95°C for 10 min. Next, 40 cycles of 95°C for 15 s and 60°C for 1 min were performed. Reactions were previously standardized using as template serial promastigote DNA dilutions. The cycle threshold value C T was indicative of the quantity of the target gene.
DNA sequencing
The RT PCR and real-time PCR product (from ticks) were used for DNA sequencing after purification using a “Wizard SV gel and PCR Clean-up System kit” (PROMEGA) according to the manufacturer's instructions. The fragments were sequenced using forward and reverse primers to amplify the sequence of HP(LJ31). Sequencing was done using the ABI Prism BigDye Terminator Cycle Sequencing Kit. Reaction and electrophoreses were made in an ABI Prism 377 DNA Sequencer. Nucleotide sequences were analyzed, manually assembled, and aligned for comparison using BioEdit Sequence Alignment Editor. The sequence alignments were compared to the sequences in the GeneBank database using basic local alignment search tool (BLAST) from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST).
Results
Occurrence of CVL and frequency of infestation with ticks and fleas in dogs
An overview of the occurrence of CVL in the canine population studied as well as the frequency of infestation with ticks and fleas is shown in Table 1. The presence of anti-Leishmania antibodies (by ELISA) and L. (L.) infantum DNA molecules in the lymph node fragments (by PCR using the marker RV1/RV2) indicated 60 of the 73 dogs were positive for CVL. The majority of the animals were infested with a large number of fleas and ticks (around 10 to 50 specimens per animal). From the 73 dogs, 40 (55%) hosted fleas (32 had CVL). Tick nymphs were shown in 24 dogs (33%), of which 18 had CVL. Male ticks were present in 41 dogs (56%), of which 34 had CVL. Females were extremely abundant in this group of dogs. A substantial portion of dogs (46–63%) were infected with female ticks with a large quantity of dog blood. CVL was observed in 39 of them.
Prevalence of L. (L.) infantum in ticks and fleas collected from dogs with CVL
The percent of ticks and fleas infected with L. (L.) infantum collected from dogs with CVL is shown in Table 2. Out of the 60 dogs with CVL, 12 harbored ectoparasites negative for L. (L.) infantum in PCR. The other 48 dogs (80%) harbored ectoparasites infected with L. (L.) infantum. From them, 17 (28.3%) had positive fleas. Positive nymph and female and male ticks were present in 14 (23.3%), 20 (33.3%) and 30 (50%) dogs, respectively. No fleas and ticks collected from the 13 dogs without CVL were infected with L. (L.) infantum.
Investigation of the viability of L. (L.) infantum into ticks collected from dogs with CVL
The second part of our study was to investigate the viability of L. (L.) infantum, using gene expression, in ticks collected from naturally infected dogs. As the presence of RNA of L. (L.) infantum indicates live parasites in the ticks, paired samples (DNA and RNA from the same group of ticks) were tested. The experiments were made by RT PCR, testing the internal contents from ticks in pool per dog, since they have greater blood volume than the fleas. L. (L.) infantum was determined using the molecular marker RV1-RV2. As shown in Fig. 1a, both products were amplified, suggesting that the parasites remain alive inside the ticks.
L. (L.) infantum viability inside the ticks determined by RT PCR and real-time PCR. Amplified products indicated in panels a and b were analyzed in electrophoresis 2% agarose gel. Panel a shows two 145-bp products amplified by the RV1/RV2 marker which is specific to a region of kDNA minicircles from the L. donovani complex. Lane 2, DNA and lane 3, RNA extracted from two different pools of female ticks collected from the same dog. Panel b shows the RT PCR using a sample of cDNA from RNA extracted from a group of recently molting adult ticks. Line 5, 272-bp product amplified from Leishmania paraflagellar rod protein using the marker PRP; line 6, 145-bp product amplified by the RV1/RV2; and line 7, positive control using DNA of an infected dog (using the RV1/RV2 marker). Lanes 4 and 7, standard molecular size of 100-bp ladder. Panel c shows the concentrations (in picograms per microliter) of L. (L.) infantum cDNA extracted from a group of fleas (F), adult female ticks (AFT), adult male ticks (AMT), nymph ticks (NT), and recently molting adult ticks (MAT). The results are expressed in means and standard deviations of 5 experiments per group, except MAT that was tested only in one sample. *Concentrations of L. (L.) infantum cDNA were previously standardized, correlating the C T value and known concentrations of L. (L.) infantum promastigotes (MHOM/BR/72/LD/strain 46–WHO reference strain) maintained in culture
Next, in order to investigate whether parasites were able to remain alive after tick ecdysis, a group of 25 ticks in the nymph stage was kept alive in laboratory conditions until molting. Then, RNA molecules were extracted from 10 recently molting adult ticks and subsequent synthesis of cDNA. As shown in Fig. 1b, two amplified PCR products from two markers (RV1-RV2 and PRP), which amplify different regions of L. (L.) infantum regions, were amplified. These results suggested that parasites remain alive inside the ticks after molting. These data were further confirmed by real-time PCR after determination of L. (L.) infantum cDNA concentrations. As shown in Fig. 1c, real-time PCR detected 1.3 ± 0.47, 2.7 ± 0.7, 1.4 ± 0.15, and 2.5 ± 1.04 pg/μl in 5 RNA samples/group of fleas, adult female, adult male, and nymph ticks, respectively. A single RNA sample from the group of the recently molting adult ticks presented the concentration of 1.1 pg/μl. These data suggest the ability of ticks to preserve viable genetic material after ecdysis from the nymph stage to adult.
The high sensitivity and specificity of RV1-RV2 molecular marker were previously investigated in samples collected from dogs living in the Mediterranean region (Lachaud et al. 2002) and Brazil (Gomes et al. 2007). Specificity was further confirmed by testing DNA extracted from other trypanosomatids, such as Leishmania (Leishmania) amazonensis, Leishmania (Viannia) braziliensis, and Trypanosoma cruzi, and other canine disease-causing bacteria such as Rickettsia rickettsii and Ehrlichia canis (Gomes et al. 2007; Pereira-Chioccola 2009). These experiments were also complemented by analysis of the 140-bp sequence amplified by HPLJ31 marker by DNA sequencing. The alignments evaluated in NCBI/nucleotide–nucleotide BLAST revealed 89% homology with the following sequences in Genebank: L. donovani donovani strain AG83, differentially regulated gene 1 (DRG1), and gene 2 (DRG2) (accession numbers GQ214330.1 and GQ214331.1, respectively). L. infantum JPCM5 hypothetical proteins (LinJ31.1310 and LinJ31.1270) (accession numbers XM_001467352 and XM_001467348, respectively).
Discussion
Visceral leishmaniasis has been increasing worldwide, principally due to a substantial rise in human and domestic animal traffic contributing to spreading leishmanial infection in low- or non-endemic areas (Stuart et al. 2008; Aagaard-Hansen et al. 2010; WHO 2010). Canine infection is considered a major potentially fatal zoonotic infection in regions of Europe, Africa, Asia, and South America. Particularly in South America, millions of dogs are infected. In some endemic regions, the prevalence of CVL is around 63–80% (Dantas-Torres 2006; Dantas-Torres 2007; Baneth et al. 2008; MSB 2010). This situation has led in recent years to diagnosing CVL in previously non-endemic areas such as different regions of São Paulo state in Brazil. In addition, the occurrence of L. longipalpis with low rates of Leishmania infection have been reported in different Brazilian endemic areas (Missawa and Dias 2007; Michalsky et al. 2009; Savani et al. 2009). Similar situations have been reported in other countries. Autochthonous transmission in the absence of the natural vector in dogs has occurred in the USA, Canada, and Netherlands (Díaz-Espińeira and Slappendel 1997; Gaskin et al. 2002; Duprey et al. 2006). All these data together suggest that other transmission means may be involved in the Leishmania spp. cycle. As one hypothesis proposes transmission by other arthropods, the prevalence of L. (L.) infantum in ticks, as well as their viability, was evaluated herein.
Previous studies experimentally demonstrated the capacity of ectoparasites to be infected by Leishmania, as well as their ability to promote the infection by means of the inoculation of ticks or fleas triturates in rodents (Coutinho et al. 2005; Coutinho and Linardi 2007; Ferreira et al. 2009).
In our study from the 73 dogs, the laboratory diagnosis confirmed that 83% were infected, since this region is endemic for CVL with many cases per year. Interestingly, the occurrence of L. longipalpis is usually low in certain seasons of the year (Odorizzi and Galati 2007; CVE 2010), and the majority of the studied animals were infested with a large number of fleas and ticks. Fleas were collected in 55% of the 73 studied dogs. Nymph, male and female ticks were collected in the majority of them.
The high infection rate in the ectoparasites found in our study is related to the ectoparasite's habitat, as this parasite is mostly found living on the skin of dogs. Around 80% of the studied dogs with CVL harbored ectoparasites (fleas and/or ticks) infected with L. (L.) infantum. Differently, sand flies occasionally have contact with the dog's skin and, therefore, may represent fewer chances for infection. In addition, previous studies demonstrated that the infection rate of sand flies in endemic areas has been estimated as less than 1% (Missawa and Dias 2007; Michalsky et al. 2009; Savani et al. 2009). However, Leishmania could be adapting to other vectors independent of the occurrence or non-occurrence of the natural vector (Costa et al. 2001; Camargo-Neves 2004; Dantas-Torres et al. 2005; Odorizzi and Galati 2007). In addition to our data being consistent with other studies (Coutinho et al. 2005; Coutinho and Linardi 2007; Ferreira et al. 2009; Paz et al. 2010), they also could indicate a higher infection rate by L. (L.) infantum ticks and fleas, compared with that found in L. longipalpis. These findings do not conclusively demonstrate whether these ectoparasites can act as vectors of CVL, but they show a high incidence of infection by L. (L.) infantum.
As the occurrence of L. (L.) infantum DNA from ticks and fleas is expected, given their blood-feeding habits, the second part of this study was directed at investigating whether parasites remain viable inside ticks. The results showed that parasites were alive for a long period (7 to 10 days after removal from the dogs). In addition, live parasites could be detected inside ticks after ecdysis, which occurred in laboratory conditions.
These findings, combined with a recent study that detected Leishmania kDNA in the salivary glands of R. sanguineus (Dantas-Torres et al. 2010a, b), suggest that during blood feeding, ticks could release live parasites, since they excrete a large amount of liquid during the feeding. The presence of viable L. (L.) infantum in ticks suggests the possible importance of dog ectoparasites in CVL dissemination.
References
Aagaard-Hansen J, Nombela N, Alvar J (2010) Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop Med Int Health 15:1281–1288
Aragão HB (1936) Ixodidas brasileiros e de alguns paizes limitrophes. Mem Inst Oswaldo Cruz 31:759–844
Armstrong TC, Patterson JL (1994) Cultivation of Leishmania braziliensis in an economical serum-free medium containing human urine. J Parasitol 80:1030–1032
Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer F (2008) Canine leishmaniasis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol 24:324–330
Blanc G, Caminopetros J (1930) La transmission du Kala—Azar méditerranéen par une tique: Rhipicephalus sanguineus. Compt Rendus Acad Sci 191:1162–1164
Camargo-Neves VLF (2004) A leishmaniose visceral americana no Estado de São Paulo. Estado atual. Bol Epid Paul 6. At: http://www.cve.saude.sp.gov.br
Costa CHN, Gomes ACG, Costa JML, Vieira JBF, Lima JWO, Dietz R (2001) Changes in the control program of visceral leishmaniasis in Brazil. Rev Soc Bras Med Trop 34:223–228
Coutinho MT, Linardi PM (2007) Can fleas from dogs infected with canine visceral leishmaniasis transfer the infection to other mammals? Vet Parasitol 147:320–325
Coutinho MT, Bueno LL, Sterzik A, Fujiwara RT, Botelho JR, Maria M, Genaro O, Linardi PM (2005) Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Vet Parasitol 128:149–155
CVE–Centro de Vigilância Epidemiológica do Estado de São Paulo (Surveillance Epidemiological Center of Sao Paulo State) (2010). Site in Portuguese. At: http://www.cve.saude.sp.gov.br
Dantas-Torres F (2006) Current epidemiological status of visceral leishmaniasis in Northeastern Brazil. Rev Saude Pub 40:537–541
Dantas-Torres F (2007) The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol 10:139–146
Dantas-Torres F, Faustino MAG, Lima OC, Acioli RV (2005) Epidemiologic surveillance of canine visceral leishmaniasis in the municipality of Recife, Pernambuco. Rev Soc Bras Med Trop 38:444–445
Dantas-Torres F, Lorusso V, Testini G, de Paiva-Cavalcanti M, Figueredo LA, Stanneck D, Mencke N, Brandão-Filho SP, Alves LC, Otranto D (2010a) Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol Res 106:857–860
Dantas-Torres F, Martins TF, de Paiva-Cavalcanti M, Figueredo LA, Lima BS, Brandão-Filho SP (2010b) Transovarial passage of Leishmania infantum kDNA in artificially infected Rhipicephalus sanguineus. Exp Parasitol 125:184–185
Degrave W, Fernandes O, Campebell D, Bozza M, Lopes U (1994) Use of molecular probes and PCR for detection and typing of Leishmania—a mini review. Mem Inst Oswaldo Cruz 89:463–469
Díaz-Espińeira MM, Slappendel RJ (1997) A case of autochthonous canine leishmaniasis in the Netherlands. Vet Q 19:69–71
Duprey ZH, Steurer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, Schantz PM (2006) Canine visceral leishmaniasis, United States and Canada, 2000-2003. Emerg Infect Dis 12:440–446
Ferreira MG, Fattori KR, Souza F, Lima VM (2009) Potential role for dog fleas in the cycle of Leishmania spp. Vet Parasitol 165:150–154
Freitas E, Melo MN, Costa-Val AP, Michalick MS (2006) Transmission of Leishmania infantum via blood transfusion in dogs: potential for infection and importance of clinical factors. Vet Parasitol 137:159–167
Gaskin AA, Schantz P, Jackson J, Birkenheuer A, Tomlinson L, Gramiccia M, Levy M, Steurer F, Kollmar E, Hegarty BC, Ahn A, Breitschwerdt EB (2002) Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med 16:34–44
Giraud P, Ranque J, Cabassu H (1954) Epidemiologie de la leishmaniose viscérale humaine mediterranéenne, en particulier dans sés rapports avec la leishmaniose canine. Arch Fr Ped 11:337–353
Gomes AH, Ferreira IM, Lima ML, Cunha EA, Garcia AS, Araujo MF, Pereira-Chioccola VL (2007) PCR identification of Leishmania in diagnosis and control of canine leishmaniasis. Vet Parasitol 144:234–241
Grimaldi G, Tesh RB (1993) Leishmaniasis of the New World: current concepts and implications for the future research. Clin Microbiol Rev 6:230–250
Kullberg M, Nilsson MA, Arnason U, Harley EH, Janke A (2006) Housekeeping genes for phylogenetic analysis of eutherian relationships. Mol Biol Evol 23:1493–1503
Lachaud L, Marchergui-Hammami S, Chabbert E, Dereure J, Dedet JP, Bastien P (2002) Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol 40:210–215
Lainson R, Rangel EF (2005) Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil–a review. Mem Inst Oswaldo Cruz 100:811–827
Lainson R, Shaw JJ (1998) New world leishmaniasis–the neotropical Leishmania species. In: Collier L, Balows A, Sussman M (eds) Topley & Wilson's microbiology and microbial infections, vol 5, 9th edn. Wiley, London
Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J (1999) Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol 37:1953–1957
Linari PM, Guimarães LR (2000) Sifonápteros do Brasil. Ed. Museu de Zoologia USP/FAPESP. 291pp
Michalsky EM, Fortes-Dias CL, França-Silva JC, Rocha MF, Barata RA, Dias ES (2009) Association of Lutzomyia longipalpis (Diptera: Psychodidae) population density with climate variables in Montes Claros, an area of American visceral leishmaniasis transmission in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 104:1191–1193
Missawa NA, Dias ES (2007) Phlebotomine sand flies (Diptera: Psychodidae) in the municipality of Várzea Grande: an area of transmission of visceral leishmaniasis in the state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz 102:913–918
MSB–Ministerio da Saude do Brasil (Health Ministry of Brazil) (2010) Manual de vigilância e controle da leishmaniose visceral (visceral leishmaniasis: surveillance and control. Technical manual) (in Portuguese). At http://portal.saude.gov.br/portal/svs
Odorizzi RM, Galati EA (2007) Sand flies in the Aguapeí river floodplain, northwest area of State of São Paulo, Brazil. Rev Saude Publ 41:645–652
Paz GF, Ribeiro MF, Michalsky EM, da Rocha Lima AC, França-Silva JC, Barata RA, Fortes-Dias CL, Dias ES (2010) Evaluation of the vectorial capacity of Rhipicephalus sanguineus (Acari: Ixodidae) in the transmission of canine visceral leishmaniasis. Parasitol Res 6:523–528
Pereira-Chioccola VL (2009) Molecular diagnosis of leishmaniasis: contribution to the American visceral leishmaniasis surveillance program in Sao Paulo state. Bol Epidemiol Paul 6:4–13. doi:http://www.cve.saude.sp.gov.br
Ravel S, Cuny G, Reynes J, Veas F (1995) A highly sensitive and rapid procedure for direct PCR detection of Leishmania infantum within human peripheral blood mononuclear cells. Acta Trop 59:187–196
Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS (2005) Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol 91:970–972
Savani ES, Nunes VL, Galati EA, Castilho TM, Zampieri RA, Floeter-Winter LM (2009) The finding of Lutzomyia almerioi and Lutzomyia longipalpis naturally infected by Leishmania spp. in a cutaneous and canine visceral leishmaniases focus in Serra da Bodoquena, Brazil. Vet Parasitol 160:18–24
Schantz PM, Steurer FJ, Duprey ZH, Kurpel KP, Barr SC, Jackson JE, Breitschwerdt EB, Levy MG, Fox JC (2005) Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc 226:1316–1322
Sherlock IA (1964) Nota sobre a transmissão da leishmaniose visceral no Brasil. Rev Bras Malariol Doenças Trop 16:19–26
Silva FL, Oliveira RG, Silva TM, Xavier MN, Nascimento EF, Santos RL (2009a) Venereal transmission of canine visceral leishmaniasis. Vet Parasitol 160:55–59
Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MSM (2009b) First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol 3:159–162
Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, McKerrow J, Reed S, Tarleton R (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest 118:1301–1310
Troughton DR, Levin ML (2007) Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J Med Entomol 44:732–740
WHO–World Health Organization (2010) At: http://gamapserver.who.int/mapLibrary/Files/Documents/DD_visceral.pdf
Acknowledgments
This study was supported by grants from Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo, Brazil (FAPESP) (proc 08/00520-6). FAC was supported by fellowships from FAPESP (proc-08/57245-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colombo, F.A., Odorizzi, R.M.F.N., Laurenti, M.D. et al. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res 109, 267–274 (2011). https://doi.org/10.1007/s00436-010-2247-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2247-6