Abstract
Phytophthora species are highly destructive phytopathogens, associated with massive damage in natural ecosystems and agriculture. Citrus production is also affected, mainly by the hemibiothrophic oomycete Phytophthora parasitica, which causes root rot and gummosis. Poncirus trifoliata and Citrus sunki (two rootstocks widely used in citrus orchards) pose a resistance and a susceptible interaction with P. parasitica, respectively, which makes them suitable models to study plant defense mechanisms. Gene expression analysis is a very important tool in this type of study, in particular PCR (RT-qPCR). Hence, it is crucial to use appropriate reference genes for expression normalization. Our aim was to evaluate the stability of several candidate reference genes to determine which set is best suited for normalization in citrus infected with P. parasitica. We evaluated five candidate reference genes selected from the database CitEST. GeNorm and NormFinder algorithms were used to assess the best reference genes. We found that the more stable genes to be used for RT-qPCR analysis in P. trifoliata plants were GAPC2 and F-BOX, while EGIDH and GAPC2 were more suitable to C. sunki. These four genes were found to be excellent normalizers, being stable throughout the infection regardless of pathogen attack or symptom development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world’s citrus production has been widely damaged by several diseases caused by viruses, bacteria, fungi, oomycetes, and nematodes. Phytophthora parasitica (Dastur) (syn. P. nicotianae) is a destructive oomycete capable of infecting more than 250 plant families (Kamoun et al. 2015. In citrus, it is associated with root rot and gummosis, which affects the productivity but can also lead to the declining of the tree (Panabieres et al. 2016). It is estimated that in average 10 to 30% of the total production of citrus orchards is affected due to P. parasitica-associated diseases (Timmer et al. 2000).
During infection, pathogens secrete hundreds of effector proteins, some of which are recognized by plant proteins activating defense systems, while others are fundamental to the disease establishment and pathogen virulence (Osswald et al. 2014). In any case, there are changes in the transcription program of the plants, which could lead to susceptibility or resistance. These alterations in the genetic expression profile of plants are still obscure, especially in citrus.
In the interaction between P. parasitica and citrus, two genotypes, widely used as rootstocks, stand out because of their opposite outcomes regarding development of symptoms. While Poncirus trifoliata (L.) Raf. presents a clearly resistant interaction, Sunki mandarin (Citrus sunki (Hayata) hort. ex. Tanaka) is highly susceptible to the pathogen. These distinct responses to infection make the two species very convenient to study mechanisms of attack and defense, particularly at the molecular level. Gene expression analysis has been a very important tool in this type of study. One of the techniques with great accuracy, high sensitivity and specificity for this type of work is quantitative reverse transcription-PCR (RT-qPCR) (Gachon et al. 2004; Nicot et al. 2005). However, for improved efficiency and accuracy it is necessary to use appropriate reference genes to normalize the expression levels of target genes (Nicot et al. 2005; Jain et al. 2006; Czechowski et al. 2005; Vandesompele 2002; Radonic et al. 2004; Brunner et al. 2004; Iskandar et al. 2004).
Genes are considered good normalizers when their expression does not change under different conditions or treatments, different tissues or cell types, developmental stages or stresses (biotic or abiotic) (Wong and Medrano 2005; Nolan et al. 2006; Borges et al. 2012). Many housekeeper genes encoding proteins involved in basic cellular processes, such as ACTIN (Zheng et al. 2011), EF1 (Sharifi-Sirchi et al. 2011), TUBULIN (Tan and Swain 2007), GAPDH (Fan et al. 2010) and 18 s-RNA (Albrecht and Bowman 2008) have been used as normalizers to study gene expression in citrus under different conditions and stresses without first testing them for their stability (Mafra et al. 2012). (Volkov et al. 2003) and (Matta et al. 2011) have demonstrated that several genes involved in basic cellular processes are not always stable under different conditions, which could lead to deviated results in gene expression analyses (Czechowski et al. 2005; Nicot et al. 2005; Remans et al. 2008). Nolan et al. (2006) published a protocol for reliable RT-qPCR and concluded that for each different experimental set-up, internal reference genes should be validated. To our knowledge, in the citrus-P. parasitica interaction, the stability of candidate reference genes was never tested in experiments in which the infection was performed via inoculation of zoospores in roots. The inoculation of zoospores in roots would be the experimental condition more related to what happens in nature, and therefore, the experimental set-up of choice in future studies on this pathosystem in detriment of stem or leaf wounding.
In the present study we aimed to evaluate the stability of candidate reference genes and determine which set of these genes is best suited for transcript normalization in resistant and susceptible citrus genotypes during infection with zoospores of P. parasitica. We evaluated five candidate reference genes (CYP, DIM1, EGIDH, FBOX, GAPC2), which were selected from the database CitEST. GeNorm and NormFinder algorithms were used to find the best reference genes in all the conditions evaluated.
Material and methods
Zoospore production and infection of plants
P. parasitica isolate IAC 01–95 was maintained in Petri dishes with carrot-agar medium, in the dark, at 20 °C. After the colony reached up to 80% of the plates, sporangia development was induced by pouring autoclaved water on the plate. The water was replaced daily for 1 week. After 7 days, zoospore release was induced by placing the Petri dishes at 4 °C for 1 h. The amount of zoospores released was recorded using a Neubauer chamber. The zoospore suspension was set to 1 × 105 zoospores/mL.
Seeds of C. sunki and P. trifoliata were germinated and grown in root containers with sterile substrate for 3 months in the greenhouse. Three days before initiating the experiment, the seedlings were carefully removed from the containers. The roots were rinsed off the substrate and placed in 50 mL Falcon tubes containing 50 mL of distilled water and sealed with parafilm. Roots were inoculated as follows: zoospores (1 × 105 zoospores per plant) were carefully pipetted from the zoospore suspension and transferred to the water surrounding the roots inside the Falcon tubes. Plants were maintained in growth chambers at 20 °C, light conditions of 250 μmol/m2/s photosynthetic photon flux density (PPFD) and photoperiod of 12 h. Six plants were used for each treatment. The experiment was repeated twice.
Disease assessment
The plants were monitored daily for root necrosis, growth of visible mycelia on the root surface, wilting of leaves and mortality.
Genomic DNA was extracted from 100 mg frozen root material using the DNeasy Plant Mini kit (Qiagen) and was further purified using the Wizard DNA Clean Up System (Promega) according to the manufacturers’ protocols. The DNA was diluted 1:10 in water to prevent the inhibition of the PCR. The amount of P. parasitica DNA in 5 μL of root extract was determined by Sybr Green quantitative PCR using an SDS7700 sequence detection system (Applied Biosystems), with the primer pair PN5b/PN6 (Ippolito et al. 2002). All analyses were performed in three technical replicates using Promega Sybr Green chemicals and performing 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 62 °C for 60 s. The Ct values of the samples were compared with a standard curve that was generated for pure P. parasitica genomic DNA extracted from mycelia grown in liquid culture (mineral medium M1) supplemented with 10 g/L glucose and 2 g/L L-asparagine). The standard curve concentrations ranged from 1 pg DNA/mL to 10 ng DNA/mL in five steps.
For the extraction of total RNA, 50 mg of roots were ground in liquid nitrogen using a mortar and pestle. Total RNA was extracted using the MasterPure Plant RNA Purification kit (Epicentre Biotechnologies) according to the manufacturer’s protocol, including a DNAse I treatment. The concentration and quality of extracted RNA was measured using a nanodrop (NanoDrop 8000; Thermo Scientific) in which the samples were analyzed at 260 and 280 nm wavelengths. cDNA was reverse transcribed using 1 μg of total RNA with oligo-dT primers and the MMLV Reverse Transcriptase First Strand cDNA Synthesis kit (Epicentre Biotechnologies). The transcript levels of the genes were analysed by using 0.05 μg of cDNA (analyzed by NanoDrop 8000) by qRT-PCR in three technical replicates using the ABsolute SYBRGreen ROX chemicals (ABgene) and performing 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30s. For all amplifications performed in RT-qPCR, we produced dissociation curves to check for nonspecific amplification resulting from possible contamination.
Light microscopy analysis
Root tip samples were taken after 96 h post infection and fixed in Karnovsky solution (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer pH 7.4). After 3 days, samples were washed in phosphate buffer (pH 7.4) and embedded in resin (Leica embedding kit) following the manufacturer’s protocol. The cuttings were processed in a microtome (longitudinal cuts, 1 nm), colored with Toluidine blue (0.05% solution, pH 6.0, for eight minutes) and analyzed by light microscopy.
Reference genes selection
The five candidate genes evaluated in this experiment were selected from the CitEST (http://limonia.centrodecitricultura.br/blast/blast.html) and HarvEST (http://www.harvest-web.org/) citrus databases according to one or more of the following criteria: (1) reference genes traditionally used in citrus for transcript normalization; (2) citrus homologues of reference genes tested for transcript level normalization and quantification in Arabidopsis. The information regarding all the genes used in this work is summarized in Table 1.
Data analysis and statistics
Data analysis was performed similarly to Mafra et al. 2012. Primer efficiency for each experimental set was estimated using an algorithm in real-time PCR Miner software (http://www.miner.ewindup.info/) that calculates primer efficiency and quantification cycle (Cq) values based on the kinetics of individual reactions without the need for a standard curve. Cq values, determined by the second derivative maximum for each biological sample, were converted into non-normalized relative quantities using the formula Q = EΔCq, where E represents the arithmetic mean of efficiency of all samples for each gene, and ΔCq represents the difference between the arithmetic mean Cq value across all samples for this gene and the Cq value of the sample in question, as recommended by Hellemans et al. 2007. These quantities were imported into geNorm v3.5 (Vandesompele et al. 2002) and NormFinder (Andersen et al. 2004) for reference gene selection. First, we performed a global analysis composed of all biological samples in geNorm. Then, we analyzed each experimental condition individually in an attempt to identify specific reference genes according to the two citrus species. The geNorm software calculated the average of expression stability yielding an M value and the variation of the stability of the better gene pairs (V). This allowed us to exclude the less suitable genes, i.e., genes with the lowest M value (Vandesompele et al. 2002). Finally, NormFinder calculated both inter- and intra-group variation in the expression stability, thus identifying the best combination of reference genes. This software is based on the Ct method, in which data are linearized through transformation of the Ct values in relative quantity (Q), fixing the highest Ct value and using the amplicon average efficiency in the treatment (E). To do that, we have applied the following equation, called delta-Ct (Andersen et al. 2004): Q = (E gene )∆Ct(lowest-highest)
To test the sets of optimal reference genes we have selected as target the gene CC-NBS, which is a coile-coil resistance-type gene, known to display differences in expression when comparing P. trifoliata and C. sunki. To calculate the expression of the CC-NBS gene we have used the method of 2-∆∆Ct (Livak and Schmittgen 2001). We also applied the Kruskal-Wallis non-parametric test to check the significance among the treatments and time-points (Kruskall and Wallis 1952).
Results and discussion
In this work we present two sets of candidate reference genes suitable to analyze gene expression of P. trifoliata and C. sunki upon root infection with zoospores of P. parasitica.
Successful infection by P. parasitica in both species after inoculation via zoospores was confirmed by microscopy and/or pathogen DNA found in roots (Figs. 1 and 2). At 48 h post-inoculation (hpi) in C. sunki, the pathogen colonized the root tissues damaging the cortex and central cylinder. It is possible to visualize hyphae and sporangia near the central cylinder (Fig. 1c). Figure 2a shows P. parasitica colonizing the cortex of C. sunki. The infection is also confirmed by P. parasitica DNA quantification in roots, with the highest value at 48 hpi (Fig. 1d). P. trifoliata was also infected by P. parasitica but to a lower degree compared to C. sunki. It was not possible to find hyphae in P. trifoliata root cuttings (Fig. 2b) but the infection was confirmed by the DNA analysis (Fig. 1d). This pattern was expected since P. trifoliata shows resistance/tolerance against P. parasitica, while C. sunki is highly susceptible. Similar results were reported by Boava et al. 2011.
a Light microscopy view of mycelium in Petri dish evidencing clusters of sporangia of P. parasitica cultivated in carrot agar. b Neubauer chamber picture showing zoospores of P. parasitica (1 × 105 zoospores/mL). c Light microscopy of C. sunki roots infected with P. parasitica. The arrows show P. parasitica colonization in the central cylinder of the damaged root and the asterisks show the tissue necrosis process, characterizing root rot in citrus (48 h post inoculation). Staining: toluidine blue. d Heat map showing P. parasitica genomic DNA values along the colonization of C. sunki and P. trifoliata seedlings (DNA expressed in μg/g FW), monitored by RT-qPCR over 3, 6, 12, 24, 48 and 96 h after inoculation
Longitudinal section of C. sunki (a) and P. trifoliata (b) roots infected with 1 × 105 zoospores of P. parasitica. a The arrows point mycelia growing in root tissue of C. sunki. b Mycelia of P. parasitica was not found in P. trifoliata root tissues, despite qPCR confirmation of roots infection. Asterisk shows the central cylinder of C. sunki seedlings. Staining: toluidine blue
Internal reference genes have been extensively analyzed over their capability to normalize transcripts in gene expression in citrus under different treatments and pathogen attack. The most used genes have been EF-1α (Endo et al. 2006; Cernadas et al. 2008; Nishikawa et al. 2009; Sharifi-Sirchi et al. 2011; Martinelli et al. 2012), ACTIN (Liu et al. 2007; Huerta et al. 2008; Liu et al. 2009; Chai et al. 2011; Zheng et al. 2011; Miao et al. 2011; Zhong et al. 2015), 18 s rRNA (Albrecht and Bowman 2008; Aritua et al. 2013), UBQ and CYP (Boava et al. 2011; Mafra et al. 2012; Rodrigues et al. 2013). Specifically for the interaction between P. parasitica and citrus, the genes already evaluated are ETEF2, EGIDH, CYP, UBQ and TUB (Boava et al. 2011) and FBOX, GAPC2, SAND, UPL7, EF1, ADP, PTB1, TUB, UBC21, UBC9, ACT2, DIM1, CtP, CYP (Mafra et al. 2012). For this study we have selected the following genes: CYP, DIM1, EGIDH, FBOX, GAPC2. All genes selected were proven to be stable in the citrus-P. parasitica interaction, however, the experimental set up, citrus genotypes and P. parasitica inoculation were different in those previous works.
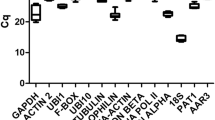
In order to check the stability of the selected candidate reference genes, we have performed RT-qPCR to obtain Cq values of these genes following the inoculation of P. trifoliata and C. sunki. For most of the genes, the Cq values were maintained with low variability when compared to Cq values of control plants at 0 and 96 h for both genotypes. Even at the latest time points (48 and 96 hpi), when the infection was fully established in C. sunki, imposing a great stress to the plant, the Cq values of the genes were comparable to the Cq values of control plants (Fig. 3), indicating that the majority of the genes were stable throughout the experiment.
Cq values of endogenous candidate genes. The arithmetic mean (n = 6) of Cq values of the genes of plants infected by P. parasitica over time were plotted in the graphs. a P. trifoliata values. b C. sunki values. Time points: 0, 3, 6, 12, 24, 48, 96 h (96 hpi: infected plants at 96 h; 96 h: control plants at 96 h). Bars represent standard deviation
To ensure the stability of the genes, all Cq values of all target candidate reference genes were used as input in the NormFinder algorithm. After NormFinder analysis, the most stable genes were (in order): GAPC2 (0.146), EGIDH (0.286), DIM1 (0.287), F-BOX (0.300) and CYP (0.477), for C. sunki, and F-BOX (0.092), CYP (0.096), GAPC2 (0.140), DIM1 (0.169) and EGIDH (0.225) for P. trifoliata (Table 2).
The geNorm analysis showed that all five genes showed stable values (M < 1.5) for both species, except for CYP in C. sunki with an M value of 2.642 (Table 3). For P. trifoliata, the software ranked F-BOX (M < 0.60) and GAPC2 (M < 0.56) as the more stable genes, while the genes F-BOX (M < 1.26) and EGIDH (M < 1.27) were ranked for C. sunki. The stability values of the genes tested in the geNorm software are shown in Fig. 4.
Mafra et al. (2012) analyzed endogenous genes in citrus plants infected with several pathogens, including P. parasitica. However, the experiment, despite having the same objective, was different from ours. The authors used older plants, different citrus genotypes in other rootstocks and they infected the stem using mycelia discs in wounded tissues. Furthermore, the infected plants were compared with wounded control plants. The infection in roots via zoospores, as in our work, reflects better what happens in nature and does not impose further stress to the plant by artificially wounding its tissues. Therefore, we expected differences in the ranking of the normalizing genes, which was indeed the case for both C. sunki and P. trifoliata. However, some similarities were also found, such as the F-BOX gene. We have found that the F-BOX gene is stable under all conditions for C. sunki as well as P. trifoliata, and was high-ranked by geNorm and ranked fourth by Normfinder (despite having a high stability value) (Fig. 4; Tables 2, 3 and 4). The F-BOX genes are grouped in a very large multigenic superfamily controlling several important biological functions in plants. At least 694 and 687 F-BOX genes were found in the genome of Arabidopsis thaliana and Oryza sativa, respectively (Gagne et al. 2002; Jain et al. 2007). In Citrus sinensis (L.) Osbeck, 363 F-BOX domain ontologies were found (phytozome.org). The main functions of F-BOX proteins are related to ubiquitination and protein degradation, embryogenesis, hormone responses, seedling development, senescence and floral ontology. All these fundamental processes render this gene a good reference gene status (Lechner et al. 2006).
The CYP (Cyclophilin) gene was also found to be a suitable normalizer and was well-ranked by geNorm and Normfinder for P. trifoliata analysis. However, for C. sunki, CYP received the lowest M score in geNorm and the least stable value by Normfinder (Fig. 4; Tables 2, 3 and 4). In the study of Mafra et al. (2012), the CYP gene was not found as a stable normalizer in any condition or genotype. The CYP gene encodes a molecular chaperone protein which is involved in protein folding by peptidyl prolil isomerization in the plant cell (Maruyama et al. 2004). Since these functions are essential for the physiology of the plant, this gene can also be considered a good candidate reference gene.
The genes DIM1 (18S rRNA dimethylase) and EGIDH (NADP-isocitrate dehydrogenase) were found to be suitable normalizers for C. sunki in both NormFinder and geNorm algorithms (Fig. 4; Tables 2, 3 and 4). However, while with acceptable normalizing values, the DIM1 and EGIDH genes were low-ranked by both algorithms for P. trifoliata (Fig. 4; Tables 2, 3 and 4). Similar results were obtained by Boava et al. (2011). Mafra et al. (2012) found higher values for both genes in citrus plants. Several studies have been using DIM1 and EGIDH as normalizers to study gene expression in plant-pathogen interactions or plant physiology (Mafra et al. 2012; Boava et al. 2011; Boava et al. 2010; Paux et al. 2004; Legay et al. 2007; Boiffin et al. 1998).
The GAPC2 gene expresses a citosolic isoenzime GAPDH (gliceraldehide-3-fosfate Nad-dependent desidrogenase) which is involved in carbon metabolism in cells. It is frequently used as a normalizer gene. In our study, it was the most stable of all five genes tested by GeNorm and the third by NormFinder for P. trifoliata plants. In C. sunki, it was the best ranked gene in NormFinder and the fourth by geNorm (Fig. 4; Tables 2, 3 and 4). Mafra et al. (2012) also found GAPC2 to be a suitable internal reference gene for citrus.
The best reference genes for expression normalization in P. trifoliata and C. sunki, based on geNorm and NormFinder are presented in the Table 5.
In order to check if the highly ranked genes are in fact good normalizers we performed a gene expression analysis in P. trifoliata and C. sunki infected with P. parasitica. The target gene was CC-NBS (NBS-LRR protein) (Rodrigues et al. 2013), known to be differentially expressed when comparing P. trifoliata and C. sunki. As normalizers, we used F-BOX and GAPC2 for P. trifoliata and GAPC2 and EGIDH for C. sunki., since those genes were highly ranked by both GeNorm and NormFinder (Table 5). The results are shown in Fig. 5.
CC-NBS (a resistance gene) differential expression in P. trifoliata and C. sunki seedlings infected with zoospores of P. parasitica. Genes F-BOX, GAPC2 of P. trifoliata and GAPC2, EGIDH of C. sunki were used as normalizers. The Kruskal-Wallis statistical test shows highly significant differences in gene expression levels for the CC-NBS in P. trifoliata seedlings in comparison with C. sunki
As expected, the CC-NBS gene was differentially expressed in citrus roots comparing the two rootstocks at 24, 48 and 96 hpi. This result validates the selected reference genes for the study of gene expression in the citrus-P. parasitica interaction.
In summary, most of the genes studied were found to be suitable normalizers of citrus plants infected with P. parasitica. We recommend the use of F-BOX (best ranked in NormFinder) and GAPC2 (best ranked in GeNorm) for P. trifoliata, and EGIDH (second best ranked in GeNorm; the first ranked, F-BOX, has a low score in NormFinder) and GAPC2 (first ranked in NormFinder) for C. sunki. If only two genes were to be used for normalization of both P. trifoliata and C. sunki concomitantly, we suggest the use of F-BOX and GAPC2 (overall best ranked genes).
References
Albrecht U, Bowman KD (2008) Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci 175:291–306
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative recerse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon câncer data sets. Cancer Res 64:5245–5250
Aritua V, Achor D, Gmitter FG, Albrigo G, Wang N (2013) Transcriptional and microscopic analyses of citrus stem and root responses to Candidatus Liberibacter asiaticus infection. PLoS ONE 8, e73742
Boava LP, Laia ML, Jacob TR, Dabbas KM, Gonçalves JF, Ferro JA, Ferro MIT, Furtado EL (2010) Selection of endogenous genes for gene expression studies in Eucalyptus under biotic (Puccinia psidii) and abiotic (acibenzolar-S-methyl) stresses using RT-qPCR. BMC Res Notes 3:43
Boava LP, Cristofani-Yaly M, Mafra VS, Kubo K, Kishi LT, Takita MA, Ribeiro-Alves M, Machado MA (2011) Global gene expression of Poncirus trifoliata, Citrus sunki and their hybrids under infection of Phytophthora parasitica. BMC Genomics 12:39
Boiffin V, Hodges M, Galvez S, Balestrini R, Bonfante P, Gadal P, Martin F (1998) Eucalypt NADP-dependent isocitrate dehydrogenase - cDNA cloning and expression in ectomycorrhizae. Plant Physiol 117:939–948
Borges A, Tsai SM, Caldas DGG (2012) Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Rep 31:827–838
Brunner AM, Yakovlev IA, Strauss SH (2004) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4:14
Cernadas RA, Camillo LR, Benedetti CE (2008) Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Mol Plant Pathol 9:609–631
Chai L, Ge X, Xu Q, Deng X (2011) CgSL2, an S-like RNase gene in ‘Zigui shatian’ pummelo (Citrus grandis Osbeck), is involved in ovary senescence. Mol Biol Rep 38:1–8
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Endo T, Shimada T, Fujii H, Omura M (2006) Cloning and characterization of five MADS-box cDNAs isolated from citrus fruit tissue. Sci Hortic 109:315–321
Fan J, Chen C, Brlansky RH, Gmitter FG Jr, Li Z-G (2010) Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol 59:1037–1043
Gachon C, Mingam A, Charrier B (2004) Real-time PCR: what relevance to plant studies. J Exp Bot 55:1445–1454
Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD (2002) The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci U S A 99:11519–11524
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis for real-time quantitative PCR data. Genome Biol 8:R19
Huerta L, Forment J, Gadea J, Fagoaga C, Peña L, Pérez-Amador MA, García-Martínez JL (2008) Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant Cell Environ 31:1620–1633
Ippolito A, Schena L, Nigro F (2002) Detection of Phytophthora nicotianae and P. citrophthora in citrus roots and soils by nested PCR. Eur J Plant Pathol 108:855–868
Iskandar H, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Report 22:325–337
Jain M, Nijhawan A, Tyagi AK (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, Kapoor S, Tyagi AK, Khurana JP (2007) F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol 143:1467–1483
Kamoun S, Furzer O, Jones JDG, Judelson H S, Ali GS, Dalio RJD, Roy SG, Schena L, Zambounis A, Panabières F, Cahill D, Ruocco M, Figueiredo A, Chen X-R, Hulvey J, Stam R, Lamour K, Gijzen M, Tyler BM, Grünwald NJ, Mukhtar MS, Tomé DFA, Tör M, Van Den Ackerveken G, McDowell J, Daayf F, Fry WE, Lindqvist-Kreuze H, Meijer HJG, Petre B, Ristaino J, Yoshida K, Birch PRJ, Govers F (2015), The Top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol 16:413–434
Kruskal HW, Wallis AW (1952) Use of Ranks in One-Criterion Variance Analysis. Journal of the American Statistical Association 47(260):583–621
Lechner E, Achard P, Vansiri A, Poutuschak T, Genschik P (2006) F-box proteins everywhere. Curr Opin Plant Biol 9:631–638
Legay S, Lacombe E, Goicoechea M, Brière C, Séguin A, Mackay J, Grimapettenati J (2007) Molecular characterization of EgMYB1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Sci 173:542–549
Liu Q, Xu J, Liu Y, Zhao X, Deng X, Guo L, Gu J (2007) A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J Exp Bot 58:4161–4171
Liu Q, Zhu A, Chai L, Zhou W, Yu K, Ding J, Xu J, Deng X (2009) Transcriptome analysis of a spontaneous mutant in sweet orange [Citrus sinensis (L.) Osbeck] during fruit development. J Exp Bot 60:801–813
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)). Methods 25:402–408
Mafra V, Kubo KS, Alves-Ferreira M, Ribeiro-Alves M, Stuart RM, Boava LP, Rodrigues CM, Machado MA (2012) Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 7, e31262
Martinelli F, Uratsu SL, Albrecht U, Reagan RL, Phu ML, Britton M, Buffalo V, Fass J, Leicht E, Zhao W, Lin D, D’Souza R, Davis CE, Bowman KD, Dandekar AM (2012) Transcriptome profiling of citrus fruit response to huanglongbing disease. PLoS ONE 7, e38039
Maruyama T, Suzuki R, Furutani M (2004) Archaeal peptidyl prolyl cis-trans isomerases (PPIases) update 2004. Front Biosci 9:1680–1720
Matta BP, Bitner-Mathe BC, Alves-Ferreira M (2011) Getting real with real-time qPCR: a case study of reference gene selection for morphological variation in Drosophila melanogaster wings. Dev Genes Evol 221:49–57
Miao HX, Qin YH, Silva JT, Ye ZX, Hu GB (2011) Cloning and expression analysis of S-RNase homologous gene in Citrus reticulata Blanco cv. Wuzisha- tangju. Plant Sci 180:58–367
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Omura M (2009) Differences in seasonal expression of flowering genes between deciduous trifoliate orange and evergreen Satsuma mandarin. Tree Physiol 29:921–926
Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time PCR. Nat Protoc 1:1559–1582
Osswald W, Fleischmann F, Rigling D, Coelho AC, Cravador A, Diez J, Dalio RJ, Horta Jung M, Pfanz H, Robin C, Sipos G, Solla A, Cech T, Chambery A, Diamandis S, Hansen E, Jung T, Orlikowski LB, Parke J, Prospero S, Werres S (2014), Strategies of attack and defence in woody plant–Phytophthora interactions. For. Path., 44:169–190.
Panabieres F, Ali GS, Allagui MB, Dalio RJD, Gudmestad NC, Kuhn M, Roy SG, Schena L, Zampounis A (2016) Phytophtora nicotianae diseases worldwide: new knowledge of a long-recognised pathogen. Phytopatologia Mediterr 55:20–40
Paux E, Tamasloukht M, Ladouce N, Sivadon P, Grima-Pettenati J (2004) Identification of genes preferentially expressed during wood formation in Eucalyptus. Plant Mol Biol 55:263–280
Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313:4856–4862
Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A (2008) Normalization of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 227:1343–1349
Rodrigues CM, De Souza AA, Takita MA, Kishi LT, Machado MA (2013) RNA-Seq analysis of Citrus reticulata in the early stages of Xylella fastidiosa infection reveals auxin-related genes as a defense response. BMC Genomics 14:1
Sharifi-Sirchi GR, Beheshti B, Hosseinipour A, Mansouri M (2011) Priming against Asiatic citrus canker and monitoring of PR genes expression during resistance induction. Afr J Biotechnol 10:3818–3823
Tan FC, Swain SM (2007) Functional characterizationof AP3, SOC1 and WUS homologues from citrus (Citrus sinensis). Plant Physiol 131:481–495
Timmer LR, Garnsey SM, Graham JM (2000) Compendium of Citrus Diseases. APS Press, Sant Paul
Vandesompele J, De Preter K, Patyn F (2002) Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:0034.1-0034.11
Volkov RA (2003). Heat-stress-dependency and developmental modulation of gene expression, the potential of house-keeping genes as internal standards in mRNA expression profiling using real-time RT-PCR. J Exp Bot 54:2343–2349
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. Biotechniques 39:1–9
Zheng TG, Qiu WM, Fan GE, Zheng BB, Guo WW (2011) Construction and characterization of a cDNA library from floral organs and fruitlets of Citrus reticulata. Biol Plant 55:431–436
Zhong Y, Cheng C-z, Jiang N-h, Jiang B, Wu B, Zhang Y-y, Hu M-l, Zeng J-w, H-x Y, G-j Y, Zhong G-y (2015) Comparative transcriptome and iTR AQ proteome analyses of citrus root responses to Candidatus Liberibacter asiaticus infection. PLoS ONE 10, e0126973
Acknowledgements
RJDD thanks FAPESP for grant number 2015/14498-6. HJM thanks Anhanguera Educacional and Kroton Group. MAM thanks INCT citrus (Fapesp 2008/57909-2, CNPq 573848/08-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Robert Miller
Heros J. Máximo and Ronaldo J. D. Dalio contributed equally to this work.
Rights and permissions
About this article
Cite this article
Máximo, H.J., Dalio, R.J.D., Rodrigues, C.M. et al. Reference genes for RT-qPCR analysis in Citrus and Poncirus infected by zoospores of Phytophthora parasitica . Trop. plant pathol. 42, 76–85 (2017). https://doi.org/10.1007/s40858-017-0134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-017-0134-8