Abstract
Managing tendon healing process is complicated mainly due to the limited regeneration capacity of tendon tissue. Mesenchymal stem cells (MSCs) have potential applications in regenerative medicine and have been considered for tendon repair and regeneration. This study aimed to evaluate the capacity of equine adipose tissue-derived cells (eASCs) to differentiate into tenocytes in response to platelet-derived growth factor-BB (PDGF-BB) and growth differentiation factor-6 (GDF-6) in vitro. Frozen characterized eASCS of 3 mares were thawed and the cells were expanded in basic culture medium (DMEM supplemented with 10% FBS). The cells at passage 5 were treated for 14 days in different conditions including: (1) control group in basic culture medium (CM), (2) induction medium as IM (CM containing l-prolin, and ascorbic acid (AA)) supplemented with PDGF-BB (20 ng/ml), (3) IM supplemented with GDF-6 (20 ng/ml), and (4) IM supplemented with PDGF-BB and GDF-6. At the end of culture period (14th day), tenogenic differentiation was evaluated. Sirius Red staining was used to assess collagen production, and H&E was used for assessing cell morphology. mRNA levels of collagen type 1 (colI), scleraxis (SCX), and Mohawk (MKX), as tenogenic markers, were analyzed using real-time reverse-transcription polymerase chain reaction (qPCR). H&E staining showed a stretching and spindle shape (tenocyte-like) cells in all treated groups compared to unchanged from of cells in control groups. Also, Sirius red staining data showed a significant increase in collagen production in all treated groups compared with the control group. MKX expression was significantly increased in PDGF-BB and mixed groups and COLI expression was significantly increased only in PDGF-BB group. In conclusion, our results showed that PDGF-BB and GDF-6 combination could induce tenogenic differentiation in eASCs. These in vitro findings could be useful for cell therapy in equine regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendon function is to transfer force from muscle to attached bone which in turn triggers active movement. The tendon tissue is designed to withstand large tensile loads. When tendon is injured, the normal tissue of the tendon cannot be restored and instead healed by scar tissue intervention. This scar tissue has substances with properties less than the original tendon tissue which makes the surgical repair of the torn tendons prone to fracture. Therapeutic strategies such as prosthetic devices, autografts, allografts, or xenografts are being utilized to treat tendon and ligament injuries [1, 2]. Mesenchymal stem cells (MSCs), because of their potential to differentiate into tenocytes, are suggested for torn tendons, tendon growth, and improving tendon healing [3, 4].
Tissue engineering is an advanced method of repairing damaged tissue by a live tissue repair process based on three principles of cellular resources, appropriate scaffolding, and growth factors [5]. Regarding cellular sources, adipose-derived mesenchymal stem cells (ASCs) are ideal for tissue engineering because of their ease of isolation, rapid proliferation, differentiation to other cells, and low immunogenicity, as well as covering a large surface area in the body, cost-effectiveness, and being extractable [6]. These beneficial characteristics of ASCs distinguish them from other MSCs that are more widely used in tissue engineering and regenerative medicine (TERM) field [7]. ASCs are potentially applicable for tendon and ligament repair [6, 8] which their differentiation is induced by using growth factors (GF) [9], or by a combination of mechanical and biochemical stimulation in vitro [10, 11].
So far, none of the treatments has been able to completely repair the tendon tissue. However, tissue engineering is one of the most recent and fastest treatments for musculoskeletal injuries in horses and other animals [12]. Compared to MSCs derived from other sources, the major merit of ASCs is that adipose tissue offers patient-derived autologous MSCs, which is possible to be easily harvested with lower morbidity [9]. The result of ASCs per gram of tissue is 500-fold higher than that obtained for bone marrow MSCs (BM-MSCs) [13]. Cultured ASCs in tissue engineering (TE) has been taken into consideration for other stem-cell-based studies in the field of cell therapy [14].
Tendon extracellular matrix (ECM) synthesis could be controlled in vitro by some major growth factors, including transforming growth factors- β (TGF-β). Of special interests are growth differentiation factors (GDFs), especially GDF 5, 6 and 7, which are members of the TGF-β superfamily and are crucial for growth, homeostasis and tendon regeneration [15]. Numerous studies have shown that other growth factors such as bone morphogenetic proteins (BMP), TGF-b, and fibroblast growth factor (FGF) are included in the tenogenic differentiation of MSCs [16,17,18]. Studies have shown that ASCs respond to BMPs. Cartilage-derived morphogenetic protein-2 (CDMP-2), named also BMP-13 and GDF-6, is a BMP that belongs to the TGF-β family [15, 19,20,21]. GDF-6/BMP-13 has been shown that proliferate and produce collagen in tendon fibroblasts in vitro [22, 23]. It has also been reported that it induces ECM collagen synthesis and even small bone and cartilage growth in new tissue [21]. Actually, this growth factor is important in the growth of many cell types in different tissues, the function of cell proliferation and differentiation, embryonic growth, and the repair of a wide range of tissues [24].
Besides differentiation potential into tenocyte, MSCs produce many paracrine factors, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [25]. PDGF is composed of 3 isoforms (PDGF-AA, PDGF-BB, and PDGF-AB) that PDGF-BB isoform has the greater potential to stimulate cell proliferation [26, 27]. PDGF probably acts as one of the factors contributing to tendon and ligament recovery by regulating cell viability and differentiation. [28]. Additionally, PDGF is expressed at the wounds, which indicate that PDGF-BB can lead to a healing process through its mitogenic effect [29]. Despite the potential of PDGF as an important factor for tendon healing, there is little compelling evidence to confirm that PDGF-BB induces cell proliferation and matrix production in tendon samples [30]. Thus, in this study, we aimed to investigate the effect of BMP13 (GDF-6) and PDGF-BB as supplementary growth factors on differentiation of equine ASCs (eASCs) into tendon-like cells when they are added to culture medium alone or in combination form.
Materials and methods
All stages and protocols of the tests have been approved by the Ethics Committee of Ferdowsi University of Mashhad. All chemicals and cell culture media were prepared from Sigma or Gibco (St. Louis, Mo., USA) and tissue culture flasks and dishes from SPL (Korea).
Culture and proliferation of eASCs
Frozen eASCs of 3 mares which were previously characterized [31] were used. First, cells at passage 3 (P3) were thawed and cultured in basic medium containing Dulbecco’s adjusted Eagle Medium (DMEM), 10% fetal bovine serum (FBS, Gibco, USA ), 100 U/ml penicillin (P), and 100 µg /ml streptomycin (S) and the medium was replaced at 2 days intervals. The frozen cells of each sample (3 mares) at P3 were thawed and expanded until P5 to get the required enough cell number for next step.
Experimental design for tenogenic differentiation of eASCs
The experimental groups were designed into four categories (at least five replicates for each sample): (1) control group: cells were cultured in basic culture medium as CM (DMEM with 10% FBS and P/S), treated groups including (2) induction medium as IM (CM containing l-Prolin (0.34 mM), and Ascorbic Acid (0.17 mM)) supplemented with PDGF-BB (20 ng/ml), (3) IM supplemented with GDF-6 (20 ng/ml), and (4) IM supplemented with PDGF-BB and GDF-6. eASCs at P5 were implanted into six-well plates (3 × 103 cells /well) and culture was continued for 14 days.
Hematoxylin and eosin (H&E staining)
In order to assess cell morphology at the end of culture period (14th day), H&E staining was employed. By using 4% paraformaldehyde for 15 min and washing it twice with PBS, cells were fixed. For completely covering the base of the wells for 7 min, first PBS was separated from the wells and then hematoxylin solution was added. After 7 min, hematoxylin solution was aspirated and each well washed with distilled H2O to remove residual eosin solution. The wells were then counterstained with hematoxylin solution for 5 min and each well washed with double-distilled H2O. The cells were covered with PBS. Images were taken with an inverted microscope (Olympus IX41).
Sirius red staining
At 14th day, the medium was changed and the cells were washed with PBS. Before Sirius red staining, cells were fixated with 70% ethanol for 30 min and washed 3 times [32]. The deposited collagen was stained with 0.1% Sirius red (Direct Red 80 sigma 365,548) in a saturated aqueous solution of picric acid. To quantify the stained nodules, the stain was solubilized with 0.5 ml of 1:1 (vol/vol) 0.1% NaOH and pure methanol for 30 min at room temperature. Solubilized stain (0.1 ml) was added to wells of a 6-well plate, and absorbance was measured at 540 nm. Data are presented as mean ± SD.
RNA extraction, cDNA synthesis and quantitative RT-PCR (qPCR)
Cells at 14th day were collected and total RNA was extracted using Roche kit (Germany) based on manufacturer’s protocol. For cDNA synthesis, 1 µg of total RNA was reverse transcribed into cDNA using cDNA Synthesis Kit (Parstous, Iran). Quantitative polymerase chain reaction (qPCR) was performed to assess the expression levels of tendon-specific markers in cells cultured with different medium. By using Primer premier (version 5.0), all primers were designed and are reviewed in Table 1. qPCR was performed using SYBR Green qPCR Master Mix (amplicon). The PCR cycling was contained 30 cycles of amplification of the template DNA, with primer annealing at 58–64 °C. GAPDH, as reference gene, was used for normalizing the CT of target genes. The efficiency of all genes was calculated using standard curve of each gene, and the efficiency for target genes and GAPDH were almost the same. So, the expression level of each target gene was determined by using the 2−∆∆Ct method, and fold change related to the expression level of control sample (untreated eASCs). ∆Ct values were attained by the difference between the Ct values of target genes and the GAPDH gene. For obtaining ∆∆Ct values, these values were normalized by subtracting the ∆Ct value of the calibrator sample (control), their respective Ct value in basic medium condition. Outcomes are defined as relative gene expression in comparison with the calibrator sample that is equal to 1.
Statistical analysis
The data was gathered from optical absorption of Sirius Red staining on three mare samples with at least five replicates. Ct values from 3 treated samples and control group (with triplicate read) was obtained. Normal distribution of data was checked by Shapiro–Wilk test. Data are expressed as the mean ± SD and statistical significancy was assessed by one-way analysis of variance (ANOVA) test followed by post-hoc Tukey test using GraphPad Prism software (version 8.0; GraphPad Software, Inc.). A value of P < 0.05 was considered statistically significant.
Results
Cell morphology
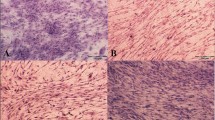
Tenocytes are similar in shape to long, narrow, and spindle-shaped morphology of fibroblasts arranged in rows one by one in the longitudinal direction of the tendon. As a result, it is important to study morphology of differentiated eASCs as an important criterion for tenogenic differentiation. The cells treated by PDGF-BB, GDF-6 or GDF-6 + PDGF-BB appeared more slender, elongated and spindle-shaped with thinner and longer cytoplasmic projections compared to control untreated cells (Fig. 1).
Morphologic appearance of eASCs in different culture conditions This H&E staining shows the change of eASC morphology under different treatments at day 14. As it can be seen, the cells appeared more slender, elongated and spindle-shaped with treatment by GDF-6 (b) and PDGF-BB (c) and also in combination of GDF-6 and PDGF (D) in comparison to cells at untreated control cells (a) which were cultured in the absence of differentiation factors. (Scale bars = 200 µm)
Collagen production assessment by Sirius Red Staining
Sirius Red is a unique method for assessing differentiation of tendons that dyes collagen I and III, with more focus on collagen I which is one of the main features of tendons. Data Analysis showed that all treated groups had higher optical absorption than the control group and the most distinguished belonged to PDGF-BB/GDF-6 group. This difference in PDGF-BB and PDGF-B/GDF-6 groups was significant compared to other groups (Fig. 2).
Production of collagen in eASCs induced with various growth factors. a Sirius red staining for collagen deposition evaluation in eASCs cultured in 6 well in the absence of growth factors (Ctrl) or the presence of GDF-6 (G), PDGF-BB (P) and mix of the two factors GDF-6 + PDGF-B (M) at day 14, which show the increased collagen production in the treatment groups in comparison to control group. b Quantification data of Sirius red staining. Data are presented as mean ± SD. According to OD, collagen levels increased in all three treatment groups compared to the control group According to these data, the amount of collagen in groups PDGF-BB and GDF-6/PDGF-BB has increased significantly compared to group GDF-6, and control. Ctrl control, OD optical density
Expression level of tendon specific markers by qPCR
Using qPCR, tendon markers including Scleraxis (SCX), Collagen type 1 (COLI), and Mohawk (MKX) were analyzed. qPCR results showed that PDGF-BB and also GDF-6 (alone or in combination) were able to activate tendon marker gene expression in eASCs.
In conclusion, the results indicated that both PDGF-BB and GDF-6 are capable of inducing tendon marker gene expression in eASCs. According to Fig. 3, with adding factors in culture medium and creating a distinctive environment for 14 days, these factors, alone or in combination, caused an increase in gene expression in comparison with the control group. The expression of COLI was significantly increased only in the PDGF-BB group in compare with control group (P = 0.008). MKX expression in the presence of factors was increased compared to the control group, and this increase in the presence of PDGF-BB and PDGF-BB/GDF-6 was significant (P = 0.001 and P = 0.000, respectively). Increased expression of SCX in treated groups was not significant in comparison with control group (P = 0.57).
The mRNA expression level of tendon markers including SCX, COLI and MKX. The expression of collagen 1a1 only in PDGF-BB group was significantly increased compared to the control group at day 14 (P < 0.5). Mkx significantly increased under the influence of PDGF-BB and GDF-6/PDGF-B groups compared to control and GDF-6 (P < 0.5). Data are shown mean ± SD
Discussion
The purpose of this study was to develop a reliable method to enhance the tenogenic differentiation of eASCs using growth factors in vitro. Two potential tenogenic factors, PDGF-BB and GDF-6, were chosen to assess their single or combined effects on the tenogenic differentiation of eASCs. Our results showed that PDGF-BB and GDF-6 particularly in combination form are efficient and effective for the induction of tenogenic differentiation of eASCs in vitro.
Although MSCs to be extracted from variety of tissues have significant similarities in distinctive traits, sometimes there are differences between them, and it seems that the origin of stem cells can be important in its distinctiveness. Also, using different cocktail of inductive factors play an important role in the degree of differentiation. The attainment of stem cells has raised great hopes for the treatment of many incurable degenerative diseases, however, it is necessary to examine their differentiation potential into different tissues (e.g. tendon) in the laboratory before their clinical use. Furthermore, one of the strategies for regeneration and tissue engineering is use of optimized, in vivo pre-differentiated stem cells to avoid unwanted differentiation, such as bone formation [33, 34].
Differentiated cells such as fibroblasts can also be a good cell source for regeneration of tendon. Although beneficial effects of fibroblasts for tendon repair has been reported [35], cutaneous fibroblasts have disadvantage of producing fibrotic ECM, which is involved in scarring. ASCs have a higher advantage in tissue engineering due to their differentiation potential, self-renewal capacity, being easily accessible from various tissues such as adipose, cord blood and bone marrow, with minor donor site complications. However, their inherent tendency toward fat may preclude the use of ASCs in tendon reconstruction [36]. To this end, intensive research was conducted to overcome this obstacle. Yu et al. (2016) showed that hypoxia or activation of factor-induced hypoxia-1 expression could improve wound healing by ASCs [37]. In addition, several studies have shown that growth factor supplementation can increase ASC cell proliferation and improve tendon repair efficiency, production of ECM components of tendon and TNMD [38].
Since repairing damaged tendon takes long time and this repair is interrupted with scar tissue, the new formed tissue has usually poorer quality than natural tendon. So, using an appropriate cell source in regenerative medicine such as MSCs could prevent the formation of scar tissue and a more effective tendon tissue will be produced. Despite the good characteristics of fibroblasts in tendon cell differentiation compared to other cells, cutaneous fibroblasts are harmful because they may produce fibrotic ECM, which is involved in scarring [36]. In addition, Ho et al. have compared potential of different cells (i.e. autologous tenocytes, fibroblasts, and autologous MSC) for the treatment of tendon lesions in clinical setting and introduced MSCs as the best choice [39].
Although many studies with successful results used growth factor supplementation for tenogenic differentiation of ASCs, it is hard to evaluate efficacy because of differences in cell sources, species, duration, and readout indices [11, 40, 41]. So, it is necessary to compare the potential growth factors shoulder-to-shoulder to determine which one is the most dominant factor for tenogenic differentiation in equine ASCs. Specific markers of most stages of tendon development need to be identified; nonetheless, so far we know that the process is associated with the initial emergence of tendon progenitor cells followed by differentiation and maturation [42]. During differentiation of MSC into a tendon-like cell, different molecules have priority in synthesis. SCX is a central transcription factor expressed at the early stages of tendon formation and known to stimulate the formation of tendon progenitors. MKX and COLI are downstream molecules, respectively, that are directly induced by SCX. Actually, SCX is required to stimulate the cell to become a tendon progenitor [9, 41, 43]. Via our approach, we could show comparable results with significant increases in expression of MKX and COL1 after 14 days of treatment. However, the expression of SCX was not significant in treated groups compared to the control group at 14th day. It is possible that its expression was increased at early days and was decreased at 14th day. Based on our results, we considered that these changes show the molecular mechanisms involved in eASCs undergoing tendon differentiation. Norelli et al. have studied the effect of GDF-5, 6 and 7 and PDGF-BB growth factors alone and in combination on rat ASCs and showed a time priority increase in expression of SCX and COLI in combination with two growth factors and in a combination of PDGF-BB and GDF-6 [38]. In another study, differentiation of rat bone marrow MSCs with GDF-6 at concentration of 20 ng/ml caused significant expression of the SCX gene [44]. These results may reflect the differences of molecular mechanisms in rat versus equine MScs during tendon differentiation process in vitro.
Many of tendon and ligament damages in race equine are similar to damages in athletes [35]. Therefore, equine has been used as an appropriate animal model and that is why we chose this model. It has been previously proven that 20 ng/ml concentration of the GDF-6 growth factor is effective to induce expression of tendon-specific gene markers [40]. A study by Gonçalves et al. (2013) regarding tenogenic differentiation of stem cells from human amniotic membrane (hHAFSCs) and from human adipose tissue (hASCs) with different growth factors has been reported. In 10 ng/ml concentration of PDGF-BB in both cell groups on the 21st-day, COLI and SCX had a significant increase in comparison with the control group, while in the 14th-day, CLOI and SCX were less than or equal to the control group [9] which shows the effect of time on tenogenic differentiation.
Mohanty et al. showed that stimulation of MSCs from the umbilical cord stimulated by BMP-12 leads to an increase in MKX expression [45]. It was also reported that human bone marrow MSCs under treatment by BMP-12 led to an increase in MKX expression as well [46]. In agreement with these studies, our results showed the increased expression of MKX as an important tendon transcription factor in PDGF-BB group.
Some recent studies suggest the new combinations of factors and culture conditions for induction of tenogenic differentiation of MSCs. Stanco et al. have shown that using serum-free medium (SF) or a xenogenic-free human pooled platelet lysate medium (hPL) in the presence of CTGF, TGFβ-3, BMP-12 and ascorbic acid (AA) promoted differentiation of hASCs into tenocyte-like cells [47]. The results of another study showed that BMP-12 causes late expression of SCX and MKX, while TGF-b1 leads to their earlier expression. Moreover, the addition of ascorbic acid with BMP-12 or TGF-b1 resulted in increased collagen I deposition [48]. Rajpar et al. induced tenogenic differentiation of equine BM-MSC in the presence of collagen I hydrogels and growth factors FGF-2, TGF-β1, IGF-1 and BMP-12 alone or as novel combinations over 10 days. Their results showed higher potential of BMP-12 as a tendon differentiation factor, while FGF-2, TGF-β1, and IGF-1 was described as better inducers of matrix synthesis and/or cell proliferation [49].
In conclusion, our results showed that PDGF-BB and GDF-6 could induce tenogenic differentiation in eASCs in combination together during a 14 days culture period. Moreover, the novel combinations of different growth factors are guaranteed for more effective induction of tenogenic differentiation. It is hoped that these in vitro findings could be useful for equine regenerative medicine in treatment of tendon injuries.
References
Bagnaninchi PO, Yang Y, El Haj AJ, Maffulli N (2007) Tissue engineering for tendon repair. Br J Sports Med. https://doi.org/10.1136/bjsm.2006.030643
Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF et al (2006) Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res 24(11):2124–32. https://doi.org/10.1002/jor.20271
Awad HA, Butler DL, Boivin GP, Smith FNL, Malaviya P, Huibregtse B et al (1999) Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 5(3):267–277. https://doi.org/10.1089/ten.1999.5.267
Lui PPY, Chan KM (2011) Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev Rep. https://doi.org/10.1007/s12015-011-9276-0
Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T et al (2008) Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells 26(3):819–830. https://doi.org/10.1634/stemcells.2007-0671
Uysal CA, Tobita M, Hyakusoku H, Mizuno H (2012) Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthetic Surg 65(12):1712–1719. https://doi.org/10.1016/j.bjps.2012.06.011
Liu G, Zhou H, Li Y, Li G, Cui L, Liu W et al (2008) Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology 57(1):18–24. https://doi.org/10.1016/j.cryobiol.2008.04.002
Kim YS, Lee HJ, Ok JH, Park JS, Kim DW (2013) Survivorship of implanted bone marrow-derived mesenchymal stem cells in acute rotator cuff tear. J Shoulder Elb Surg 22(8):1037–1045. https://doi.org/10.1016/j.jse.2012.11.005
Gonçalves AI, Rodrigues MT, Lee SJ, Atala A, Yoo JJ, Reis RL et al (2013) Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS ONE 8(12):e83734. https://doi.org/10.1371/journal.pone.0083734
Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS (2013) Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34(37):9295–9306. https://doi.org/10.1016/j.biomaterials.2013.08.054
Raabe O, Shell K, Fietz D, Freitag C, Ohrndorf A, Christ HJ et al (2013) Tenogenic differentiation of equine adipose-tissue-derived stem cells under the influence of tensile strain, growth differentiation factors and various oxygen tensions. Cell Tissue Res 352(3):509–521. https://doi.org/10.1007/s00441-013-1574-1
Koch TG, Berg LC, Betts DH (2009) Current and future regenerative medicine-principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can Vet J 50:155–65
Kuhbier JW, Weyand B, Radtke C, Vogt PM, Kasper C, Reimers K (2010) Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol 123:55–105. https://doi.org/10.1007/10_2009_24
Pikuła M, Marek-Trzonkowska N, Wardowska A, Renkielska A, Trzonkowski P (2013) Adipose tissue-derived stem cells in clinical applications. Exp Opin Biol Ther 13:1357–1370
Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N et al (1997) Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J Clin Invest 100(2):321–330
Brent AE, Schweitzer R, Tabin CJ (2003) A somitic compartment of tendon progenitors. Cell 113(2):235–248. https://doi.org/10.1016/S0092-8674(03)00268-X
Wang QW, Chen ZL, Piao YJ (2005) Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng 100(4):418–422. https://doi.org/10.1263/jbb.100.418
Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U et al (2005) Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 11(1–2):41–49. https://doi.org/10.1089/ten.2005.11.41
Sieber C, Kopf J, Hiepen C, Knaus P (2009) Recent advances in BMP receptor signaling. Cytokine and Growth Factor Reviews. 20:343–55. https://doi.org/10.1016/j.cytogfr.2009.10.007
Liu J, Tao X, Chen L, Han W, Zhou Y, Tang K (2015) CTGF positively regulates BMP12 induced tenogenic differentiation of tendon stem cells and signaling. Cell Physiol Biochem 35(5):1831–1845. https://doi.org/10.1159/000373994
Haddad-Weber M, Prager P, Kunz M, Seefried L, Jakob F, Murray MM et al (2010) BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 12(4):505–13. https://doi.org/10.3109/14653241003709652
Aspenberg P, Forslund C (1997) Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 70(1):51–54. https://doi.org/10.3109/17453679909000958
Wong YP, Fu SC, Cheuk YC, Lee KM, Wong MWN, Chan KM (2005) Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop 76(3):421–427. https://doi.org/10.1080/17453670510041330
Ducy P, Karsenty G (2005) The family of bone morphogenetic proteins. Kidney Int 57(6):2207–2214. https://doi.org/10.1046/j.1523-1755.2000.00081.x
Mehanna RA, Nabil I, Attia N, Bary AA, Razek KA, Ahmed TAE et al (2015) The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically delivered in fibrin glue on chronic wound healing in rats. Biomed Res Int. https://doi.org/10.1155/2015/846062
Randelli P, Randelli F, Ragone V, Menon A, D’Ambrosi R, Cucchi D et al (2014) Regenerative medicine in rotator cuff injuries. BioMed Res Int. https://doi.org/10.1155/2014/129515
Lepistö J, Laato M, Niinikoski J, Lundberg C, Gerdin B, Heldin CH (1992) Effects of homodimeric isoforms of platelet-derived growth factor (PDGF-AA and PDGF-BB) on wound healing in rat. J Surg Res 53(6):596–601. https://doi.org/10.1016/0022-4804(92)90260-7
Oliva F, Via AG, Maffulli N (2011) Role of growth factors in rotator cuff healing. Sports Med Arthrosc Rev. https://doi.org/10.1097/JSA.0b013e3182250c78
Nedeau AE, Bauer RJ, Gallagher K, Chen H, Liu ZJ, Velazquez OC (2008) A CXCL5- and bFGF-dependent effect of PDGF-B-activated fibroblasts in promoting trafficking and differentiation of bone marrow-derived mesenchymal stem cells. Exp Cell Res 314(11–12):2176–2186. https://doi.org/10.1016/j.yexcr.2008.04.007
Yoshikawa Y, Abrahamsson SO (2001) Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand 72(3):287–292. https://doi.org/10.1080/00016470152846646
Alipour F, Parham A, Kazemi Mehrjerdi H, Dehghani H (2015) Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials. Cell J 16(4):456–465
Yin Z, Guo J, Wu T, Chen X, Xu L, Lin S, Sun Y, Chan K, Ouyang H, Li G (2016) Stepwise differentiation of mesenchymal stem cells augments tendon-like tissue formation and defect repair in vivo. Stem cells translational medicine 5(8):1106–1116. https://doi.org/10.5966/sctm.2015-0215
Naderi H, Matin M, Bahrami A (2011) Critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26(4):383–417. https://doi.org/10.1177/0885328211408946
Naderi H, Bahrami A, Bidkhori H, Mirahmadi M, Ahmadiankia N (2015) Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int 39(1):23–34. https://doi.org/10.1002/cbin.10378
Alves A, Stewart AA, Dudhia J, Kasashima Y, Goodship AE, Smith RK (2011) Cell-based therapies for tendon and ligament injuries. Vet Clin 27(2):315–333. https://doi.org/10.1016/j.cveq.2011.06.001
Qi F, Deng Z, Ma Y, Wang S, Liu C, Lyu F, Wang T, Zheng Q (2020) From the perspective of embryonic tendon development: various cells applied to tendon tissue engineering. Ann Transl Med. https://doi.org/10.21037/atm.2019.12.78
Yu Y, Zhou Y, Cheng T, Lu X, Yu K, Zhou Y, Hong J, Chen Y (2016) Hypoxia enhances tenocyte differentiation of adipose-derived mesenchymal stem cells by inducing hypoxia‐inducible factor‐1α in a co‐culture system. Cell Prolif 49(2):173–184. https://doi.org/10.1111/cpr.12250
Norelli JB, Plaza DP, Stal DN, Varghese AM, Liang H, Grande DA (2018) Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. https://doi.org/10.1177/2041731418811183
Ho JO, Sawadkar P, Mudera V (2014) A review on the use of cell therapy in the treatment of tendon disease and injuries. J Tissue Eng 5:2041731414549678. https://doi.org/10.1177/2041731414549678
Jiang D, Gao P, Zhang Y, Yang S (2016) Combined effects of engineered tendon matrix and GDF-6 on bone marrow mesenchymal stem cell-based tendon regeneration. Biotechnol Lett 38(5):885–892. https://doi.org/10.1007/s10529-016-2037-z
Dale T, Mazher Sh, Webb W, Zhou J, Maffulli N, Chen G, El Haj A, Forsyth N (2018) Tenogenic differentiation of human embryonic stem cells. Tissue Eng Part A 24(5–6):361–368. https://doi.org/10.1089/clo.2006.8.16
Nourissat G, Berenbaum F, Duprez D (2015) Tendon injury: from biology to tendon repair. Nat Rev Rheumatol 11:223–233. https://doi.org/10.1038/nrrheum.2015.26
Shojaee A, Parham A, Ejeian F, Esfahani M (2019) Equine adipose mesenchymal stem cells (eq-ASCs) appear to have higher potential for migration and musculoskeletal differentiation. Res Vet Sci 125:235–243. https://doi.org/10.1016/j.rvsc.2019.06.015
Wei CH, Ming N, Rui Y, Zhang K, Zhang Q, Xu L, Chan K, Gang LI, Wang Y (2013) Effect of growth and differentiation factor 6 on the tenogenic differentiation of bone marrow-derived mesenchymal stem cells. Chin Med J. https://doi.org/10.3760/cma.j.issn.0366-6999.20123351
Mohanty N, Gulati BR, Kumar R, Gera S, Kumar P, Somasundaram RK et al (2014) Immunophenotypic characterization and tenogenic differentiation of mesenchymal stromal cells isolated from equine umbilical cord blood. Vitr Cell Dev Biol 50(6):53848. https://doi.org/10.1007/s11626-013-9729-7
Matsukawa T, Otabe K, Muneta T, Inui M, Nakahara H, Sekiya I et al (2014) Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Orthop Res 33(1):1–8. https://doi.org/10.1002/jor.22750
Stanco D, Caprara Ch, Ciardelli G, Mariotta L, Gola M, Minonzio G, Soldati G (2019) Tenogenic differentiation protocol in xenogenic-free media enhances tendon-related marker expression in ASCs. PLoS ONE 14(2):e0212192. https://doi.org/10.1371/journal.pone.0212192
Falcon N, Riley G, Saeed A (2019) Induction of tendon-specific markers in adipose-derived stem cells in serum-free culture conditions. Tissue Eng Part C 25(7):389–400. https://doi.org/10.1089/ten.tec.2019.0080
Rajpar I, Barrett JG (2019) Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells. J Tissue Eng. https://doi.org/10.1177/2041731419848776
Acknowledgements
This study was financially supported by Ferdowsi University of Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All methods and procedures were approved by The Ethics Committee of the Ferdowsi University of Mashhad, Mashhad, Iran.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javanshir, S., Younesi Soltani, F., Dowlati, G. et al. Induction of tenogenic differentiation of equine adipose-derived mesenchymal stem cells by platelet-derived growth factor-BB and growth differentiation factor-6. Mol Biol Rep 47, 6855–6862 (2020). https://doi.org/10.1007/s11033-020-05742-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05742-7