Abstract
Passion fruit (Passiflora edulis), an important tropical and subtropical fruit, has a high edible and medicinal value. Stem rot disease is one of the most important diseases of passion fruit. An effective way for control and prevention of this disease is to identify the genes associated with resistance to this disease. Quantitative real-time PCR (RT-qPCR) has mainly been widely applied to detect gene expression because of its simplicity, fastness, low cost and high sensitivity. One of the requirements for RT-qPCR is the availability of suitable reference genes for normalization of gene expression. However, currently, no Passiflora edulis reference genes have been identified andthus it has hindered the gene expression studies in this plant. The present study aimed to address this issue. We analyzed sixteen candidate reference genes, including nine common (GAPDH, UBQ, ACT1, ACT2, EF-1α-1, EF-1α-2, TUA, NADP, and GBP) and seven novel genes (C13615, C24590, C27182, C10445, C21209, C22199, and C22526), in different tissues (stem, leaf, flower and fruit) of two accessions under stem rot condition. We calculated the expression stability in twenty-four samples using the ΔCt, GeNorm, NormFinder, BestKeeper and RefFinder. The results showed that both C21209 and EF-1α-2 were sufficient to normalize gene expression under stem rot, whereas the commonly used reference genes, GAPDH and UBQ, were the least stable ones. The expression patterns of PeUFC under stem rot condition normalized by stable and unstable reference genes indicated the suitability of using the optimal reference genes. To our knowledge, this is the first systematic study of reference genes in Passiflora edulis, which identified a number of reliable reference genes suitable for gene expression studies in Passiflora edulis by RT-qPCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The passion fruit (Passiflora edulis) is an herb or vine plant belonging to the Passifloraceae family. This family includes of 530 species, which are classified into 16 genera [1]. Currently, purple passion fruit and yellow passion fruit are the main cultivatars. Passion fruit have an aromatic smell and are rich in sugar, vitamins, mineral elements and other substances. Thus, they attract a vast number of consumers.
Stem rot disease is one of the main diseases of Passiflora edulis. This disease manifests itself as the initial symptoms of water-stained decay in the stem base. After being infected, the plants gradually wither, their leaves drop, and later the whole plant becomes yellow and withered to death, leading to the destruction of the garden. Due to the serious destruction caused by this disease in Passiflora edulis planting areas in southern China, it is highly necessary to identify the genes related to resistance to stem rot disease for the development of the cultivars resistant to this disease.
RT-qPCR has become one of the main methods for studying gene expression because of its simplicity, fastness, low cost and high sensitivity [2]. However, due to the large differences in RNA quality, reverse transcription efficiency and copy number of genes in different samples, two relatively stable internal reference genes are required for correction and standardization when the RT-qPCR is used. The reference gene usually participates in the basic biological activities of the cells and its expression is relatively stable among various tissues of the organism and under different experimental and environmental conditions [3]. To date, the commonly used reference genes in plants include glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [4], ubiquitin-conjugating enzyme (UBQ) [5], Actin1 (ACT1) [6], Actin2 (ACT2) [7], elongation factor 1-alpha (EF-1α) [8], α-tubulin (TUA) [9], NADP-isocitrate dehydrogenase (NADP) [10], and GTP-binding protein (GBP) [11]. However, the stability of the reference gene is relative and gene expression is greatly influenced by a number of internal and external factors, such as different varieties, tissues and environmental conditions. In order to ensure reliable experimental results, it is necessary to screen and identify the most suitable reference genes according to the specific experimental conditions.
With the rapid development of genomics and molecular genetics, increasing researches on Passiflora edulis genes have carried out [12,13,14,15]. To date, four reference genes have been reported in Passiflora edulis [13, 16]. However, the systematic screening of reference genes has not been reported yet. Thus, we analyzed the expression stability of genes in different developmental stages and accessions under stem rot condition using ΔCt [17], GeNorm [18], NormFinder [19], Bestkeeper [20], and RefFinder [21], and finally selected C21209 and EF-1α-2 as the most reliable reference genes. Our study identified a number of reliable reference genes for the future quantification of gene expressions in Passiflora edulis.
Materials and methods
Plant materials
Passiflora edulis is caused by Fusarium oxysporum Schlecht. At the beginning, water-stained lesions appeared on the infected part of plant by the pathogen, and then the lesions spread in stripes along the stem, and finally the whole branch rotted (Fig. 1). Cultivar Jinlingziguo (JLZG) is susceptible to while the other cultivar Huangguoyuanshengzhong (HGYSZ) is resistant to stem rot disease. Two accessions were planted at the germplasm garden of the Institute of Biotechnology of Guangxi Academy of Agricultural Sciences (22.77° N, 108.15° E), Nanning, Guangxi, China. The cutting seedling heights ranged from 29 to 38 cm, and the seedlings were transplanted on May 25, 2019, using a single-line hedge planting model. The distance between the plants was 200 cm. About 100 kg of pure nitrogen per hectare was applied. The ratio of nitrogen (N) fertilizer: phosphorus fertilizer (P2O5): potassium fertilizer (K2O) was 3: 1.5: 1 for application.
The samples of pathogenic fungi that causes stem rot disease were collected from the infected plants. The fungi were isolated, placed on the potato glucose agar medium (PDA), separated and purified. The fungal pathogens were identified mainly as Fusarium oxysporum Schlecht. Disease-resistant determination was performed using exosometric inoculation method: the purified and preserved pathogen was activated and inoculated to the PDA medium and cultured at 28 °C for 5 days, and the fungal filament was picked up by punching with a 1000 ul-pipetting tip.
When the first bud appeared in the plant, the stem base was artificially stung, and then the prepared piece of mycelium was picked up and its front side was inoculated to the wound site. Three days later, water-stained spots began appearing on the inoculated site. The stems, leaves, flowers and fruits (7 DAF) were collected from JLZG and HGYSZ with three biological replicates per sample. A total of 24 samples were stored in the − 80 °C freezer.
Total RNA extraction and cDNA synthesis
Total RNA was extracted with RNAprep Pure (Tiangen, Beijing, China) according to the manufacturer’s instructions. The concentration and purity of the RNA samples were detected using the BioSpec-nano UV–visible spectrophotometer (Shimadzu, Japan) and the integrity was evaluated by 1% agarose gel electrophoresis. Reverse transcription was performed with HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) and the first chain cDNA synthesis was performed with superMix according to the use instructions. All synthesized cDNA samples were diluted at 1:10 with RNase-free water and then stored at − 80 °C for subsequent analysis.
RNA-seq data analysis and selection of candidate reference genes
Pingtang 1’ is cold-tolerant variety of P. edulis [13]. The transcriptome data of ‘Pingtang 1’ was derived from published research by Xu et al. [12], details are as follows: cuttings of ‘Pingtang 1’ were cultivated in L rocky desertification areas and sandy D rocky desertification areas, and the two plots are located in Kedu town, Pingtang Country, Guizhou Province (25.72° N, 106.8° E). Two root samples selected from those three biological replicates for RNA extraction were immediately frozen in liquid nitrogen and stored at − 80 °C in an ultralow temperature freezer, and four cDNA libraries (T01, T02, T03 and T04) were generated. RNA-seq data were downloaded from the NCBI database ( https://www.ncbi.nlm.nih.gov/sra/SRP150688 ). The Trinity [22] was used for the assembly of the sequence. The expression level of the predicted sequence was analyzed using RSEMs [23]. Functional annotations for gene sequences were performed using BLAST and databases NR, COG, KOG, EggNOG, KEGG, GO, Pfam and Swiss-prot. The expression stability of each gene in T01, T02, T03 and T04 was calculated and evaluated using a coefficient of variation (CV). The candidate References. There are no sources in the current document. genes were selected based on their expression levels detected in this study and those reported by previous studies [4,5,6,7,8,9,10,11].
Primer design
Specific primers were designed using Primer3 software and synthesized by BGI (Beijing, China). The length of primers varied from 20 to 22 bp. The length of the amplified products ranged from 119 to 498 bp and the annealing temperatures ranged from 58 to 61 °C.
Quantitative real time PCR
All RT-qPCR assays were carried out in 96-well plates using qTOWER 2.2 Quantitative Real-Time PCR Thermal Cycler (Analytik Jena, Germany). The reaction system included: 10 µl of 2 × TransStart SYBR Green Master Mix (Vazyme, Nanjing, Jiangsu, China), 1 µl of each primer, 1 µl of template cDNA, complemented by ddH2O to 20 µl. The cycle program for product amplification was as follows: 94 °C for 5 min followed by 40 cycles of 94 °C for 30 s (denaturation), 55 °C for 30 s (annealing), and 72 °C for 30 s (extension). Tripricates were set for each sample. When the reaction was completed, the melting curve was analyzed and specificity of the product was determined based on the melting curve.
Analysis of expression stability of candidate reference genes
Each candidate reference gene was analyzed and evaluated with the method of ΔCt [17], GeNorm [18], NormFinder [19], Bestkeeper [20], and RefFinder [21]. The ΔCt, M value, SV value and CV/SD value of the candidate genes were calculated. These genes were ranked based on their stabilities in the order from low to high. The lower the value is, more stable the corresponding gene is. Finally, based on the ranking results obtained with software ΔCt, GeNorm, Normfinder, Bestkeeper, and RefFinder, the comprehensive ranking for the expression stability of each internal reference gene was calculated.
Validation of the selected candidate reference genes
The Passiflora edulis gene PeUFC (protein UPSTREAM OF FLC in Passiflora edulis) was cloned based on homology of ZmAuxRP1 [24]. To validate the selected reference genes (C21209, EF-1α-2, GAPDH and UBQ), RT-qPCR was performed to analyze PeUFC expression profile in stem, leaf, flower and fruit of JLZG and HGYSZ with three biological replicates per sample, under stem rot condition via normalization with these reference genes. The relative gene expression level was calculated by reference to the 2−ΔΔCt method [25].
Results
Identification of candidate reference genes with RNA-seq data
The transcriptome data showed that 10,052, 20,580, 12,584, 17,888, 21,037, 20,925, 28,700 and 29,286 genes were significantly matched the unigenes in the COG, GO, KEGG, KOG, Pfam, Swissport, EggNOG and NR database, respectively. The genes with the length > 1000 bp accounted for 55.75%, 49.90%, 46.71%, 48.89%, 55.02%, 52.37%, 48.75%, and 48.73% of total unigenes in respective databases. The NR database gained the highest proportion of the matched unigenes, while the COG database gained the lowest one.
We analyzed the expression levels of 30,499 genes in T01, T02, T03 and T04, then selected the candidate reference genes based on their CV values. Firstly, the CV value of each of the common reference genes, including GAPDH, UBQ, ACT1, ACT2, EF-1α-1, EF-1α-2, TUA, NADP and GBP, was calculated, leaving only the one with the lowest CV value for each type of gene. Secondly, the gene with high expression value (FPKM > 25) was analyzed and the gene with a smaller CV value was selected.After analysis, we obtained a total of 16 candidate genes (Fig. 2).
Amplification efficiency and specificity evaluation of primers
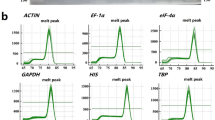
We used Primer3 to design primers targeting 16 candidate reference genes for RT-PCR (Table 1). The results of amplification efficiency and specificity evaluation of primers showed that the R2 values of these candidate reference genes varied from 0.9683 to 0.9822, indicating that there is a linear relationship between the cDNA level as the template and Ct values, and that the resulting linear equations are reliable. The amplification rates for all candidate genes varied from 92.65% (NADP) to 102.37% (C21209). The melting curves obtained by RT-qPCR all displayed a single peak, indicating that 16 primer pairs are specific to the targeted region (Fig. S1).
Expression profiling of candidate reference genes
The expression levels of the 16 candidate reference genes were evaluated in 24 samples collected from different tissues in two accessions using their Ct values. The Ct values of 16 candidate reference genes varied from 18.07 to 36.16 in all samples. Because the gene expression level is negatively correlated with the Ct value, EF-1α-1 was the most highely expressed gene with the lowest mean Ct value (19.75), while NADP was the lowest abundant gene with the highest mean Ct value (33.60) in JLZG (Fig. 3a); C10445 was the most highely expressed gene with the lowest mean Ct value (19.04), while NADP was the lowest abundant gene with the highest mean Ct value (32.80) in HGYSZ (Fig. 3b). The Ct values varied from 19.48 (EF-1α-1) to 33.20 (NADP) in all samples (Fig. 3c).
Expression stability of candidate reference genes
In this study, each reference gene was evaluated in three groups: (1) JLZG (stem, leaf, flower and fruit of JLZG with three biological replicates per sample), (2) HGYSZ (stem, leaf, flower and fruit of HGYSZ with three biological replicates per sample), and (3) All (stem, leaf, flower and fruit of JLZG and HGYSZ with three biological replicates per sample). Then, the expression stabilities of the 16 candidate reference genes were analyzed by the ΔCt, GeNorm, NormFinder, BestKeeper, and RefFinder.
The genes with the lowest ΔCt values showed the most stable expression profiles. Therefore, the top five reference genes were C22526, EF-1α-1, C27182, C24590 and C13615 in JLZG, those were EF-1α-2, C21209, C27182, EF-1α-1 and C24590 in HGYSZ; and those were C21209, EF-1α-2, EF-1α-1, C27182 and C13615 in All (Table 2, Fig. 3), which indicated that seven reference genes, C22526, EF-1α-1, C27182, C24590, C13615, EF-1α-2 and C21209 would be stably expressed in our RT-qPCR normalization. Especially, EF-1α-2 and C21209 all in the top two rankings might be more stably expressed in HGYSZ and All. TUA, GBP and UBQ were the least stably expressed reference genes because they were all in the last five rankings.
GeNorm based on the calculated candidate gene expression stability (M) in different samples was used to determine the most stable reference candidate gene, M value is inversely proportional to gene expression stability. M value of 1.5 is generally considered a stable expression [19]. GeNorm analysis showed that EF-1α-2, C27182, C21209, C10445 and EF-1α-1 were the most stable reference genes in JLZG, C24590, C22199, EF-1α-2, EF-1α-1 and C13615 were the most stable ones in HGYSZ, and EF-1α-2, C27182, C21209, EF-1α-1 and C22199 in All (Table 2, Fig. 4) were the most stableones. These results indicated that EF-1α-1 and EF-1α-2 might be more stably expressed in HGYSZ, HGYSZ and All, whereas GBP, TUA, UBQ and GAPDH were the least stable reference genes because they were all in the last five rankings. Pairwise variation analysis showed that V2/3 was 0.106 in JLZG, V3/4 was 0.145 in HGYSZ, and V3/4 was 0147 in all samples. The results indicated that the most suitable reference genes are 2 or 3 in different samples (Fig. 5).
According to the NormFinder analysis, the gene with the lowest stability value is the most stable reference gene for RT-qPCR [20], C10445, C21209, EF-1α-2, C27182 and C24590 showed higher stability values in JLZG, while C21209, C27182, NADP, EF-1α-2 and EF-1α-1 showed higher stability values in HGYSZ, and C21209, TUA, EF-1α-1, C27182 and C24590 showed higher stability values in All (Table 2) and thus, they could serve as the top five reliable reference genes. The results showed that C21209 and C27182 might be more stably expressed in HGYSZ, HGYSZ and All. UBQ and GAPDH were the least stable reference genes because they were all in the last five rankings.
Bestkeeper reflects the stability of the internal reference gene by calculating the CV and the standard deviation (SD), and the gene that has the smaller difference between the coefficient of variation and the standardization is the most stable reference gene [26]. Bestkeeper analysis showed that EF-1α-1, C24590, C22526, C27182 and C13615 were the stably expressed genes in JLZG, while EF-1α-2, C21209, C27182, EF-1α-1 and C24590 were the stably expressed genes in HGYSZ, C21209, EF-1α-1, EF-1α-2, C27182 and C24590 were the stably expressed genes in All (Table 2), which indicated that the seven reference genes, EF-1α-1, C24590, C22526, C27182, C13615, EF-1α-2 and C21209 would be stably expressed in qRT-PCR normalization. EF-1α-1, C24590, and C27182 might be more stably expressed in JLZG, HGYSZ and All than the other four reference genes because they were all listed in the top five rankings. GBP, UBQ, TUA and GAPDH were the least stable reference genes because they were all lisred in the last five rankings.
The RefFinder calculates the geometric mean of the stability rankings obtained from the analysis of GeNorm, NormFinder, and BestKeeper and Ct obtain a comprehensive ranking. The five most stable reference genes were C27182, EF-1α-1, EF-1α-2, C24590 and C21209 in JLZG; EF-1α-2, C21209, C27182, EF-1α-1 and C24590 in HGYSZ, C21209, EF-1α-2, EF-1α-1, C27182 and C24590 in All. These genes were the same in JLZG, HGYSZ and All (Table 2). Especially, both C21209 and EF-1α-2 were listed as the top two in the rankings in HGYSZ and All. They might be more stably expressed than the other three reference genes. GADPH, GBP, UBQ and TUA were the least stable reference genes because they were all listed in the last five rankings. In addition, GADPH and UBQ were listed as the least stable reference genes because they were all in the last five rankingsin HGYSZ and All.
Validation of candidate reference genes
To validate the accuracy and reliability of our results, the relative expression patterns of PeUFC in different tissues under stem rot disease were analyzed. The two most stable reference genes (C21209 and EF-1α-2) and two most unstable genes (GAPDH and UBQ) were selected for normalizing RT-qPCR data. The expression level of reference gene in the stem of JLZG was used as a control. Using C21209 as the reference gene, the expression levels of PeUFC in HGYSZ tissues (stem, leaf, flower and fruit) were much higher than those in JLZG. This result is consistent with the resistance to stem rot disease in HGYSZ. Similar trend was seen in the analysis of PeUFC expression data with EF-1α-2 normalization. However, the expression level of PeUFC in the leaf of JLZG with GAPDH normalization was much higher than those in the stem and flower of HGYSZ. Meanwhile, the expression level of PeUFC in the leaf of JLZG with UBQ normalization was much higher than that in the stem and fruit of HGYSZ (Fig. 6).
In summary, the expression patterns of PeUFC were nearly the same when reference genesC21209 and EF-1α-2 were used for normalization, while the expression levels in all tissues were higher using EF-1α-2 than using C21209 as the normalizer. Expression level in leaf was higher when C21209, EF-1α-2, GAPDH and UBQ were used as the normalizers.
Discussions
Because of its numerous advantages, RT-qPCR has been widely used in gene expression research and has become an effective method for quantifying the transcriptional expression of gene, revealing the gene expression patterns [2]. It is generally believed that the ideal internal reference gene is the one whose expression is stable at different developmental stages, in different tissue/organs, and under different stress conditions, and whose expression level is similar to that of the target gene. A number of house-keeping genes, such as GAPDH [4], UBQ [5], ACT1 [6], ACT2 [7], EF-1α [8], TUA [9], NADP [10], and GBP [11], are usually chosen as the internal reference genes. However, an increasing body of research has shown that the expression levels of the house-keeping genes vary under different experimental conditions [27, 28]. Therefore, it is particularly important to select the genes whose expression levels are usually stable as the reference genes for analysis of gene expression based on specific experimental conditions. The stable reference genes have been identified in mamy plants such as Jatropha curcas [29], Lilium regale [30], acerola (Malpighia emarginata) [31], and birch (Betula platyphylla)[32] etc.. However, there have been no reports on systematic identification and selection of the most suitable internal reference genes for the different tissues and organs of the passion fruityet.
The previous studies have shown that RT-qPCR is an effective method widely applied to identify and select the suitable internal reference genes using transcriptome sequence screening [28, 33,34,35]. By applying the previously described research methods in combination with the transcriptomes database resources, we selected 9 common house-keeping genes and 7 highly and stably expressed genes as the internal reference gene candidates, and analyzed them based on their Ct values of RT-qPCR. By using three specialized software, GeNorm, NormFinder and BestKeeper, to conduct the comprehensive comparison of the expression stabilities of 16 candidate genes in different varieties and different tissue organs. Finally, we selected C21209 and EF-1α-2 as the best internal reference genes. We found that among 16 genes that were stably expressed under two different vegetative growth conditions, only C21209 and EF-1α-2 genes were stably expressed after JLZG and HGYSZ were infected with Fusarium oxysporum while UBQ was the least stable reference gene.
The function of gene C21209 is annotated as eukaryotic initiation factor 1 (NR), which participates in the initiation process of eukaryotic translation. For instance, Wang et al. [36] reported the expression stabilities of eight candidate reference genes, including eukaryotic translation initiation factor 4 (eIF-4), in garlic under salt stress. Feng et al. [37] also found the stable expression of eIF-4 in celery under abiotic stress and hormone treatment. Cheng et al. [38] also observed the stable expression of eIF-4 in Miscanthus lutarioriparia. Zhang et al. [39] confirmed the expression stability of eIF-4 in Carex rigescens under abiotic stress. Phule et al. [40] confirmed the stable expression of that eIF-5C in rice under aerobic condition. Our findings, together with those reported by the studies mentioned above, indicate that the C21209 gene can be used as a suitable internal reference gene in the passion fruit.
EF-1α is another commonly used reference gene that and has been confirmed in many plant species [32, 36, 37]. In this study, we analyzed the expression stabilities of two EF-1α genes (EF-1α-1 and EF-1α-2) and found that the expression of EF-1α-2 was more stable than EF-1α-1. This indicates that expression stabilities vary widely among different members of the same gene family.
The GAPDH gene is expressed at a high level in almost all tissues, and is widely used as a reference gene for gene expression normalization. However, some studies have shown that its expression levels in different tissues are not stable [32, 41].UBQ is also a commonly used reference gene. Li et al. [42] found that UBQ was the most stably expressed gene in Paeonia ostii. Cheng et al. [38] also confirmed that UBQ was stably expressed in Miscanthus lutarioriparia. However, Hou et al. [43] showed that expression stability of UBQ was poor in different developmental stages, organs and accessions in long yellow daylily. In this study, our findings also indicate that GAPDH is not suitable as a reference gene in Passiflora edulis under stem rot condition.
To our knowledge, only a few genes related to stem rot have been cloned [24, 44]. For instance, ZmAuxRP1(GRMZM2G063298) encoding a plastid stroma-localized auxin-regulated protein in maize responded quickly to pathogen challenge with a rapid yet transient reduction in expression, leding to the arrested root growth but the enhanced resistance to Gibberella stalk rot and Fusarium ear rot [24]. Recently, by using ZmAuxRP1 homologous sequence, Shen et al. cloned AtAuxRP3 in Arabidopsis and observed that it enhanced the expression of the auxin-responsive reporter DR5:GUS near the vegetative shoot apex, leding to ectopic activation of auxin signaling [45]. As we know, auxin plays an important role in biotic and abiotic stress [46, 47]. From Swissprot and NCBI database, we found five genes in Passiflora edulis using the ZmAuxRP1 homologous from maize,including C26623 (protein UPSTREAM OF FLC), which named PeUFC. Under stem rot condition, we identified the function of PeUFC in 74 cultivated passion fruits using absolute quantification PCR, and found that the expression level of it was significantly negatively correlated with the incidence rate of Passiflora edulis. Based on this result, we infered that PeUFC may be associated with resistance to stem rot in Passiflora edulis. In this study, we used C21209, EF-1α-2, GAPDH and UBQ to normalize the expression level of PeUFC, and the result showed that the normalization with C21209 and EF-1α-2 was consistent with the resistance of stem rot disease in HGYSZ.
Conclusions
In this study, GAPDH, UBQ, ACT1, ACT2, EF-1α-1,EF-1α-2, TUA, NADP, GBP, C13615, C24590, C27182, C10445, C21209, C22199, and C22526 genes were selected as candidate reference genes based on Passiflora edulis RNA-seq data and previous reports in other species. We systematically evaluated the expression stabilities of 16 candidate reference genes in different tissues of two Passiflora edulis varieties under stem rot condition. We found that C21209 and EF-1α-2 were the best reference genes for normalizing RT-qPCR gene expression data. The reliability was validated by PeUFC gene. It is worth noting that this work is the first systematic study of the most suitable reference genes that will facilitate further research into the molecular biology of Passiflora edulis.
References
Munhoz CF, Costa ZP, Cauz-santos LA et al (2018) A gene-rich fraction analysis of the Passiflora edulis genome reveals highly conserved microsyntenic regions with two related Malpighiales species. Sci Rep 8:13024. https://doi.org/10.1038/s41598-018-31330-8
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. Biotechniques 39:75–85. https://doi.org/10.2144/05391RV01
Kozera B, Rapacz M (2013) Reference genes in real-time PCR. J Appl Genet 54:391–406. https://doi.org/10.1007/s13353-013-0173-x
Rapacz M, Stępień A, Skorupa K (2012) Internal standards for quantitative RT-PCR studies of gene expression under drought treatment in barley (Hordeum vulgare L.): the effects of developmental stage and leaf age. Acta Physiol Plant 34:1723–1733. https://doi.org/10.1007/s11738-012-0967-1
Monteiro F, Sebastiana M, Pais MS et al (2013) Reference gene selection and validation for the early responses to downy mildew infection in susceptible and resistant Vitis vinifera cultivars. PLoS ONE 8:e72998. https://doi.org/10.1371/journal.pone.0072998
Li QF, Sun SM, Yuan DY et al (2010) Validation of candidate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol Biol Rep 28:49–57. https://doi.org/10.1007/s11105-009-0124-1
Zhao Y, Luo J, Xu S et al (2016) Selection of reference genes for gene expression normalization in Peucedanum praeruptorum Dunn under abiotic stresses, hormone treatments and different tissues. PLoS ONE 11:e0152356. https://doi.org/10.1371/journal.pone.0152356
Carvalho KD, Bespalhok FJ, Santos TD et al (2013) Nitrogen starvation, salt and heat stress in coffee (Coffea arabica L.): identification and validation of new genes for qPCR normalization. Mol Biotechnol 53:315–325. https://doi.org/10.1007/s12033-012-9529-4
Wan H, Zhao Z, Qian C et al (2010) Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem 399:257–261. https://doi.org/10.1016/j.ab.2009.12.008
Chen H, Yang ZQ, Hu Y et al (2016) Reference genes selection for quantitative gene expression studies in Pinus massoniana L. Trees 30:685–696. https://doi.org/10.1007/s00468-015-1311-3
Liu D, Shi L, Han C et al (2012) Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 7:e46451. https://doi.org/10.1371/journal.pone.0046451
Xu M, Li A, Teng Y et al (2019) Exploring the adaptive mechanism of Passiflora edulis in karst areas via an integrative analysis of nutrient elements and transcriptional profiles. BMC Plant Biol 19:185. https://doi.org/10.1186/s12870-019-1797-8
Liu S, Li AD, Chen CH et al (2017) De Novo transcriptome sequencing in Passiflora edulis sims to identify genes and signaling pathways involved in cold tolerance. Forests 8:435. https://doi.org/10.3390/f8110435
Araya S, Martins AM, Junqueira N et al (2017) Microsatellite marker development by partial sequencing of the sour passion fruit genome (Passiflora edulis Sims). BMC Genomics 18:549. https://doi.org/10.1186/s12864-017-3881-5
Costa ZD, Munhoz CF, Vieira M (2017) Report on the development of putative functional SSR and SNP markers in passion fruits. BMC Res Notes 10:445. https://doi.org/10.1186/s13104-017-2771-x
Munhoz CF, Santos AA, Arenhart RA, Santini L et al (2015) Analysis of plant gene expression during passion fruit–xanthomonas axonopodis interaction implicates lipoxygenase 2 in host defence. Ann Appl Biol 167:135–155. https://doi.org/10.1111/aab.12215
Xie F, Sun G, Stiller JW et al (2011) Genome-wide functional analysis of the cotton transcriptome by creating an integrated EST database. PLoS ONE 6:e26980. https://doi.org/10.1371/journal.pone.0026980
Vandesompele J, De PK, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11. https://doi.org/10.1186/gb-2002-3-7-research0034
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496
Pfaffl MW, Tichopad A, Prgomet C et al (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. https://doi.org/10.1023/b:bile.0000019559.84305.47
Xie F, Xiao P, Chen D et al (2012) miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol 80:75–84. https://doi.org/10.1007/s11103-012-9885-2
Grabherr MG, Haas BJ, Yassour M et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. https://doi.org/10.1038/nbt.1883
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323. https://doi.org/10.1186/1471-2105-12-323
Ye J, Zhong T, Zhang D et al (2019) The auxin-regulated protein ZmAuxRP1 coordinates the balance between root growth and stalk rot disease resistance in maize. Mol Plant 12:360–373. https://doi.org/10.1016/j.molp.2018.10.005
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Untergasser A, Cutcutache I, Koressaar T et al (2012) Primer3–new capabilities and interfaces. Nucleic Acids Res 40:e115. https://doi.org/10.1093/nar/gks596
Sun HF, Meng YP, Cui GM et al (2009) Selection of housekeeping genes for gene expression studies on the development of fruit bearing shoots in Chinese jujube (Ziziphus jujube Mill.). Mol Biol Rep 36:2183–2190. https://doi.org/10.1007/s11033-008-9433-y
Mughal BB, Leemans M, Spirhanzlova P et al (2018) Reference gene identification and validation for quantitative real-time PCR studies in developing Xenopus laevis. Sci Rep 8:496. https://doi.org/10.1038/s41598-017-18684-1
Karuppaiya P, Yan XX, Liao W et al (2017) Identification and validation of superior reference gene for gene expression normalization via RT-qPCR in staminate and pistillate flowers of Jatropha curcas-A biodiesel plant. PLoS ONE 12:e0172460. https://doi.org/10.1371/journal.pone.0172460
Du W, Hu F, Yuan S et al (2019) Selection of reference genes for quantitative real-time PCR analysis of photosynthesis-related genes expression in Lilium regale. Physiol Mol Biol Plants 25:1497–1506. https://doi.org/10.1007/s12298-019-00707-y
Dos Santos CP, Da Cruz SK, Batista MC et al (2019) Identification and evaluation of reference genes for reliable normalization of real-time quantitative PCR data in acerola fruit, leaf, and flower. Mol Biol Rep 47:953–965. https://doi.org/10.1007/s11033-019-05187-7
Li Z, Lu H, He Z et al (2019) Selection of appropriate reference genes for quantitative real-time reverse transcription PCR in Betula platyphylla under salt and osmotic stress conditions. PLoS ONE 14:e0225926. https://doi.org/10.1371/journal.pone.0225926
Wang X, Wu Z, Bao W et al (2019) Identification and evaluation of reference genes for quantitative real-time PCR analysis in Polygonum cuspidatum based on transcriptome data. BMC Plant Biol 19:498. https://doi.org/10.1186/s12870-019-2108-0
Hu X, Zhang L, Nan S et al (2018) Selection and validation of reference genes for quantitative real-time PCR in Artemisia sphaerocephala based on transcriptome sequence data. Gene 657:39–49. https://doi.org/10.1016/j.gene.2018.03.004
Gao D, Kong F, Sun P et al (2018) Transcriptome-wide identification of optimal reference genes for expression analysis of Pyropia yezoensis responses to abiotic stress. BMC Genomics 19:251. https://doi.org/10.1186/s12864-018-4643-8
Wang G, Tian C, Wang Y et al (2019) Selection of reliable reference genes for quantitative RT-PCR in garlic under salt stress. Peer J 7:e7319. https://doi.org/10.7717/peerj.7319
Feng K, Liu JX, Xing GM et al (2019) Selection of appropriate reference genes for RT-qPCR analysis under abiotic stress and hormone treatment in celery. Peer J 7:e7925. https://doi.org/10.7717/peerj.7925
Cheng T, Zhu F, Sheng J et al (2019) Selection of suitable reference genes for quantitive real-time PCR normalization in Miscanthus lutarioriparia. Mol Biol Rep 46:4545–4553. https://doi.org/10.1007/s11033-019-04910-8
Zhang K, Li M, Cao S et al (2019) Selection and validation of reference genes for target gene analysis with quantitative real-time PCR in the leaves and roots of Carex rigescens under abiotic stress. Ecotoxicol Environ Saf 168:127–137. https://doi.org/10.1016/j.ecoenv.2018.10.049
Phule AS, Barbadikar KM, Madhav MS et al (2018) Genes encoding membrane proteins showed stable expression in rice under aerobic condition: novel set of reference genes for expression studies. 3 Biotech 8:383. https://doi.org/10.1007/s13205-018-1406-9
Borkowska P, Zielińska A, Paul-samojedny M et al (2020) Evaluation of reference genes for quantitative real-time PCR in Wharton's Jelly-derived mesenchymal stem cells after lentiviral transduction and differentiation. Mol Biol Rep 47:1107–1115. https://doi.org/10.1007/s11033-019-05207-6
Li C, Hu L, Wang X et al (2019) Selection of reliable reference genes for gene expression analysis in seeds at different developmental stages and across various tissues in Paeonia ostii. Mol Biol Rep 46:6003–6011. https://doi.org/10.1007/s11033-019-05036-7
Hou F, Li S, Wang J et al (2017) Identification and validation of reference genes for quantitative real-time PCR studies in long yellow daylily Hemerocallis citrina Borani. PLoS ONE 12:e0174933
Wang C, Yang Q, Wang W et al (2017) A transposon-directed epigenetic change in ZmCCT underlies quantitative resistance to Gibberella stalk rot in maize. New Phytol 215:1503–1515. https://doi.org/10.1111/nph.14688
Shen L, Zhong T, Wang L et al (2019) Characterization the role of a UFC homolog, AtAuxRP3, in the regulation of Arabidopsis seedling growth and stress response. J Plant Physiol 240:152990. https://doi.org/10.1016/j.jplph.2019.152990
Huot B, Yao J, Montgomery BL et al (2014) Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7:1267–1287. https://doi.org/10.1093/mp/ssu049
Wang W, Wang ZY (2014) At the intersection of plant growth and immunity. Cell Host Microbe 15:400–402. https://doi.org/10.1016/j.chom.2014.03.014
Acknowledgements
This work was supported by Guangxi Natural Science Foundation of China (2018GXNSFBA281024, 2019GXNSFAA245002), Guangxi’s Ministry of Science and Technology (AB18294007), Guangxi Academy of Agricultural Sciences (2018YT19, TS2016010). Thanks to Xu et al. for the transcriptome data.
Author information
Authors and Affiliations
Contributions
YW, HM and XY. contributed to study design, QT contributed to qPCR analysis, WH and JL. contributed to data analysis, XX. contributed to primer design, YW. wrote this manuscript, XY. revised the manuscript. All authors read and approve the paper.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Primer specificity and melting curve analysis of the sixteen candidate reference genes. (a-p): C13615, C24590, C27182, C10445, C21209, C22199, C22526, GAPDH, UBQ, Actin1, Actin2, EF-1α-1, EF-1α-2, TUA, NADP, and GBP. Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Tian, Q., Huang, W. et al. Identification and evaluation of reference genes for quantitative real-time PCR analysis in Passiflora edulis under stem rot condition. Mol Biol Rep 47, 2951–2962 (2020). https://doi.org/10.1007/s11033-020-05385-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05385-8