Abstract
Objectives
To examine whether an engineered tendon matrix (ETM) environment and growth and differentiation factor-6 (GDF-6) have synergistic effects on the tenogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and the quality of tendon repair.
Results
ETM and GDF-6 promote tenogenic differentiation of BMSCs in vitro. Implantation of GDF-6-incorporated ETM containing BMSCs into a tendon injury model significantly improved the histological and mechanical properties of the repaired tendon.
Conclusions
GDF-6-incorporated ETM containing BMSCs represents a promising strategy for tendon injury repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The repair of injured tendons is a significant challenge in orthopedics, and the use of tissue-engineering-based procedures to treat tendon injuries is increasing (Butler et al. 2004; Hogan et al. 2015). Bone marrow mesenchymal stem cells (BMSCs) and tendon stem cells have been used in the repair of injured tendons (Ni et al. 2012; Huang et al. 2013). However, non-tenogenic differentiation of these stem cells may occur after transplantation (Tan et al. 2012). Therefore, development of improved tissue-engineering strategies is required and may provide novel opportunities for tendon regeneration.
Incorporation of BMSCs in tendon repair procedures can improve early tendon healing based upon both histological and biomechanical criteria (Chong et al. 2007; Yao et al. 2012). However, regulating the tenogenic differentiation of BMSCs is important for the successful application of BMSCs to tendon therapy. Growth and differentiation factor-6 (GDF-6) can induce tenogenic differentiation of BMSCs by increasing the expression of tenomodulin and scleraxis (Chai et al. 2013) and the extracellular matrix (ECM) is one of the most important factors influencing the growth and function of stem cells (Pei et al. 2011). ECM generated from fibroblasts has also been used to construct engineered tendon tissue (Jiang et al. 2014). Additionally, engineered tendon matrix (ETM) derived from decellularized tendon tissue can enhance the bioactivities of seeded cells, thereby promoting reconstruction of injured tendons (Zhang et al. 2011). ETM-based biomaterial is of great interest for tendon tissue engineering applications because of its biocompatibility and biodegradability. Moreover, ETM yields a combined scaffold with superior biological and mechanical properties.
We hypothesized that a combination of an ETM environment and GDF-6 would stimulate tendon-lineage differentiation of BMSCs. A combination of ETM, GDF-6, and BMSCs transplantation may represent a promising strategy for tendon injury repair. We therefore have cultured BMSCs on ETM in the presence of GDF-6 and investigated cell morphology and tenogenic differentiation. We also examined the effectiveness of GDF-6-incorporated ETM containing BMSCs on tendon repair using a rat patellar tendon injury model. Nude rats were used to demonstrate the effects of engineered tendon on the formation of a neo-tendon in vivo.
Materials and methods
Isolation and culture of BMSCs
BMSCs were isolated from the bone marrow of six adult rabbits and eight rats. All animal procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine and performed strictly according to established institutional guidelines. Briefly, the tibiae and femurs of rabbits and rats were removed and dissected free of muscle tissue. Bones were rinsed in sterilized phosphate-buffered saline (PBS) before being cut in half, and bone marrow was aspirated from the medial aspect of the proximal tibia. Bone marrow extract was placed into a centrifuge tube and centrifuged at 800×g for 15 min at room temperature. Mononuclear cells were isolated by density gradient centrifugation using Lymphoprep. Isolated cells were resuspended in alpha modified Eagle medium (α-MEM) containing 10 % (v/v) fetal bovine serum (FBS), and 1 % (v/v) penicillin/streptomycin. Cells were plated at 105/cm2 in T-75 flasks and culture medium was changed every 3 days. The stem cell identities of BMSCs were routinely confirmed by their clongenicity and multi-differentiation potentials before being used for experimental procedures.
Preparation of engineered tendon matrix (ETM)
ETM was prepared from rat patellar tendon tissues. Harvested tendons were frozen at −80 °C until processing. ETM was obtained using the method as previously described (Zhang et al. 2011). Briefly, tendons were cut into small pieces and immersed in liquid N2 for 2 min before being grounded into powder. This powder was then treated with a 0.5 % trypsin/PBS for 24 h at 37 °C. Following three 30 min washes in PBS lacking EDTA, the powder was incubated in nuclease solution (50 U DNase/ml and 1 U RNase/ml in 10 mM Tris/HCl, pH 7.5) for 12 h at 37 °C. Finally, the powder was treated with 1 % Triton X-100 for 24 h and rinsed in PBS at room temperature with gentle agitation. Rinsing was repeated six times. The powder was then frozen at −80 °C and stored for subsequent cell culture or implantation experiments.
Culture of BMSCs on ETM-coated surfaces
Rat-derived ETM powder was mixed with 3 % (v/v) acetic acid to make a 5 % solution prior to culture of BMSCs. Six-well plates were coated with ETM/acetic acid solution (0.8 ml/well) for 12 h under UV light, and then washed once with PBS. Rat BMSCs at passage 3 were plated at 5000 cells/cm2 in six-well plates with ETM and/or GDF-6 (20 ng/ml). Culture medium was changed every 3 days. BMSCs were also cultured in six-well plates without ETM and GDF-6 as control. The four groups were control, ETM, GDF-6, and ETM + GDF-6.
After culture for 14 days, the morphology of BMSCs was examined directly using an inverted microscope
The effects of rat-derived ETM and GDF-6 on rat BMSCs were evaluated by examining expression of the tenocyte-related genes collagen type I, tenascin C, tenomodulin, and scleraxis using qRT-PCR after 2 weeks treatment. Total RNA was extracted using the RNeasy mini kit (Qiagen). cDNA was synthesized using the First-strand cDNA synthesis kit (Invitrogen) according to manufacturer’s instructions. qRT-PCR was carried out with the QuantiTect SYBR Green RT-PCR kit (Qiagen). Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. Relative gene expression levels were calculated using the 2−ΔCT formula. Gene expression levels were normalized to those of controls. Rat-specific primers (Supplementary Table 1) were used for expression of collagen type I, tenascin C, tenomodulin, scleraxis, and GAPDH.
Implantation of BMSCs
After treatment with 20 ng GDF-6/ml for 2 weeks, rabbit-derived BMSCs were mixed with rat-derived ETM gel. Eight nude rats (180–200 g) were then used to demonstrate the effects of BMSCs on the formation of neo-tendons in vivo. Surgery was performed under general inhalation anesthesia with isofluorane. Incisions were made on the dorsum and two subcutaneous pockets were created in each rat. Approx. 106 passage-3 rabbit BMSCs from a randomly selected experimental group were implanted into each pocket. BMSCs without ETM and/or GDF-6 served as the control group and were implanted into subcutaneous pockets by injection. 3 weeks after implantation, tissue samples were harvested and stored at −80 °C for later histological examination.
Sprague Dawley rats were used to demonstrate the effects of BMSCs on the histological and mechanical properties of regenerated tendons. The central third (10 mm length × 2 mm width) of the patellar tendon was removed from the distal apex of the patella to the insertion of the tibial tuberosity to create a tendon defect. Rat BMSCs treated with or without GDF-6 were then implanted into the tendon defect along with ETM. Window injury without any treatment served as control. At week 2 after surgery, eight rats in each group were sacrificed and the patellar tendon tissues taken for histological examination. Twelve rats from each group were sacrificed at week 4 after surgery, and the regenerated patellar tendons harvested for biomechanical testing.
Histological examination
The neo-tendon and regenerated patellar tendon tissues were sectioned longitudinally into 5 μm sections. Neo-tendon and regenerated patellar tendon tissue sections were then processed for histological examination by hematoxylin and eosin (H&E) staining. The organization of fibrous connective tissue within regenerated patellar tendon tissues was evaluated using fiber alignment scoring. The fiber alignment score was as follows: 3 = 75–100 % parallel fiber alignment, 2 = 50–75 % parallel fiber alignment, 1 = 25–50 % parallel fiber alignment, and 0 = 0–25 % parallel fiber alignment. Sections from neo-tendons were also used for immunohistochemical staining of collagen type I. Sections were incubated with mouse anti-rabbit collagen type I antibody (1:400; Santa Cruz Biotechnology) for 2 h at room temperature. After three washes with PBS, Cy3-conjugated goat anti-mouse IgG (1:500; Santa Cruz Biotechnology) was used as a secondary antibody. Nuclei were counterstained with Hoechst 33,342 (1 mg/ml).
Mechanical examination
Rats were sacrificed at 4 weeks for biomechanical testing of tendons. Patella–patellar tendon–tibia complexes were dissected from the knee and all surrounding soft tissues were carefully transected. The regenerated tissue (10 mm length × 2 mm width) in the window defect was isolated according to the procedure described in a previous study (Ni et al. 2013). The patella/patellar tendon/tibia complex was then mounted in a biomaterial testing machine (Z010, Zwick, Germany). The composite was distracted along the longitudinal axis of the tendon at 0.6 mm/s until gross failure of the healed tendon occurred. A preload of 0.1 N was applied to each tendon. The load-elongation curve was recorded. Stress was calculated as the ultimate force divided by the cross-sectional area as measured using an animal ultrasound system. Young’s modulus was obtained from the stress–strain curve as the slope of the linear portion.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Data were compared using one-way analysis of variance followed by the Tukey’s test. Statistical analyses were carried out using the SPSS 11.0 statistical package. All values of P < 0.05 were considered statistically significant.
Results and discussion
Cell morphology of BMSCs
After 14 days’ incubation, examination of cell morphology showed that BMSCs were randomly aligned and had adopted spindle or fibro-like shapes (Fig. 1). BMSCs grown in the presence of ETM or GDF-6 were more elongated in shape (Fig. 1). BMSCs grown in the presence of combined ETM and GDF-6 adopted an especially slim morphology and were aligned in parallel. As BMSCs were grown on a smooth surface without physical or mechanical stimulation, cell orientation cannot be quantified based on the angle formed by the major axis.
Tenogenic differentiation of BMSCs
Collagen type I, tenascin C, tenomodulin, and scleraxis were selected as markers of tendon-like tissue to investigate the tenogenic effects of GDF-6 and ETM on BMSCs based on previous studies (Zhang et al. 2006; Murchison et al. 2007; Jelinsky et al. 2010; Chai et al. 2013). Expression of scleraxis, an early marker for tenogenic differentiation, was significantly higher in ETM and GDF-6 groups compared with control (Fig. 2d). Expression of tenomodulin, a mature marker for tenogenic differentiation, was also significantly higher in ETM and GDF-6 groups compared with control (Fig. 2c). Additionally, expression levels of the tendon-related genes tenascin C and collagen type I were also higher in the ETM. There were no significant differences in tenascin C and collagen type I expression levels between the GDF-6 and control groups (Fig. 2a, b). Furthermore, combination treatment with ETM and GDF-6 significantly increased expression of all tenocyte-related genes compared with other groups. These findings suggest that the combination of ETM with GDF-6 effectively induces tenogenic differentiation of BMSCs.
Expression levels of tenocyte-related genes determined by qRT-PCR. Expression levels of collagen type I (a), tenascin C (b), tenomodulin (c), and scleraxis (d) in BMSCs after treatment of ETM and GDF-6. *P < 0.05, n = 6. Expression levels were normalized to that of GAPDH. Data are shown as mean ± SD
Histological characteristics of neo-tendon tissues in nude rat model
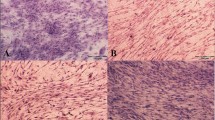
H&E staining of neo-tendon tissue was performed 3 weeks after implantation to examine the structure of neo-tendon tissues in nude rats. Mature and tight collagen fibers formed in the ETM + GDF-6 group, and cells were longitudinally aligned along with collagen fibers in neo-tendon tissues (Fig. 3). Meanwhile, cells in the ETM- and GDF-6-alone groups were relatively randomly aligned (Fig. 3). There was no formation of tendon-like tissue after implantation of BMSCs alone (Fig. 3).
Immunohistochemical staining for rabbit collagen type I was also performed to detect the formation of neo-tendon tissues. Extensive expression of collagen type I was apparent after implantation of BMSCs along with ETM and GDF-6 (Fig. 4). However, implantation of BMSCs without either ETM or GDF-6 resulted in weaker expression of collagen type I (Fig. 4).
Histology of regenerated tendon tissue
We next investigated the effects of GDF-6-incorporated ETM containing BMSCs on tendon regeneration by collecting healing tendon samples for H&E staining 2 weeks after implantation. Most of the healing tendon cells were oriented randomly in healing tissue after implantation of BMSCs alone (Fig. 5). Remodeling of fiber alignment was improved in ETM and GDF-6 groups, either alone or in combination. Cells were also arranged longitudinally between collagen fibers in the control group. The detailed mechanisms underlying the decreased cell alignment after implantation of BMSCs alone requires further investigation, and 2 weeks may be too short a period to evaluate the final organization of collagen fibers at a healing site. Additionally, the fiber alignment score was significantly greater for the group treated with ETM + GDF-6 compared with all other groups (Fig. 6). Our results from the histological evaluation of tissues from the patellar tendon injury model suggest that transplantation of BMSCs in combination with ETM and GDF-6 improves tendon healing by promoting organization of collagen fibers.
Mechanical properties of regenerated tendon tissue
The improved tissue quality observed using engineered tendon tissue treatment was further supported by biomechanical testing results. The mean ultimate stress and Young’s modulus for the regenerated tendon tissue are shown in Fig. 7. The ultimate stress and Young’s modulus of healing patellar tendons were significantly lower in the control group than in other groups (Fig. 7). Additionally, tendons from the ETM + GDF-6 group withstood significantly increased ultimate stress and had a Young’s modulus at 4 weeks after transplantation when compared with ETM- or GDF-6-alone groups. These findings suggest that the combination of ETM with GDF-6 significantly improves biomechanical function of injured tendon tissue. The improved biomechanical properties of tendons observed with combination therapy suggest a complementary effect of ETM and GDF-6 on the functional performance of a healing tendon.
In conclusion ETM accelerates the differentiation of BMSCs into tenocytes. This induction is further enhanced by addition of GDF-6 that promotes stronger expression of tendon-associated genes. Transplantation of GDF-6-incorporated ETM containing BMSCs enhanced tendon regeneration by promoting formation of well-organized collagen fibers and extensive deposition of collagen. This resulted in improved tendon mechanical function. The use of ETM, GDF-6, and BMSCs in combination is therefore a promising approach to improve the healing of injured tendons. However, a potential limitation is that the detailed mechanisms underlying ETM-enhanced tenogenic potential of BMSCs have not been elucidated and these require further investigation.
References
Butler DL, Juncosa N, Dressler MR (2004) Functional efficacy of tendon repair processes. Annu Rev Biomed Eng 6:303–329
Chai W, Ni M, Rui YF, Zhang KY, Zhang Q, Xu LL, Chan KM, Li G, Wang Y (2013) Effect of growth and differentiation factor 6 on the tenogenic differentiation of bone marrow-derived mesenchymal stem cells. Chin Med J (Engl) 126:1509–1516
Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH (2007) Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am 89:74–81
Hogan MV, Walker GN, Cui LR, Fu FH, Huard J (2015) The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy 31:1017–1021
Huang TF, Yew TL, Chiang ER, Ma HL, Hsu CY, Hsu SH, Hsu YT, Hung SC (2013) Mesenchymal stem cells from a hypoxic culture improve and engraft achilles tendon repair. Am J Sports Med 41:1117–1125
Jelinsky SA, Archambault J, Li L, Seeherman H (2010) Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res 28:289–297
Jiang D, Xu B, Yang M, Zhao Z, Zhang Y, Li Z (2014) Efficacy of tendon stem cells in fibroblast-derived matrix for tendon tissue engineering. Cytotherapy 16:662–673
Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R (2007) Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134:2697–2708
Ni M, Lui PP, Rui YF, Lee YW, Lee YW, Tan Q, Wong YM, Kong SK, Lau PM, Li G, Chan KM (2012) Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res 30:613–619
Ni M, Rui YF, Tan Q, Liu Y, Xu LL, Chan KM, Wang Y, Li G (2013) Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials 34:2024–2037
Pei M, He F, Kish VL (2011) Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17:3067–3076
Tan Q, Lui PP, Rui YF, Wong YM (2012) Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A 18:840–851
Yao J, Woon CY, Behn A, Korotkova T, Park DY, Gajendran V, Smith RL (2012) The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 37:1639–1645
Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE (2006) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98:1436–1449
Zhang J, Li B, Wang JH (2011) The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 32:6972–6981
Acknowledgments
This work was supported by grants from the National Natural Sciences Foundation of China (No.30901516, 81472085).
Supporting information
Supplementary Table 1—Primer sequences of target genes for real-time RT-PCR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, D., Gao, P., Zhang, Y. et al. Combined effects of engineered tendon matrix and GDF-6 on bone marrow mesenchymal stem cell-based tendon regeneration. Biotechnol Lett 38, 885–892 (2016). https://doi.org/10.1007/s10529-016-2037-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2037-z