Abstract
Ethanol consumption can generate free radicals and cause oxidative stress, tissue inflammation, and kidney impairment. It has long been known that saponins from natural sources have numerous therapeutic benefits in African herbal and traditional medicine. However, the study aimed to the study aimed to evaluate the ameliorative effect of saponin-rich extract of Citrullus lanatus seed against ethanol-induced kidney injury. Rats were divided into six groups: a normal control, an ethanol control (10 ml/kg of 50% ethanol), a saponin control (100 mg/kg/day), an ethanol plus low and high doses of saponin (ET + 50 and 100 mg/kg SCL), and an ethanol plus Silymarin (ET + 100 mg/kg SLY) group. Serum urea, creatinine, and uric acid were measured, as were oxidative stress markers and renal tissue inflammatory markers. The ethanol-induced increases in creatinine, urea, and uric acid were significantly (p < 0.05) mitigated by Citrullus lanatus seed saponins (SCL). When rats administered SCL were compared to untreated ethanol rats, CAT, SOD, and GPx levels increased significantly (p < 0.05), whereas MDA decreased. Likewise, SCL dramatically lowered IL-1β and TNF-α levels in ethanol-treated rats. A histopathological study strongly supported the result of the biochemical assay. The results of this study reveal that by reducing the rise in renal function, inflammatory markers, and oxidative stress, the saponin-rich fraction of Citrullus lanatus seed has therapeutic potential against ethanol-induced kidney damage. SCL may therefore act as a plant-based natural remedy to reduce kidney damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Excessive alcohol use is becoming a global issue, and researchers have discovered a link between chronic kidney disease (CKD) and alcohol intake (Fan et al. 2019). According to the World Health Organization, 140 million people worldwide suffer from alcoholism and more than 55% of adults consume alcohol (Kępka et al. 2016). The kidneys have an equal role in the metabolism and excretion of ethyl alcohol, even though the liver is where the majority of alcohol is first broken down. Alcohol dehydrogenase, CYP2E1, and CYP24A1 are a few of the enzymes found in the kidneys that are necessary for the metabolism of ethanol (Bulle et al. 2016; Meza et al. 2022). As a result, drinking too much alcohol puts a lot of stress on the kidneys' regular metabolic functions (Hosseini et al. 2017). Reactive oxygen species and reactive nitrogen species (ROS/RNS) can be produced by alcohol, which may lead to oxidative stress in the kidneys and a risk of renal damage from hemodynamic disturbances and inflammation (Leal et al. 2017; Elkomy et al. 2018; Gao et al. 2022). However, according to certain research, ethanol can harm the kidneys without also harming the liver (Cohen et al. 2011; Costa-Valle et al. 2018). In the absence of substantial liver impairment, Latchoumycandane et al. (2014) found that the effects of excessive ethanol metabolism alone were sufficient to considerably compromise kidney function. Chronic alcohol use, on the other hand, can stimulate the sympathetic nervous system and activate the renin-angiotensin system (RAS), raising blood pressure and impairing glomeruli morphology (Leal et al. 2017; Varga et al. 2017).
Saponins, a structurally varied class of glycosides with a steroid, steroidal alkaloid, or triterpenoid aglycone backbone that are mostly found in the plant kingdom but also exist in some marine invertebrates, are one form of defence chemical (Osbourn et al. 2011; Claereboudt et al. 2019). Furthermore, the wide range of biological activity reflects their structural and functional variety, making them appealing compounds for the cosmetics and pharmaceutical industries (Osbourn et al. 2011; Gholami et al. 2014). However, not all saponins have the same hemolytic action. Previous studies found significant differences in hemolytic activity among saponins depending on the polarity and chemistry of their aglycones and sapogenins (Top et al. 2017; Vo et al 2017). For example, the triterpenoid saponin extracted from the bark of Quillaja saponaria Molina (Quillajaceae) was shown to be non-haemolytic and was licenced as an immunostimulant adjuvant in commercial vaccines (De Groot and Muller-Goymann 2016). Saponins derived from various extracts have been shown to have hepatoprotective properties. However, there is no data to support Citrullus lanatus (Watermelon) saponin's therapeutic potential against experimental renal damage produced by ethanol. Hence, the purpose of this study was to determine the therapeutic effects of Citrullus lanatus total saponin extract against ethanol induced kidney damage in male Wistar rats.
Methods
Chemicals and reagents
Silymarin (70 mg/tablet) was purchased from a reputable pharmaceutical store in Zaria, Kaduna State (M.U.B. Pharmaceutical Enterprises Ltd.). The following materials were obtained from Sigma Aldrich and purchased at the Steve Moore Laboratory Chemical Store at Emanto Zaria: ethanol at 100%, sodium hydroxide (NaOH), isobutyl alcohol, magnesium carbonate (MgCO3), iron (III) chloride (FeCl3), and hematoxylin and eosin (H&E) staining solution (St. Louis, MO, USA). Ketamine hydrochloride (Sigma-Aldrich Co., LLC, St. Louis, USA). The following rat ELISA kits were used: superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), reduced glutathione (GSH), nitrite oxide, TNF-α, and IL-1β (Shanghai Enzyme-Linked Biotechnology Ltd Company, Shanghai, China). All of the compounds used were of analytical grade.
Plant material and preparation of Saponin extract
The Citrullus lanatus fruits were obtained from a fruit vendor at a neighbourhood market in Samaru, Zaria, Kaduna State, Nigeria. The taxonomic identification and authentication were carried out by a taxonomist in the Department of Botany, Ahmadu Bello University (ABU), Zaria. The department's herbarium has the plant's voucher specimen, 34122. The seeds were extracted manually from the fruits, washed, and air-dried at 40 °C before being ground into powder in an electric blender. Methanol was used to extract and filter the dried powdered seed (3.5 kg). The extract (430 g) was dissolved in water and subsequently portioned with petroleum ether, chloroform, ethyl acetate, and n-butanol. Methanol was used to separate the bioactive petroleum ether fraction. The methanolic fraction was then re-extracted with diethyl ether until all chlorophyll pigments were removed. The aqueous phase was then partitioned using n-butanol. The n-butanol fraction was concentrated to dryness, yielding a saponin mixture of 4.60 g (Kothavade et al. 2015).
Animal handling
Thirty male Wistar rats weighing 170–190 g were obtained from the Department of Pharmacology at Ahmadu Bello University (ABU), Zaria, Nigeria. The animals were housed in polypropylene cages under conventional laboratory conditions of relative humidity (45 ± 5%), temperature (25 ± 2 °C), and a 12-h light/dark cycle, and were fed with a pelletized form of rat chow and allowed free access to tap water ad libitum. The principles of laboratory animal care were followed (NIH publication no. 85–23, amended in 1985). The Animal Use and Care Committee (ABUCAUC/2022/088) authorised the study. Before the experiment began, the rats were given two (2) weeks to acclimatise to the animal house setting.
Experimental design
Thirty (30) rats were used in this study and were separated into six groups of five rats each (n = 5):
-
Group 1 (NC) received distilled water, (2 ml/kg) daily for 4 weeks.
-
Group 2 (ET) received 10 ml/kg of 50% ethanol daily for 4 weeks;
-
Group 3 (100 mg/kg SCL) group receive 2 ml/kg of distilled water for 3 weeks daily and 100 mg/kg of SCL for one week.
-
Group 4 (ET + 50 mg/kg SCL) group receive 10 ml/kg of 50% ethanol daily for 3 weeks daily and 50 mg/kg of SCL for one week.
-
Group 5 (ET + 100 mg/kg SCL) group receive 10 ml/kg of 50% ethanol daily for three weeks daily and 100 mg/kg of SCL for one week.
-
Group 6 (ET + 100 mg/kg SLY) group receive 10 ml/kg of 50% ethanol daily for three weeks followed by 100 mg/kg of silymarin for 1 week.
All treatments (SCL extract and ET) were given orally, and the experiment lasted 28 days. The SCL dose used in this study was based on previous research (Ejelonu et al. 2021). ET (50%) at a dosage of 10 ml/kg/day was used; this is almost comparable to the amount previously associated with organ damage (Jiang et al. 2016).
Determination of body weight gain and kidney somatic index
The weights of control and treated rats were recorded using a 9001 scale (Satorius, Hertfordshire, U.) at the end of each week. The difference between the final body weight on the day of sacrifice and the initial body weight prior to the start of administration was utilised for determining each rat's body weight gain. The kidneys were promptly removed when the rats were humanely sacrificed, cleaned with ice-cold normal saline, blotted with filter paper, and weighed to calculate the renal somatic index (RSI). RSI = (the weight of the kidney (g)) ÷ (the final weight of the body (g)) × 100 (Perera, et al. 2020).
Preparation of serum biochemistry
After the four-week treatment period, the rats were given an intramuscular injection of ketamine hydrochloride USP at a dose of 50 mg/kg to anesthetised them. The rats were euthanized, and blood samples were collected by puncturing the heart with a 22-gauge needle and a 5-ml syringe. Blood samples obtained in plain blood tubes were allowed to clot at room temperature. The clotted blood samples were centrifuged at 1500 g for 15 min, and serum samples were aspirated, placed in cryovials, and kept at -20 degrees Celsius until biochemical assay. The kidney function parameters of serum creatinine, urea, and uric acid were measured using an automated chemistry analyzer (Labmax Plenno Co., Ltd., Lagoa Santa, Brazil) with commercial assay kits.
Oxidative stress and antioxidant defence system assay
The kidney organs were excised, weighed, and homogenised in a 50 mmol/l Tris–HCl buffer (pH 7.4) before being centrifuged at 1500 g for 15 min for biochemical analysis. Supernatants obtained were immediately placed in the freezer at -20 °C, frozen, and stored until needed. The homogenates were used to quantify oxidative stress and inflammatory markers. The concentration of lipid peroxidation in the kidney was determined by measuring malondialdehyde (MDA) production using the Zhang et al. (2008) technique. Nitric oxide (NO) was measured using a reagent kit acquired from Biodiagnostics (Egypt) and determined following the technique of Montgomery and Dymock (1961). The antioxidant enzymes superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT) were evaluated using the techniques of Marklund and Marklund (1974), Kar and Mishra (1976), and Cohen et al. (1970), respectively.
Cytokines in kidney homogenate assay
The levels of tumour necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) in kidney homogenate were determined using specialised ELISA kits (ACON Labs, USA) and the manufacturer's instructions. The cytokine concentrations were quantified spectrophotometrically at 450 nm. Standard curves were created using standard cytokines, and standard plots were used to calculate the concentrations of the unknown samples.
Histopathological examination
Specimens from the kidney were fixed in 10% phosphate buffered formalin, dehydrated in alcohol, and embedded in paraffin for light microscopy. For general histological evaluation, five-micron tissue slices were stained with hematoxylin and eosin (H&E). A light microscope was used to examine the slides (Olympus CHNB107MVR, Tokyo, Japan).
Statistical analysis
The data were analysed statistically, with the mean and standard error of the mean (SEM) being calculated. A one-way ANOVA was used to analyse the data, and Tukey's multiple comparison test was used for the post hoc test. These statistical analyses were performed using GraphPad Prism Software, version 5 (La Jolla, CA). At p < 0.05, the difference was considered significant.
Result
Body weight gain and kidney somatic index
Table 1 shows the differences in kidney somatic index and body weight gain of rats in different groups. When compared to the control group, the ET-treated group had a significant increase (p < 0.001) in kidney somatic index and a decrease in rats' body weight gain. In contrast, therapeutic treatment with saponin-rich fractions of CL at both higher and lower dosages, as well as Sylimarin, significantly decreased (p < 0.001) the kidney somatic index as well as increased body weight gain in the rats compared to ET-treated rats. However, there was no difference in response between the two dosages of CL (50 mg/kg and 100 mg/kg body weight), and likewise, no significant difference was observed in kidney somatic index between the ET-treated rats and the Sylimarin-treated rats. Furthermore, when comparing the rats treated with just CL to the control group, no statistically significant difference (p > 0.05) in values was observed.
Changes in kidney function parameters and nitrite oxide
When compared to the control group, rats given just ethanol (ET) treatment had significantly higher blood levels of urea, creatinine, and uric acid (p < 0.001). Rats in the co-treatment groups (50 ET+SCL, 100 ET+SCL, and ET+SLY) showed urea, creatinine, and uric acid levels that were significantly (p < 0.05) reduced compared to ET-treated rats. Comparing the rats in the SCL-only treatment group to the control group's rats, all kidney function parameters did not indicate any significant differences (p > 0.05). Apart from creatinine, all of the kidney function markers evaluated demonstrated a dose-dependent response. When compared to rats in the low-dose saponin-rich fraction group and the silymarin-treated group, the high dosage of SCL significantly (p < 0.05) lowered urea, creatinine, and uric acid levels (Fig. 1A-C). The ET treated rat's mean nitrite oxide level in the homogenate increased significantly when compared to rats in the normal control group (p < 0.001). In comparison to the normal control group, it did not differ substantially in only the SCL-treated rats (p > 0.05). The mean nitrite oxide level in the homogenate decreased significantly in all ET+SCL and ET+SLY groups (P < 0.001) when compared to the ethanol control group (Fig. 1D).
Bar charts of kidney function parameters A Creatinine, B Urea, C Uric acid, and D Nitrite oxide. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a statistically significant difference as compared to the Normal control; #P < 0.033; ## P < 0.002; ### P < 0.0001, indicates a statistically significant difference as compared to the ET Control group. n = 5, NC = Control, ET = Ethanol, SCL = Saponin Rich Fraction of Citrullus Lanatus seed, SLY = Silymarin
Changes in antioxidant enzyme status in kidney tissue
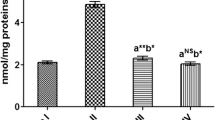
SOD and CAT enzyme activity, as well as GSH levels, were found to be significantly reduced in the ET group compared to the NC group of rats. Also, SOD, CAT, and GSH levels were significantly increased (p < 0.05) in rats treated with Citrullus lanatus and Sylimarin (ET + 50 mg/kg SCL, ET + 100 mg/kg SCL, and ET + 100 mg/kg SLY) compared to rats in the ET group. For SOD and GSH, there was no significant difference (p > 0.05) between the treatment groups; however, there was still a substantial rise in CAT levels in the ET + 100 mg/kg SCL and ET + 100 mg/kg SLY groups compared to the NC and ET + 50 mg/kg SCL groups (Fig. 2A-C). Rats in the ET group had a significant rise in MDA levels when compared to the rats in the NC group. Citrullus lanatus seed saponin and Sylimarin significantly (p < 0.05) lowered the MDA levels compared to MDA level in the ET group, but the decrease in the extract groups was in a dose-dependent manner (Fig. 2D).
Bar charts of Oxidative stress and lipid peroxidation A SOD, B CAT, C GSH, and D MDA. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a statistically significant difference as compared to the Normal control; #P < 0.033; ## P < 0.002; ### P < 0.0001, indicates a statistically significant difference as compared to the ET Control group. n = 5, NC = Control, ET = Ethanol, SCL = Saponin Rich Fraction of Citrullus Lanatus seed, SLY = Silymarin
Changes in cytokine levels
TNF-α levels were found to be significantly higher in the ET group compared to the NC group of rats. TNF-α levels were significantly reduced (p < 0.05) in rats treated with Citrullus lanatus and sylimarin compared to rats in the ET group. There was no statistically significant difference (p > 0.05) between the treatment groups; however, there was still a substantial rise in TNF-α levels in the ET + 50 mg/kg SCL, ET + 100 mg/kg SCL, and ET + 100 mg/kg SLY groups compared to the NC group (Fig. 3A). Rats in the ET group had a significant rise in IL-1β levels when compared to the rats in the NC group. Citrullus lanatus seed saponin and sylimarin significantly (p < 0.05) lowered IL-1β levels compared to rats in the ET group, but the decrease in the extract groups was in a dose-dependent manner. No significant (p > 0.05) difference was found in the IL-1β level between rats in the 100 mg/kg SCL and ET + 100 mg/kg SLY groups. However, there was still a significant rise in IL-1β levels in the ET + 50 mg/kg SCL, ET + 100 mg/kg SCL, and ET + 100 mg/kg SLY groups compared to the NC group (Fig. 3B).
Bar charts of Inflammatory markers A TNF-α and B IL-1β. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a statistically significant difference as compared to the Normal control; #P < 0.033; ## P < 0.002; ### P < 0.0001, indicates a statistically significant difference as compared to the ET Control group. n = 5, NC = Control, ET = Ethanol, SCL = Saponin Rich Fraction of Citrullus Lanatus seed, SLY = Silymarin
Histological studies of kidney
Normal control rats' kidneys have a normal histological structure, including a glomerulus and renal tubules (Fig. 4A). Tubular and glomerular necrosis were clearly seen in the renal tissues of ethanol-treated rats (Fig. 4B). However, the kidneys of rats treated with SCL (50 mg/kg) showed normal glomeruli and some minor tubular damage (Fig. 4D), whereas the kidneys of rats treated with SCL (100 mg/kg) after ethanol administration and only SCL treated both showed normal glomeruli and normal renal tubules, which were comparable to the control group (Fig. 4C, E). Rats treated with ET+SLY had moderately damaged glomeruli and renal tubules (Fig. 4F).
Composite photomicrographs of kidney of control (A) showing a normal glomerulus (G) and renal tubules, ET treated micrograph (B) exhibited dilated and degenerated renal tubules (arrow), and glomerulus degeneration and spontaneous lipid vacuolation (G), SCL (C) showed normal glomerulus (G) and renal tubules; ET + 50 mg/kg SCL (D) showed a mild glomerulus degeneration and spontaneous lipid vacuolation(G) with mild degenerated renal tubule (Arrow), ET + 100 mg/kg SCL (E) showing very normal rental tubules (Arrow) and a normal glomerulus (G), ET + 100 mg/kg SLY treated group (F), showing mild obliterative form of glomerulus (G) and mild degenerated renal tubules(arrow) (H & E, X 250)
Discussion
Ethanol has been studied for several years, and it has been shown that it can induce oxidative stress, tissue inflammation, and acute renal toxicity (Leal et al. 2017; Varga et al. 2017). There is increasing evidence that medicinal plants' phytochemical constituents, like saponin and flavonoid, have pharmacological properties with regard to anti-inflammatory and anti-oxidant activities (Ikram et al. 2021; Zhong et al. 2022). Thus, we evaluate the therapeutic efficacy of Citrullus lanatus seed saponin extract against ethanol nephrotoxicity in Wistar rats, as well as its underlying antioxidant and anti-inflammation potential.
In the current study, ethanol treatment caused body weight loss as well as significant elevations in the renal somatic index, blood urea, uric acid, and creatinine. The elevated levels of serum urea, uric acid, and creatinine levels are indicative of kidney impairment and renal tubular necrosis (Shahani et al. 2017). The findings in this study support prior research that ethanol promotes kidney impairment (Kishi et al. 2015; Wang et al. 2017). The damage causes renal dysfunction due to a decreased glomerular filtration rate, which raises blood urea, uric acid, and creatinine levels. A saponin-rich methanolic extract from Citrullus lanatus seed appears to have beneficial effects on renal dysfunction in ethanol-treated rats by considerably reducing the rise of the renal somatic index, urea, creatinine, and uric acid levels, as well as weight loss. This positive effect of the extract might be related to the antioxidant activity and membrane stabilising properties previously recorded (Ma et al. 2019). The mechanism of ethanol nephrotoxicity has been researched.
It has been reported that ROS is key to ethanol's harmful effects on renal tissue via the build-up of oxidative stress, which leads to the pathogenesis of ethanol nephrotoxicity (Comporti et al. 2010). This study shows that ethanol treatment impairs the kidneys' natural antioxidant defences. In line with previous studies, our findings show that ethanol significantly reduced renal activities of SOD, CAT, and GSH levels while significantly increasing MDA levels in rats in the control group (Pourbakhsh et al. 2014; Harris et al. 2015). However, in these studies, Citrullus lanatus seed extract and Sylimarin were able to restore SOD and CAT, renal activity, as well as GSH and MDA levels in a dose-dependent manner, with the high dose of the extract producing results equivalent to those of normal control rats. The findings of this research are consistent with other studies showing that saponin-rich extract decreases the generation of free radicals, controls the expression of antioxidant enzymes, and removes the detrimental effects of ethanol (Ma et al. 2019; Yao et al. 2019).
Furthermore, mounting evidence implicates pro-inflammatory cytokine production in ethanol-induced nephrotoxicity (Gao et al. 2022). ROS stimulate NF-B, which is essential for the initiation of inflammatory cascades. In the current study, we discovered that ethanol administration correlated with significant rises in levels of the inflammatory markers TNF-α and IL-1β. Pro-inflammatory cytokines have been shown in several studies to play a crucial role in the pathophysiology of ethanol-induced kidney damage (Xu et al. 2018). Additionally, several studies have shown that TNF-α and IL-1β are involved in the pathophysiology of ethanol-induced kidney damage (Gyamfi and Wan 2010; Ceni et al. 2014). TNF-α, in particular, causes renal function failure by causing vasoconstriction, a decrease in blood supply, and leukocyte infiltration. TNF-α reduces blood flow and glomerular filtration rate while increasing albumin permeability in the kidney (Donnahoo et al. 1999; Mahmoudzadeh et al. 2017). According to the present research findings, the oral administration of Citrullus lanatus extract and Silymarin reduced the elevated levels of kidney IL-1b and TNF-α. The anti-inflammatory action of Citrullus lanatus saponins has been widely reported. Previous research reveals that flavonoids and saponins are the principal phytochemical substances found in herbal plants, and they have anti-inflammatory properties (Hong et al. 2018; Bingqian et al. 2021; Wahid et al. 2022).
The considerable reduction in NO levels after ethanol administration as shown in this study indicates that renal endothelial protection and defence systems play a role in the pathological process of ethanol-induced kidney injury (Shi et al. 2022). According to our findings, NO levels returned to normal following the dose-dependent extract and silymarin treatment. This suggests that Citrullus lanatus saponins may protect rats' kidneys from ethanol-induced injury by modifying blood flow and maintaining renal endothelial integrity by managing NO levels.
The kidneys of rats treated with ethanol had degenerated renal tubules and a significantly shrunken glomerulus, which is similar to the findings of Shanmugam et al. (2010), who discovered that ethanol causes renal injury, including damage to glomeruli and tubules. Supplementation with Citrullus lanatus saponin extract, on the other hand, greatly reduced histological lesions and protected the kidney cells. This might be connected to saponin antioxidant effectiveness, which assists in the removal of free radicals, thereby lowering renal cell death (Kooti and Daraei 2017). Furthermore, the potential benefits of saponin extract on the kidney revealed by microscopic examination are consistent with our biochemical findings.
Conclusion
In conclusion, the current findings show that administration of Citrullus lanatus saponin extract appears to ameliorate the effect of ethanol-induced renal injury, as evidenced by a reduction in renal injury, oxidative stress, the generation of pro-inflammatory mediators, pathological changes, and improved performance in antioxidant capacity. However, further research is needed to determine the exact pathways mediating saponin's therapeutic benefits against ethanol toxicity.
Availability of data and material
The raw data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- CAT:
-
Catalase
- CKD:
-
Chronic Kidney Disease
- CL:
-
Cittrulus lanatus
- ET:
-
Ethanol
- FeCl3 :
-
Iron (III) Chloride
- GSH:
-
Reduced Glutathione
- IL-1β:
-
Interleukin 1 Beta
- MDA:
-
Malondialdehyde
- MgCO3 :
-
Magnesium Carbonate
- NaOH:
-
Sodium hydroxide
- NC:
-
Normal Control
- NO:
-
Nitrite Oxide
- RAS:
-
Renin-Angiotensin System
- RNS:
-
Reactive Nitrogen Species
- ROS:
-
Reactive Oxygen Species
- SCL:
-
Saponin-rich Fraction of Citrullus lanatus Seed
- SLY:
-
Silymarin
- SOD:
-
Superoxide Dismutase
- TNF-α:
-
Tumour Necrosis Factor Alpha
References
Bingqian Z, Wen Z, Yujia W, Ying Y, Na W, Junsong L, Tingming F, Lingchong W, Liuqing D (2021) Improved efficacy of Panax notoginseng saponin loaded into BSP/alginate microspheres for the treatment of alcoholic gastric ulcers. Int J Pharm 596:120218
Bulle S, Reddy VD, Hebbani AV, Padmavathi P, Challa C, Puvvada PK, Repalle E, Nayakanti D, Aluganti Narasimhulu C, Nallanchakravarthula V (2016) Nephro-protective action of P. santalinus against alcohol-induced biochemical alterations and oxidative damage in rats. Biomed Pharmacother 84:740–746. https://doi.org/10.1016/j.biopha.2016.09.103 (PMID: 27710898)
Ceni E, Mello T, Galli A (2014) Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol 20(47):17756–17772. https://doi.org/10.3748/wjg.v20.i47.17756 (PMID: 25548474)
Claereboudt EJS, Caulier G, Decroo C, Colson E, Gerbaux P, Claereboudt MR, Schaller H, Flammang P, Deleu M, Eeckhaut I (2019) Triterpenoids in echinoderms: fundamental differences in diversity and biosynthetic pathways. Drugs 17:E352
Cohen JI, Chen X, Nagy LE (2011) Redox signalling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal 15(2):523–534. https://doi.org/10.1089/ars.2010.3746 (PMCID: PMC3118704)
Cohen JM, Langer WD, Rosen LC, Cameron AGW (1970) Neutron star models based on an improved equation of state. Astrophys Space Sci 6:228–239. https://doi.org/10.1007/BF00651224
Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B (2010) Ethanol-induced oxidative stress: basic knowledge. Genes Nutr 5(2):101–109. https://doi.org/10.1007/s12263-009-0159-9
Costa-Valle MT, Tonieto BD, Altknecht L, Cunha CD, Fão N, Cestonaro LV, Göethel G, Garcia SC, Leal MB, Dallegrave E, Arbo MD (2018) Energy drink and alcohol combination leads to kidney and liver alterations in rats. Toxicol Appl Pharmacol 15(355):138–146. https://doi.org/10.1016/j.taap.2018.06.024 (PMID: 29959998)
De Groot C, Muller-Goymann CC (2016) Saponin interactions with model membrane systems - Langmuir monolayer studies, hemolysis and formation of ISCOMs. Planta Med 28:1496–1512
Donnahoo KK, Shames BD, Harken AH, Meldrum DR (1999) Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol 162(1):196–203
Ejelonu OC, Oluba SO, Awolokun BO, Elekofehint OO, Adanlawo IG (2021) Saponin-rich extracts reverse obesity and offer protecton against obesity-induced inflammaton in high-fat diet mice. J Med Plants Econ Dev 5(1):a101. https://doi.org/10.4102/jomped.v5i1.101
Elkomy NMIM, Ibrahim IAAE, Elshazly SM, El-Fayoumi HM (2018) Ameliorative effects of clonidine on ethanol induced kidney injury in rats: potential role for imidazoline-1 receptor. Eur J Pharmacol 5(824):148–156. https://doi.org/10.1016/j.ejphar.2018.02.001 (PMID: 29452086)
Fan Z, Yun J, Yu S, Yang Q, Song L (2019) Alcohol consumption can be a “double-edged sword” for chronic kidney disease patients. Med Sci Monit 25:7059–7072. https://doi.org/10.12659/MSM.916121
Gao Y, Jiang X, Yang D, Wang D, Gong K, Peng Y, Jiang H, Shi C, Duan Y, Chen Y, Han J, Yang X (2022) Roxadustat, a hypoxiainducible factor 1α activator, attenuates both long- and shortterm alcohol-induced alcoholic liver disease. Front Pharmacol 13:895710. https://doi.org/10.3389/fphar.2022.895710
Gholami A, De Geyter N, Pollier J, Goormachtig S, Goossens A (2014) Natural product biosynthesis in Medicago species. Nat Prod Rep 31:356–380
Gyamfi MA, Wan YJ (2010) Pathogenesis of alcoholic liver disease: the role of nuclear receptors. Exp Biol Med (Maywood) 235(5):547–560. https://doi.org/10.1258/ebm.2009.009249 (PMID: 20463294; PMCID: PMC3908670)
Harris PS, Roy SR, Coughlan C, Orlicky DJ, Liang Y, Shearn CT, Roede JR, Fritz KS (2015) Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. https://doi.org/10.1016/j.redox.2015.06.021 (PMCID: PMC4511634)
Hong MY, Beidler J, Hooshmand S, Figueroa A, Kern M (2018) Watermelon and L-argine consumption improve serum lipid profile and reduce inflammation and oxidative stress by altering gene expression in rats fed an atherogenic diet. Nutr Res 58:46–54
Hosseini SM, Taghiabadi E, Abnous K, Hariri AT, Pourbakhsh H, Hosseinzadeh H (2017) Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci 20(8):927–939. https://doi.org/10.22038/IJBMS.2017.9116 (PMID: 29085585)
Ikram M, Jo MH, Choe K, Khan A, Ahmad S, Saeed K, Kim MW, Kim MO (2021) Cycloastragenol, a triterpenoid saponin, regulates oxidative stress, neurotrophic dysfunctions, neuroinflammation and apoptotic cell death in neurodegenerative conditions. Cells 10(10):2719. https://doi.org/10.3390/cells10102719 (PMID: 34685699; PMCID: PMC8534642)
Jiang Z, Wang J, Xue H, Wang M, Jiang H, Liang Y, Dias AC, Gregory M, Chen C, Zhang X (2016) Protective effect of wild Corni fructus methanolic extract against acute alcoholic liver injury in mice. Redox Rep 22(6):338–345. https://doi.org/10.1080/13510002.2016.1239867 (PMID: 27712564)
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kępka A, Chojnowska S, Śnitko R, Zwierz K, Waszkiewicz N (2016) Renal carnitine excretion following abstinence after chronic drinking. Adv Med Sci 61(1):160–163. https://doi.org/10.1016/j.advms.2015.11.002 (PMID: 26774267)
Kishi S, Campanholle G, Gohil VM, Perocchi F, Brooks CR, Morizane R, Sabbisetti V, Ichimura T, Mootha VK, Bonventre JV (2015) Meclizine preconditioning protects the kidney against ischemia-reperfusion injury. EBioMedicine 2(9):1090–1101. https://doi.org/10.1016/j.ebiom.2015.07.035 (PMID: 26501107)
Kooti W, Daraei N (2017) A review of the antioxidant activity of celery (Apium graveolens L). J Evid Based Complementary Altern Med 22(4):1029–1034. https://doi.org/10.1177/2156587217717415 (PMCID: PMC5871295)
Kothavade PS, Bulani VD, Nagmoti DM, Deshpande PS, Gawali NB, Juvekar AR (2015) Therapeutic effect of saponin rich fraction of achyranthes aspera linn. on adjuvant-induced arthritis in sprague-dawley rats. Autoimmune Dis 2015:943645. https://doi.org/10.1155/2015/943645 (PMCID: PMC4529882)
Latchoumycandane C, Nagy LE, McIntyre TM (2014) Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free Radic Biol Med 69:403–416. https://doi.org/10.1016/j.freeradbiomed.2014.01.001 (PMID: 24412858; PMCID: PMC3960325)
Leal S, Ricardo Jorge DO, Joana B, Maria SS, Isabel SS (2017) Heavy alcohol consumption effects on blood pressure and on kidney structure persist after long-term withdrawal. Kidney Blood Press Res 42(4):664–675. https://doi.org/10.1159/000482022 (PMID: 29145198)
Ma Q, Jiang JG, Yuan X, Qiu K, Zhu W (2019) Comparative antitumor and anti-in§ammatory effects of savonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem Toxicol 125:422–429. https://doi.org/10.1016/j.fct.2019.01.020
Mahmoudzadeh L, Najafi H, Ashtiyani SC, Yarijani ZM (2017) Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology (carlton) 22(10):748–754. https://doi.org/10.1111/nep.12849 (PMID: 27381453)
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Meza V, Arnold J, Díaz LA, Ayala Valverde M, Idalsoaga F, Ayares G, Devuni D, Arab JP (2022) Alcohol consumption: medical implications, the liver and beyond. Alcohol Alcohol 57(3):283–291. https://doi.org/10.1093/alcalc/agac013 (PMID: 35333295)
Montgomery HAC, Dymock JE (1961) The determination of nitrite in water. Analyst 86:414–416
Osbourn A, Goss RJ, Field RA (2011) The saponins: polar isoprenoids with important and diverse biological activities. Nat Prod Rep 28:1261–1268
Perera T, Ranasinghe S, Alles N, Wadug R (2020) Experimental rat model for acute tubular injury induced by high water hardness and high-water fluoride: efficacy of primary preventive intervention by distilled water administration. BMC Nephrol 21:103. https://doi.org/10.1186/s12882-020-01763-3
Pourbakhsh H, Taghiabadi E, Abnous K, Hariri AT, Hosseini SM, Hosseinzadeh H (2014) Effect of Nigella sativa fixed oil on ethanol toxicity in rats. Iran J Basic Med Sci 17(12):1020–1031
Shahani S, Behzadfar F, Jahani D, Ghasemi M, Shaki F (2017) Antioxidant and anti-inflammatory effects of Nasturtium officinale involved in attenuation of gentamicin-induced nephrotoxicity. Toxicol Mech Methods 27(2):107–114. https://doi.org/10.1080/15376516.2016.1258748
Shanmugam KR, Ramakrishna CH, Mallikarjuna K, Reddy KS (2010) Protective effect of ginger against alcohol-induced renal damage and antioxidant enzymes in male albino rats. Indian J Exp Biol 48:143–149
Shi Z, Long X, Li Y, Jin J, Li J, Yuan C, Jin R (2022) Protective effect of tea saponins on alcohol-induced gastric mucosal injury in mice. ACS Omega 8(1):673–681. https://doi.org/10.1021/acsomega.2c05880 (Article ASAP)
Top H, Sarikahya NB, Nalbantsoy A, Kirmizigul S (2017) Immunomodulatory, hemolytic properties and cytotoxic activity potent of triterpenoid saponins from Cephalaria balansae. Phytochemistry 137:139–147
Varga ZV, Matyas C, Paloczi J, Pacher P (2017) Alcohol misuse and kidney injury: epidemiological evidence and potential mechanisms. Alcohol Res 38(2):283–288 (PMCID: PMC5513691)
Vo NN, Fukushima EO, Muranaka T (2017) Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J Nat Med 71:50–58
Wahid M, Saqib F, Qamar M, Ziora ZM (2022) Antispasmodic activity of the ethanol extract of Citrullus lanatus seeds: justifying ethnomedicinal use in Pakistan to treat asthma and diarrhea. J Ethnopharmacol 295:115314. https://doi.org/10.1016/j.jep.2022.115314
Wang L, Zhu Y, Wang L, Hou J, Gao Y, Shen L, Zhang J (2017) Effects of chronic alcohol exposure on ischemia-reperfusion-induced acute kidney injury in mice: the role of β-arrestin 2 and glycogen synthase kinase 3. Exp Mol Med 49(6):e347. https://doi.org/10.1038/emm.2017.76 (PMID: 28642577; PMCID)
Xu L, Yu Y, Sang R, Li J, Ge B, Zhang X (2018) Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-κB signaling pathways in mice. Oxid Med Cell Longev 23(2018):8284107. https://doi.org/10.1155/2018/8284107 (PMID: 30344887; PMCID: PMC6174809)
Yao F, Xue Q, Li K, Cao X, Sun L, Liu Y (2019) Phenolic compounds and ginsenosides in ginseng shoots and their antioxidant and anti-in§ammatory capacities in LPS-induced RAW264.7 mouse macrophages. Int J Mol Sci 20(12):2951. https://doi.org/10.3390/ijms20122951
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26(2):232–236
Zhong Y, Luo R, Liu Q, Zhu J, Lei M, Liang X, Wang X, Peng X (2022) Jujuboside a ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem Toxicol 159:112697. https://doi.org/10.1016/j.fct.2021.112697 (PMID: 34826549)
Author information
Authors and Affiliations
Contributions
SAB and WM conceptualised and designed the study; HBR, OBO, HSB, and VKJ carried out the experiments and analysed the data; SAB and HBR visualised and interpreted the histological slides. WM was responsible for drafting the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
The authors declare no specific funding for this work.
Competing interests
The authors declare there are no competing interests.
Ethics approval
The research was approved by ABU Directorate of Academic Planning and Monitoring (Approval No: ABUCAUC/2022/088) and was conducted according to the ARRIVE Guidelines.
Informed consent
For this type of study informed consent is not required.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bazabang, S.A., Makena, W., Rilwan, H.B. et al. Citrullus lanatus methanol seed extract exhibits antioxidant and anti-inflammatory potential against ethanol-induced kidney damage in Wistar rats. Comp Clin Pathol 32, 733–742 (2023). https://doi.org/10.1007/s00580-023-03479-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03479-w