Abstract
Chinar (Platanus orientalis L.) is used in folk medicine against tooth and knee pain, wounds, inflammation, and stomach discomfort; however, the effects of P. orientalis leaf (PO-leaf) infusion on the liver and kidney are unknown. The aim of this study was to investigate the phytochemical composition and antioxidant properties of an infusion obtained from dried P. orientalis leaves against ethanol-induced oxidative stress (OS) in rats. After a toxicity test, thirty male Wistar rats were divided into five groups: Control, Ethanol 20%, Ethanol 20% + Silymarin (10 mg/kg), Ethanol 20% + PO-20 mg/mL infusion, and Ethanol 20% + PO-60 mg/mL infusion. The PO-leaf infusion doses were given ad libitum during 28 days to test the biochemical and antioxidant enzyme levels. According to the results, the PO-leaf contained rich compounds such as benzaldehyde, palmitic acid, 2,4-ditert-butylphenol, stearic acid, octadecanoic acid, linoleic acid, linolenic acid, kaempferol, and kaempferol derivatives. In the Ethanol group, AST, ALT, LDH, GGT, UA, and urea in the serum and GST and malondialdehyde (MDA) in the liver and erythrocyte tissues showed a significant increase compared to the Control group. AST, LDH, GGT, UA, and LDL-C levels in the serum and MDA (all tissues) significantly decreased in the Ethanol + PO-60 mg/mL group compared to the Ethanol group. SOD, GPx, and CAT activities in the kidney tissue of the Ethanol group showed a significant decrease compared to the Control group, whereas the GPx activity in kidney tissue in all of the treatment groups increased significantly compared to the Ethanol group. These findings suggest that the administration of the determined PO-leaf infusion doses might have a protective role against ethanol-induced liver and kidney damage in rats.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platanus orientalis (Plantanaceae), also known as Chinar or Oriental plane, is a deciduous, woody and perennial tree that is a native of south-western Asia [1]. The Platanus genus consists of nine species, of which two are found in Asia and Europe and the remaining species are found in North America to southern Mexico [2]. The species P. orientalis and P. occidentalis are distributed in Turkey.
Since ancient times, Chinar has been widely used in many countries for medical purposes, shade, fuel, and against air pollution, especially in the removal of heavy metals, polluted air, and ozone gas [3,4,5]. Additionally, the leaves, fruit, seeds, and other structures of Chinar are used in public health. Phytochemical screening revealed the presence of many metabolites, like kaempferol 3-O-α-l-rhamnopyranoside, kaempferol 3-O-β-d-glucopyranoside, caffeic acid [6], platanoside, tiliroside [7], flavonol glycosides [8, 9], proanthocyanidin glycosides [10], fatty acids [11], and phytol derivatives [12]. Medicinal use of Chinar is well-known as its leaf infusion, extracts, and isolated compounds can be directly or indirectly used for the treatment of different diseases or discomfort, such as anti-HIV and anticancer [13], antiseptic and anti-inflammatory [3], hoarseness and asthma [14], opthalmia, dysentery, toothache, and dermatological and rheumatic diseases [1, 15].
Reactive oxygen species (ROS), such as superoxide (O2-), hydroxyl (HO), hydroperoxyl (HOO), lipid (L), lipid peroxyl (LOO), peroxy (ROO), lipid alkoxyl (LO), nitrogen dioxide (NO2), nitric oxide (NO), and protein (P) radicals, are highly reactive molecules that are naturally generated in small amounts during the body’s metabolic reactions and can react with and cause damage to various cell compartments [16, 17]. Ethanol is known to play a role in either the initiation or progression of carcinogenesis by inducing oxidative stress (OS) in a rodent model. OS is a complicated consecution occurring as endogenous and exogenous sources, playing an important role in the aging processes, increasing the risk of chronic disease, and causing fatal consequences [18]. In recent years, there have been significant advances in the prevention of diseases caused by OS, especially the role of free radicals and antioxidants [19]. The efforts of scientists and traditional therapists to clarify the pharmacological properties of medicinal plants and to isolate their possible active ingredients are increasing for the purpose of their use in complementary medicine [20]. Many studies have shown that medicinal plants with natural antioxidants, such as anthraquinones, flavonoids, aromatic acids, tannins [21, 22], anthocyanins [23, 24] and silymarin and derivatives [25], have inhibitory effects on ROS and lipid peroxidation. Silymarin and derivatives have many biological activities including being hepatoprotective against toxic chemicals, alcohol-induced fat infiltration, gallbladder diseases, toxin and fungal poisoning, and snake/insect bites [26, 27].
The aim of this study was to determine the possible protective or toxic effects of Chinar leaf infusion on the blood, liver, and kidney tissues of rats with ethanol-induced OS and to determine the active agents causing these effects.
Materials and methods
Chemicals
In this study, the ethanol, silymarin, thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), ethylenediaminetetraacetic acid (EDTA), reduced glutathione (GSH), metphosphoric acid, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), trihydroxymethyl aminomethane (Tris), 1-chloro-2,4-dinitrobenzene (CDNB), oxidized glutathione (GSSG), β-Nicotinamide adenine dinucleotide phosphate (NADPH), potassium dihydrogen phosphate (KH2PO4), sodium chloride (NaCl), and sodium dihydrogen citrate anhydrous (C6H7NaO7) used were of technical grade and were supplied by Sigma Chemical Co. (St. Louis, MO, USA). Kits for the antioxidant enzyme analyses were supplied by Randox Laboratories Ltd.
Analysis of volatile and fatty acid compounds
Volatile compounds and fatty acids present in the leaf material were analysed via gas chromatography mass spectrometry (GC–MS) using a head space solid-phase micro extraction and identified by the fragment ions and relative retention indices of their peaks with those of the MS library standards as described previously [18]. The headspace volume, heating temperature, and time of the extraction were optimized according to method of Dogan et al. [18].
Analysis of phenolic compounds
The identification and quantification of phenolic compounds by high-liquid chromatography-diode array-mass spectrometry (LC-DAD-MS/MS) analysis was conducted as described previously with minor modifications [18].
Plant material and preparation of the infusion
The P. orientalis leaves were collected from Haci Hamza hamlet, district of Dargeçit, city of Mardin, in the south-eastern Anatolian region of Turkey, (GPS coordinates: 37°33′19.7″N; 41°47′43.3″E) in August, 2017. The identification of the samples was confirmed by Dr. Abdullah Dalar at the Department of Pharmaceutical Botany, Faculty of Pharmacy, Van Yuzuncu Yil University, Turkey, and a voucher specimen was deposited in the university’s herbarium (Herbarium code: 340 and Collector No: A.D-761, Van Yuzuncu Yil University Faculty of Pharmacy Herbarium).
The fresh P. orientalis leaf samples were washed under tap water and dried at room temperature in the dark until dry. The powdered samples were kept in boiling water (100 °C) for about 2 min. Next, the heating was stopped and the ground leaves were allowed to remain in the water for about 15 min. Subsequently, the liquid in the container was first filtered through a gauze cloth (rough-hew) and then through a 0.45 µm hydrophilic filter (Millipore) using an injector.
Animals
Male Wistar albino rats of approximately 2 months of age and an average weight of 200 g were provided by the Experimental Animal Research Centre, Van Yuzuncu Yil University (Van, Turkey). They were divided into five groups, with each group containing six rats. The animals were housed at 25 ± 2 °C at a daily light/dark photoperiod of 10:14. All of the animals were given a wheat-soybean-based diet and water ad libitum in stainless steel cages, and received humane care according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science and published by the National Institute of Health. The ethic regulations followed were in accordance with national and institutional guidelines for the protection of animal welfare during experiments. This study was approved by the Ethics Committee of Van Yuzuncu Yil University (Protocol number: 27552122-604.01.02-E.70881).
Acute toxicity test
The study followed the acute oral toxicity method (test 423) described in the Organisation for Economic Co-operation and Development guidelines. A range of infusions (5, 10, 20, 40, 80, 160, and 320 mg/mL) was orally administrated to the six rats for 1, 2, 3, 4, 5, 6, and 7 days, respectively. The animals were given food and water ad libitum. Neither symptoms of toxicity nor mortality were observed over the period of a week.
Experimental design
The rats were randomly divided into five groups, with each containing six rats.
- Control group:
-
The rats received tap water and a standard pellet diet ad libitum.
- Ethanol group:
-
The rats received 20% ethanol water and a standard pellet diet ad libitum. The dose of ethanol was selected on the basis of a 20% concentration that was administered orally, which caused OS [28].
- Ethanol + Silymarin:
-
The rats received 20% ethanol water and silymarin (10 mg/kg, single dose per day) and were treated orally during the experimental period.
- Ethanol + PO-20 group:
-
The rats received 20% ethanol water and P. orientalis (20 mg/mL) leaf infusion during the experimental period.
- Ethanol + PO-60 group:
-
The rats received 20% ethanol water and P. orientalis (60 mg/mL) leaf infusion during the experimental period.
Preparation of the tissue supernatant and erythrocyte pellets
At the end of the 28 days experiments, the rats were anesthetized via an injection of ketamine (5 mg/100 g of body weight), intraperitoneally. The blood samples were taken using a cardiac puncture and put immediately into biochemical and hemogram tubes. Subsequently, the samples were centrifuged at 4000 ×g for 15 min at 4 °C in order to obtain serum samples for measurement of the biochemical parameters. The hemogram tubes, with EDTA as an anti-coagulant, and were centrifuged at 4000 ×g for 15 min at 4 °C and erythrocyte pellets were obtained. Next, the pellets were washed three times with physiological saline (0.9% NaCl) and finally, the erythrocytes burst when placed in cold-dH2O water.
The liver and kidney tissues were dissected and put into Petri dishes. Subsequently, the samples were taken and kept at − 78 °C until analysis. The tissues were homogenized for 5 min in 50 mM of ice-cold potassium dihydrogen phosphate (KH2PO4) solution (1:5 w/v) using a stainless steel probe homogenizer (SONOPULS HD 2200, Bandelin, Berlin, Germany), and then subsequently centrifuged at 7000 ×g for 15 min. All of the processes were carried out at 4 °C. Supernatants were used to determine antioxidant defence systems (ADS) constituents and malondialdehyde (MDA) contents as described previously [29].
Measurement of the biochemical parameters
Serum biochemical parameters aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkalane phosphatise (ALP), γ-glutamyl transferase (GGT), glucose (Glu), urea, uric acid (UA), creatinine (CREA), total bilirubin (TB), albumin, triglycerides (TRIGs), cholesterol (CHOL), HDL-cholesterol (HDL-C), and LDL-cholesterol (LDL-C) were measured using an auto analyser (ARCHITECT 16,200, Abbott Park, IL 60,064, USA) and Abbott biochemistry kits (USA).
Measurement of ADS enzymes and lipid peroxidation
Glutathione-S transferase (GST) was assayed by following the conjugation of glutathione with 1-chloro-2,4-dinitrobenzene (CDNB) at 340 nm, as described by Mannervik and Guthenberg [30]. Glutathione peroxidase (GPx) activity was measured using a method based on that of Paglia and Valentine [31]. GPx catalyses the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione is converted to a reduced form with the concomitant oxidation of NADPH. The resulting decrease in absorbance at 340 nm can be measured by a spectrophotometer. Superoxide dismutase (SOD) activity was measured at 505 nm by calculating the inhibition percentage of the formazan dye formation [32]. Catalase (CAT) activity was determined using the method described by Aebi [33], based on the rate of H2O2 consumption and as the decrease in absorbance at 240 nm. The erythrocyte and tissue MDA concentrations were determined using the method described by Jain et al. [34], based on TBA reactivity. The erythrocyte and tissue reduced glutathione (GSH) concentrations were measured using the method described by Beutler et al. [35].
Statistical analysis
All of the data were expressed as the mean ± standard deviation (SD). The statistical analyses were made using the Minitab 13 packet program for Windows. The 1-way analysis of variance (ANOVA) was used to determine the differences between the means of the experimental groups and statistical significance was accepted as P ≤ 0.05.
Results
The volatile, fatty acid and chemical compositions of PO-leaf crude material
The GC–MS analysis revealed that fatty acids were the main component of the volatile composition of the P. orientalis leaf (PO-leaf) crude material. Stearic acid (44.23% contribution) was the dominant fatty acid detected in the PO-leaf crude material, followed by palmitic, octadecanoic, linolenic, and linoleic acids (8.12%, 5.91%, 2.99%, and 1.10% contributions, respectively). In the PO-leaf crude material, 2,4-ditert-butylphenol, which is a member of the class of phenols, was determined to have a relative concentration of 15.58%, while benzaldehyde, the simplest representative of the aromatic aldehydes, had a relative concentration of 4.15% (Table 1; Fig. 1).
As a result of the high-performance liquid chromatography (HPLC) and spectral analyses shown in (Fig. 2), the major compound in the PO-leaf crude material was determined as kaempferol. As a result of the acid hydrolysis treatment, the peaks of the compounds in the chromatogram were lost and the amount of kaempferol compound significantly increased in the PO-leaf crude material. A kaempferol compound standard was also carried out in the HPLC, which was compared with the kaempferol in the extract to the spectral characteristic, co-chromatography, and delivery times. It was determined that the dominant compound in the extract was kaempferol and kaempferol derivatives.
Acute toxicity studies
Toxicity testing of new drugs or herbal substances is important for drug development or herbal use processes. The animals showed good tolerance to the testing of seven (5, 10, 20, 40, 80, 160, and 320 mg/mL) doses of PO-leaf infusion; PO-leaf infusion at a dose as high as 320 mg/mL was found to be non-lethal. The determined doses of PO-leaf infusion did not result in any noticeable signs of toxicity and mortality after ad libitum administration orally for 7 days. Therefore, the PO-leaf infusion is safe for long term administration.
PO-leaf infusion effects on live body weight and liquid consumption
As shown in Table 2, the live body weight (LBW) of the animals in the 3rd week had significantly increased compared to the 2nd and 4th weeks. In the Ethanol + PO-20 mg/mL group, the LBW in the 2nd and 4th week had decreased significantly compared to the 1st week. Moreover, in the Ethanol + PO-60 mg/mL group, the LBW in the 2nd week had significantly increased compared to the other weeks. In the ethanol group, fluid consumption in the 3rd and 4th weeks had significantly increased compared to the 1st and 2nd weeks. In the other groups, changes in the weekly fluid consumption were not statistically significant.
PO-leaf infusion effects on the biochemical parameters
Results for the levels of the serum biochemical parameters are given in Table 3. The levels of AST, ALT, LDH, GGT, UA, and urea in the Ethanol group increased significantly when compared to the Control group; however, the CHOL and HDL-C levels exhibited just the opposite effect in these groups. The LDH, GGT, TB, and LDL-C were markedly lower in the Ethanol + Silymarin group than the Ethanol group. Two doses of PO-leaf infusion resulted in a significant preventive role against Ethanol toxicity by decreasing the levels of AST, LDH, GGT, UA, LDL-C (Ethanol + PO-60 mg/mL group) ALP, UA, and HDL-C (Ethanol + PO-20 mg/mL group). Moreover, it was seen that PO-leaf infusion could be more effective than silymarin (i.e. GGT and HDL-C).
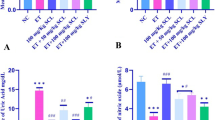
PO-leaf infusion effects on the MDA and GSH parameters
As shown in Fig. 3, following exposure in the experimental groups, the effects of ethanol, silymarin, and PO-leaf infusion on the MDA content and GSH level were evaluated. All of the groups showed exposure to ethanol in the liver tissue and erythrocytes. In the Ethanol group, the MDA content increased significantly compared to the Control, but in the liver tissue (Ethanol + Silymarin and Ethanol + PO-60 mg/mL), kidney tissue (Ethanol + PO-leaf infusion) and erythrocytes (Ethanol + PO-60 mg/mL), the MDA was significantly decreased in the groups compared to the Ethanol group. The GSH level in the liver tissue in the Ethanol and Ethanol + PO-60 mg/mL groups was significantly decreased compared to the Control, and there was no statistically significant difference between groups in the other tissues.
PO-leaf infusion effects on the antioxidant enzymes
Results for the antioxidant enzymes are shown in Table 4. GST activity in liver tissue showed a significant increase in the Ethanol group compared to the Control group. Though the PO-leaf infusion elevated the SOD, GPx, and CAT activities, Ethanol significantly reduced levels in the kidney tissue in general.
Moreover, the GPx activity in the kidney tissues of the Ethanol + PO-60 mg/mL group was significantly increased compared to the Ethanol + Silymarin and Ethanol + PO-60 mg/mL groups. The GST activity in the erythrocytes showed a significant increase in the Ethanol and all of the other treated groups compared to the Control group (Table 4).
Discussion
Platanus orientalis leaves are widely used in folk medicine as a wound-healer and ophthalmologic agent, tooth and knee pain killer, analgesic, and anti-inflammatory [3, 36], and so on; however, it is possible that positive or negative effects on some organs or tissues are not known. In this study, we aimed to determine possible protective or toxic effects of PO-leaf infusion on the blood, liver, and kidney tissues of rats with ethanol-induced OS. Our other aim was to determine the active substances present in PO-leaf infusion. Moreover, silymarin is widely accepted drug used to against liver damage; therefore, silymarin was used to set a positive control group.
Ethanol-induced OS is used in experimental animal models in pre-clinical studies to determine potential protective or toxic effects of herbal products with drug potential [37, 38]. The liver is the most important organ responsible for alcohol metabolism in the body. Alcohol dehydrogenase and Cytochrome P450 Family 2 Subfamily E Member 1 (CYP2E1) are major enzymes that catalyze alcohol into acetaldehyde, and then aldehyde dehydrogenase catalyzes acetaldehyde to acetic acid [39]. Ethanol consumption causes OS by decreasing ATP and NAD+/NADP levels in the cell and increasing cytokines, tumor necrosis factor alpha (TNFa), and Kupffer cell activation. Moreover, the ethanol treatments increase the production of ROS, lower cellular antioxidant levels, and enhance OS in many tissues, such as the liver, kidney, blood, and brain [16]. ROS production increased biochemical damage markers like AST, ALT, LDH, GGT, CREA, UA, and urea, and MDA levels are increased, while antioxidant enzyme levels are decreased. A variety of enzymatic antioxidants aid in the elimination of ROS, including SOD, CAT, GPx, GR, and CAT, and non-enzymatic antioxidants, such as GSH, α-tocopherol, ascorbate, vitamin A, ubiquinone, uric acid, and bilirubin [40].
According to the results of the HPLC and GC-MS, kaempferol and its derivatives, stearic acid, palmitic acid, octadecanoic acid, linolenic acid, linoleic acid, 2,4-Ditert-Butylphenol, and benzaldehyde compounds were determined in the PO-leaf raw materials. In the leaf, kaempferol and its derivatives were the major hydrophilic compounds, while stearic acid, 2,4-Ditert-butylphenol, and palmitic acid were the major lipophilic compounds detected. In previous studies, it was determined that P. orientalis plants had kaempferol and its derivatives, quercetin, nicotiflorin, and routine and coumaric acid contents [1, 7]. In another study, it was reported that octadecanoic acid, 2-myristynoyl pantetheine, strychane, dodecane, tetradecane, n-hexadecanoic acid, ursolic aldehyde, β-sitosterol, betulin, and many other phytochemicals were present in P. orientalis leaves [15]. In the same study, it was reported that P. orientalis has anti-oxidant, anti-microbial, anti-septic, analgesic, anti-nociceptive, anti-hepatotoxic, anti-inflammatory, and anti-cancer activities. This effect of the plant is probably the result of the active substance content. Hence, as shown in many studies, the protective effects of the plants are bound to the active substance content [21, 41, 42].
The present study showed that ingesting the PO-leaf infusion resulted in no toxicity to the liver, kidney, or blood, and no adverse effects on the growth parameters in rats over the 28 days were observed. According to our results, although the weekly fluid consumption did not change in the PO-leaf infusion treatment groups, the LBWs decreased significantly in the last weeks compared to the first weeks. Although the mechanism under the body weight reduction is unclear, as shown in our results, the PO-leaf content may be the result of the effects of kaempferol and derivatives. In some studies that support this idea, kaempferol and its derivatives have been reported to reduce the LBW and plasma lipid levels [43, 44]. According to the LBW findings, it was concluded that the PO-leaf infusion could be attempted as a diet strategy for obesity and weight balance due to the weight gain inhibiting agent contents.
The AST, ALT, LDH, and GGT activities in the serum are commonly used as biochemical markers/indicators for hepatic damage. Moreover, leakage of these indicators from hepatic cells into the blood due to damage to the liver results in increased enzyme activities. For similar reasons, CREA, UA, urea, TB, TRIG, LDL-C, HDL-C, and CHOL were also used as indicators to assess the healthy condition of the liver or kidney [21, 45]. Our findings showed that serum damage indicator parameter levels were increased in Ethanol-treated rats, which is suppressed by co-treatment with two doses of PO-leaf infusion, especially at high doses. Reference literature cannot be provided because no studies exist in the literature on P. orientalis trees being associated with this subject. However, some plants, similar to the active ingredient content of the P. orientalis tree, have been reported to significantly reduce the biomarker parameter levels of ethanol-induced liver and kidney damage [46,47,48]. The antioxidant protective properties of P. orientalis leaves might be responsible for these positive effects of the PO-leaf infusion on the liver, kidney, and blood.
Alcohol-induced OS is linked to the metabolism of ethanol, and ethanol consumption leads to increased lipid peroxidation in the cell membrane and a decrease in antioxidant enzyme activity in the cells [29, 49]. According to the obtained results, ethanol treatment drastically altered the MDA content of the model animals, while these alterations were significantly ameliorated by the PO-leaf infusion in the experimental group. In addition, the erythrocyte tissue MDA content showed a significant reduction with PO-leaf 60 mg/mL compared to all of the other groups. The reasons for such an effect of ethanol and the PO-leaf infusion treatment are not understood at present. However, the increased MDA content might have resulted from an increase of ROS as a result of stress conditions in the rats by ethanol intoxication. In many studies, it was concluded that increased ROS production and the MDA content of cell or tissue damage were observed and this damage was suppressed in the treatment groups [46, 50, 51]. It can be concluded that PO-leaf infusion prevented lipid peroxidation by clearing the ROS of kaempferol derivatives and essential fatty acids.
Live cells have important in vivo antioxidant enzyme activities, such as SOD, GPx, GST, GR, and CAT and/or non-enzymatic antioxidant compounds, such as GSH, vitamins, and so forth. These protective structure mechanisms have evolved to protect cells against ROS, including CAT and GPx, which remove H2O2; SOD, which removes O2−; GST, which can remove xenobiotics by forming thiol groups; GR, which converts oxidized glutathione (GSSG) into reduced GSH (GSH is the cofactor of many antioxidant enzymes that is found in almost all cells) [16, 29]. Therefore, the healthy functioning of the antioxidant defence system is very important to protect the organism against exogenous and endogenous pests. Studies have reported significant reductions in antioxidant enzyme activities in rats exposed to ethanol [49, 52, 53]. On the other hand, there are many studies that have shown that administration of antioxidants or antioxidant-rich extracts can prevent or ameliorate the toxic actions of ethanol [46, 54, 55]. Our findings were in parallel with previous studies. The positive effects of the PO-leaf infusion used on the antioxidant enzymes of the kidney tissue and GST in the other tissues were particularly prominent. The use of a PO-leaf infusion may contribute significantly to the prevention of kidney disease and kidney-related diseases due to its positive effects on antioxidant enzymes in kidney tissue. Moreover, CREA, which is an indicator of renal failure, was found to be close to the Control group. The increased activity of GST in the liver and erythrocytes indicated that it presents as a protective response to the elimination of xenobiotics [56]. The reasons for such an effect from the PO-leaf infusion may be due to the presence of various compounds, such as kaempferol and its derivatives, essential fatty acids, and benzaldehyde. In various studies, these compounds have been reported to increase antioxidant enzyme activities against OS and reduce lipid peroxidation [57,58,59,60]. In brief, our results indicated that the use of a PO-leaf infusion at determined doses possessed potential antioxidant, hepatoprotective, and nephroprotective properties, and has therapeutic potential for the treatment of liver and kidney diseases.
Conclusion
When all of the data were evaluated, it was determined that P. orientalis leaves possess antioxidant-rich kaempferol and its derivatives, benzaldehyde, palmitic acid, 2,4-ditert-butylphenol, stearic acid, octadecanoic acid, linoleic acid, and linolenic acid. It was revealed for the first time herein that ingesting a PO-leaf infusion might have positive effects on weight loss, hepatoprotective, nephroprotective, and various blood parameters against ethanol-induced OS in rats. As a further study, PO-leaf infusion should be tested for immunotoxicity and norotoxicity in rodent models.
Abbreviations
- ADS:
-
Antioxidant defence systems
- ALP:
-
Alkalane phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CAT:
-
Catalase
- CHOL:
-
Cholesterol
- CREA:
-
Creatinine
- GC-MS:
-
Gas chromatography mass spectrometry
- GGT:
-
Gama glutamyl transferase
- Glu:
-
Glucose
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathine
- GST:
-
Glutathione S-transferase
- HDL-C:
-
High density lipoprotein-cholesterol
- HPLC:
-
High performance liquid chromatography
- LDH:
-
Lactate dehydrogenase
- LDL-C:
-
Low density lipoprotein-cholesterol
- MDA:
-
Malondialdehyde
- OS:
-
Oxidative stress
- PO-leaf infusion:
-
Platanus orientalis leaf infusion
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TB:
-
Total bilirubin
- TRIG:
-
Total triglyceride
- UA:
-
Uric acid
References
Khan AS (2017) Woody Plants with Possible Anti-HIV Activity. In: Khan AS (ed) Medicinally important trees. Springer International Publishing, Switzerland, pp 109–131
Carpenter RJ, Hill RS, Jordan GJ (2005) Leaf cuticular morphology links Platanaceae and Proteaceae. Int J Plant Sci 166(5):843–855
Haider S, Nazreen S, Alam MM et al (2012) Anti-inflammatory and anti-nociceptive activities of Platanus orientalis Linn. and its ulcerogenic risk evaluation. J Ethnopharmacol 143(1):236–240
Khosropour E, Attarod P, Shirvany A et al (2017) Response of Platanus orientalis leaves to urban pollution by heavy metals. J For Res 1–9
Janković B, Dodevski V, Stojmenović M et al. (2018) Characterization analysis of raw and pyrolyzed plane tree seed (Platanus orientalis L.) samples for its application in carbon capture and storage (CCS) technology. J Therm Anal Calorim 133(1):465–480
Mitrokotsa D, Mitaku S, Demetzos C et al. (1993) Bioactive compounds from the buds of Platanus orientalis and isolation of a new kaempferol glycoside. Planta Med 59(06):517–520
Dimas K, Demetzos C, Mitaku S et al. (2000) Cytotoxic activity of kaempferol glycosides against human leukaemic cell lines in vitro. Pharmacol Res 41(1):83–86
El-Alfy TS, El-Gohary HMA, Sokkar NM et al. (2008) Two novel acylated flavonol glycosides from Platanus orientalis L. leaves. Nat Prod Commun 3:1899–1902
Tantry MA, Akbar S, Dar JA et al. (2012) Acylated flavonol glycoside from Platanus orientalis. Fitoterapia 83(2):281–285
Nishanbaev SZ, Kuliev ZA, Khidyrova NK et al. (2010) New oligomeric proanthocyanidin glycosides Platanoside A and Platanoside B from Platanus orientalis trunk bark. Chem Nat Compd 46:357–362
Khidyrova NK, Rashkes YV, Rashkes AM et al. (1995) Shed plane leaves as a source of α-tocopherol. Chem Nat Compd 31:312–314
Abdullaev UA, Rashkes YV, Khidyrova NK et al. (1994) Mass-spectrometric analysis of phytol derivatives from the leaves of Platanus orientalis. Chem Nat Compd 30(3):332–338
Bastos DZ, Pimentel IC, de Jesus DA et al. (2007) Biotransformation of betulinic and betulonic acids by fungi. Phytochemistry 68(6):834–839
Asadbeigi M, Mohammadi T, Rafieian-Kopaei M et al. (2014) Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: an ethnobotanical study in the Urmia. Asian Pac J Trop Med 7:364–368
Shende S, Joshi KA, Kulkarni AS et al (2018) Platanus orientalis Leaf Mediated Rapid Synthesis of Catalytic Gold and Silver Nanoparticles. J Nanomed Nanotechnol 9(494):2
Cederbaum AI, Lu Y, Wu D (2009) Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83(6):519–548
Gülçin I (2012) Antioxidant activity of food constituents: an overview. Arch toxicol 86(3):345–391
Dogan A, Dalar A, Sadullahoglu C et al (2018) Investigation of the protective effects of horse mushroom (Agaricus arvensis Schaeff.) against carbon tetrachloride-induced oxidative stress in rats. Mol Biol Rep 45:787–797
Nwozo SO, Ajagbe AA, Oyinloye BE (2012) Hepatoprotective effect of Piper guineense aqueous extract against ethanol-induced toxicity in male rats. J Exp Integr Med 2(1):71–76
Ntchapda F, Abakar D, Kom B et al (2014) Acute and sub-chronic oral toxicity assessment of the aqueous extract leaves of Ficus glumosa Del. (Moraceae) in rodents. J Intercult Ethnopharmacol 3(4):206–213
Dogan A, Celik I, Kaya MS (2015) Antidiabetic properties of lyophilized extract of acorn (Quercus brantii Lindl.) on experimentally STZ-induced diabetic rats. J ethnopharmacol 176:243–251
Nath P, Yadav AK (2015) Acute and sub-acute oral toxicity assessment of the methanolic extract from leaves of Hibiscus rosa-sinensis L. in mice. J Intercult 4(1):70–73
Wu T, Tang Q, Yu Z et al (2014) Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int J Food Sci Nutr 65(3):351–359
Wu T, Qi X, Liu Y et al (2013) Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem 141(1):482–487
Abenavoli L, Capasso R, Milic N et al (2010) Milk thistle in liver diseases: past, present, future. Phytother Res 24:1423–1432
Ding TM, Tian SJ, Zhang ZX et al (2001) Determination of active component in silymarin by RP-LC and LC/MS. J Pharmacol Biomed Anal 26(1):155–161
Kocaman N, DÖ D (2015) Hepatoprotektif bir ajan: silymarin. Firat Med J 20(3):128–132
Yayalacı Y, Celik I, Batı B (2014) Hepatoprotective and antioxidant activity of linden (Tilia platyphyllos L.) infusion against ethanol-induced oxidative stress in rats. J Membr Boil 247(2):181–188
Dogan A, Celik I (2012) Hepatoprotective and antioxidant activities of grapeseeds against ethanol-induced oxidative stress in rats. Br J Nutr 107(1):45–51
Mannervik B, Guthenberg C (1981) Glutathione S-transferase (human placenta). Methods Enzymol 77:231–235
Paglia DE, Valentine WN (1967) Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
McCord JM, Fridovich I (1969) Superoxide dismutase, an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6053
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York-London, pp 673–684
Jain SK, McVie R, Duett J et al (1989) Erythrocyte membrane lipid peroxidation and glycolylated hemoglobin in diabetes. Diabetes 38:1539–1543
Beutler E, Dubon OB, Kelly M (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Ebn-e-Sina A (1988) Ghanoon Dar Teb, vol 2. Soroosh Press, Tehran, pp 119–120
Azevedo LF, da Silva SM, Navarro LB et al (2016) Evidence of anti-inflammatory and antinociceptive activities of Plinia edulis leaf infusion. J ethnopharmacol 192:178–182
Oyeleke SA, Ajayi AM, Umukoro S et al (2018) Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J ethnopharmacol 222:239–248
Pari L, Suresh A (2008) Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem Toxicol 46(5):1627–1634
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, USA
Dalar A, Dogan A, Bengu AS et al (2018) Screening in vivo antioxidant and haematological properties of sumac and acorn bioactive rich extracts. Ind Crops Prod 124:20–27
Zakaria NNA, Okello EJ, Howes MJ et al (2018) In vitro protective effects of an aqueous extract of Clitoria ternatea L. flower against hydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother Res 32(6):1064–1072
Chang CJ, Tzeng TF, Liou SS et al (2011) Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels. Planta med 77(17):1876–1882
Zang Y, Zhang L, Igarashi K et al (2015) The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Func 6(3):834–841
Abliz A, Aji Q, Abdusalam E et al (2014) Effect of Cydonia oblonga Mill. leaf extract on serum lipids and liver function in a rat model of hyperlipidaemia. J Ethnopharmacol 151(2):970–974
Abarikwu SO, Njoku RC, Lawrence CJ et al (2017) Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharm Biol 55(1):2161–2169
Choi SH, Lee AY, Park CH et al (2018) Protective effect of Carthamus tinctorius L. seed on oxidative stress and cognitive impairment induced by chronic alcohol consumption in mice. Food Sci Biotechnol 27(5):1475–1484
Zhang H, Ma Z, Luo X et al (2018) Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: a mini-review. Antioxidants 7(5):69
Bati B, Celik I, Dogan A (2015) Determination of hepatoprotective and antioxidant role of walnuts against ethanol-induced oxidative stress in rats. Cell Biochem Biophys 71(2):1191–1198
Turan A, Celik I (2016) Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. Int J Biol Macromol 91:554–559
Das M, Basu S, Banerjee B et al (2018) Hepatoprotective effects of green Capsicum annum against ethanol induced oxidative stress, inflammation and apoptosis in rats. J Ethnopharmacol 227:69–81
Cao YW, Jiang Y, Zhang DY et al (2015) Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J Ethnopharmacol 161:92–98
Elkomy NM, Ibrahim IAH, Elshazly SM et al (2018) Ameliorative effects of clonidine on ethanol induced kidney injury in rats: Potential role for imidazoline-1 receptor. Eur J Pharmacol 824:148–156
Chen X, Ying X, Sun W et al (2018) The therapeutic effect of fraxetin on ethanol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and modulating inflammatory mediators in rats. Int Immunopharmacol 56:98–104
Hamid A, Ibrahim FW, Ming TH et al (2018) Zingiber zerumbet L.(Smith) extract alleviates the ethanol-induced brain damage via its antioxidant activity. BMC Complement Altern Med 18(1):101
Panigrahi GK, Yadav A, Yadav A et al (2014) Hepatic transcriptional analysis in rats treated with Cassia occidentalis seed: Involvement of oxidative stress and impairment in xenobiotic metabolism as a putative mechanism of toxicity. Toxicol Lett 229(1):273–283
Pan PH, Lin SY, Ou YC et al (2010) Stearic acid attenuates cholestasis-induced liver injury. Biochem Biophys Res Commun 391(3):1537–1542
Vieira AED, Araujo GL, Galassi CM et al (2013) Toxicological, toxicokinetic and gastroprotective evaluation of the benzaldehyde semicarbazone. Food Chem Toxicol 55:434–443
Langeswaran K, Selvaraj J, Ponnulakshmi R et al (2018) Protective Effect of Kaempferol on Biochemical and Histopathological Changes in Mercuric Chloride Induced Nephrotoxicity in Experimental Rats. J Biol Act Prod Nat 8(2):125–136
Mota NS, Kviecinski MR, Zeferino RC et al (2018) In vivo antitumor activity of by-products of Passiflora edulis f. flavicarpa Deg. Rich in medium and long chain fatty acids evaluated through oxidative stress markers, cell cycle arrest and apoptosis induction. Food ChemToxicol 118:557–565
Acknowledgements
This work was financially supported by the Van Yuzuncu Yil University Scientific Research Project Commission (Grant No: TYL-2018-6774). The authors are grateful to Dr. Abdullah Dalar for his invaluable contributions to the determination of the active substance, and to Van Yuzuncu Yil University for providing financial assistance for this study. A. Dogan was the main moderator of the study. O.O. Anuk performed the biochemical investigation and treatment in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of the authors, the corresponding author states that there are no conflicts of interest.
Research involving human and animal participants
Rodents were used in this study. Male Wistar albino rats of approximately 2 months of age and an average weight of 200 g were provided by the Experimental Animal Research Center, Van Yuzuncu Yil University (Van, Turkey). They were divided into five groups, with each group containing six rats. The animals were housed at 25 ± 2 °C at a light/dark photoperiod of 10:14. All of animals were given a wheat-soybean-based diet and water ad libitum in stainless steel cages, and received humane care according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science and published by the National Institute of Health. The ethic regulations followed were in accordance with national and institutional guidelines for the protection of animal welfare during experiments. This study was approved by the Ethic Committee of Van Yuzuncu Yil University (Protocol number: 27552122-604.01.02-E.70881). No human participants were involved in the present research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogan, A., Anuk, O.O. Investigation of the phytochemical composition and antioxidant properties of chinar (Platanus orientalis L.) leaf infusion against ethanol-induced oxidative stress in rats. Mol Biol Rep 46, 3049–3061 (2019). https://doi.org/10.1007/s11033-019-04741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04741-7