Abstract
Radish (Raphanus sativus L.), an important annual or biennial root vegetable crop, is widely cultivated in the world for its high nutritive value. Isolated microspore culture (IMC) is one of the most effective methods for rapid development of homozygous lines. Due to imperfection of the IMC technology system, it is particularly important to establish an efficient IMC system in radish. In this study, the effects of different factors on radish microspore embryogenesis were investigated with 23 genotypes. Buds with the largest population of late-uninucleate-stage microspores were most suitable for embryogenesis, with a ratio of petal length to anther length (P/A) in buds of about 3/4 ~ 1. Cold pretreatment was found to be genotype specific, and the highest microspore-derived embryoid (MDE) yield occurred for treatment of the heat shock of 48 h. In addition, the supplement of 0.75 g/L activated charcoal (AC) could increase the yield of embryoids. It was found that genotypes, bud size, as well as temperature treatments had significant effects on microspore embryogenesis. Furthermore, somatic embryogenesis–related kinase (SERK) genes were profiled by reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis, which indicated that they are involved in the process of MDE formation and plantlet regeneration. The ploidy of microspore-derived plants was identified by chromosome counting and flow cytometry, and the microspore-derived plants were further proved as homozygous plants through expressed sequence tags-simple sequence repeats (EST-SSR) and genetic-SSR markers. The results would facilitate generating the large-scale double haploid (DH) from various genotypes, and promoting further highly efficient genetic improvement in radish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radish (Raphanus sativus L.), a typical cross-pollinated crop, is one of the most important root vegetable crops around the world, especially in East Asia with abundant nutrition and medicinal value. For vegetable crop genetic improvement, homozygotes with stable characters can be achieved through haploid induction, which significantly reduces the generations of parent selfing and accelerates the development process of elite cultivars. Haploid and double haploid (DH) plant induction technology provides an excellent system for accelerating plant breeding, genetic analysis, physiological investigation, genetic transformation, and QTL mapping (Brew-Appiah Rhoda et al. 2013). It is well known that radish is difficult to obtain homozygous plants for advanced inbred lines due to its self-incompatibility. Conventional breeding methods take a long time, more evidences indicate that modern biotechnology including haploid induction could shorten the gap as soon as possible and promote the genetic gains in crop breeding programs. Throughout the practical technology of vegetable crop breeding in recent years, isolated microspore culture (IMC) technology has the advantage of the achievement of rapid homozygosity, providing a large number of homozygotes for breeding in relative short time (Zhang and Zheng 2013). Nowadays, regeneration of haploid or DH plants through IMC technology has become an advanced and useful method for haploid production in various species (Shariatpanahi and Ahmadi 2016). However, due to the unavailability an efficient protocol for large-scale induction of haploid plants through IMC, microspore culture technology is difficult to be widely applied in breeding programs (Reeta et al. 2018).
Since Lichter (1982) firstly induced embryoid in Brassica napus, microspore culture has been widely employed to develop double haploid plants in many vegetable crops including B. napus L. (Ahmadi et al. 2014a; Solís et al. 2016), B. oleracea var. capitata L. (Reeta et al. 2018), and B. oleracea var. acephala (Chen et al. 2019). Radish is considered to be one of the most recalcitrant species in Brassicaceae for IMC (Takahata et al. 1996). It was reported that 13 out of 19 genotypes produced embryoids with the yield of embryos varied from 0.125 to 10 per bud (Zhou et al. 2007). Similarly, only three regenerated plants were obtained from 22 radish genotypes through IMC (Wang et al. 2013a). In the process of microspore embryoid induction, the cold pretreatment time, washing medium, and medium composition were investigated by Chun et al. (2011). Later, Han et al. (2014) explored the correlation between the length or structure of flower bud and the stage of microspore development in radish; they found that flower bud of different sizes has different embryoid yields. Recently, the effects of nutrient medium composition and heat treatment time on embryogenesis of radish microspore culture were studied, and the doubled haploid of radish was successfully obtained in in vitro microspore culture (Kozar et al. 2020). Moreover, the various stages of embryogenesis of European radish were investigated (Kozar et al. 2021). It was found that efficient induction of microspore-derived embryoid (MDE) depends on various factors such as genotype dependency, low embryoid yield, and germination rate (Testillano 2019). Furthermore, embryogenesis induction is accompanied by many biochemical and morphological changes, which have been proved to be closely related to the alterations in gene expression patterns (Munoz-Amatriain et al. 2009). Therefore, it is particularly important to investigate the factors affecting microspore embryogenesis. Recently, a number of factors were reported to affect critical embryogenesis in microspore culture, which included the growth condition of donor plants, pretreatment of microspores, pollen developmental stage, the growth regulators in induction medium, the concentration of microspores in culture media, and temperature shock (Wang et al. 2013b; Makowska et al. 2015; Bhatia et al. 2016, 2017). Meanwhile, several genes are differentially expressed during microspore culture (Segui-Simarro and Nuez 2008; Hosp et al. 2014). In particular, the somatic embryogenesis receptor–like kinase (SERK) genes were found to be involved in embryogenesis (Podio et al. 2014). Therefore, the SERK gene might play an important role during embryogenesis development.
In this study, to identify the critical factors for IMC in radish, the effects of several factors including genotype of donor plants, pretreatment of the microspores, developmental stages of microspore, and the supplement of activated charcoal (AC) were investigated. Moreover, the expression profiles of RsSERK genes during the MDE development and plantlet regeneration were conducted in radish, as well as the ploidy levels and the homozygosity of microspore-derived plantlets were determined. The aim of this study was to provide valuable information for the establishment of microspore culture system of radish and facilitate generating abundant DH lines for genetic improvement in radish.
Materials and methods
Plant materials
A total of 23 radish genotypes were used for microspore culture (Table 1). All genotypes were cultivated in the greenhouse with a photoperiod cycle of 14 h/24–26 ℃ light and 10 h/14–16 ℃ dark, and the light illumination was set at 240–260 µmol m−2 s−1. Regular watering, fertilization, insect pest, and disease management were conducted.
Isolation of microspore
Microspore isolation followed the procedure of Bhatia et al. (2018) with a slight modification. Floral buds at 1.5 ~ 4.5 mm were collected from young inflorescences and then classified into three types by size. The developmental stages of the microspores were analyzed using 4′, 6-diamidino-2-phenylindole (DAPI) (H-1200) solution and observed using a fluorescence microscope (Olympus) (Winarto et al. 2011). The buds were put into a beaker containing a few drops of water, kept at 4 ℃ for 0 ~ 4 days, and then surface-sterilized with 75% (v/v) ethanol for 30 s and 1% (w/v) sodium hypochlorite for 12 min, and rinsed with sterile distilled water 3 times. Sterilized buds were transferred to B5-13 medium supplied with 13% (w/v) sucrose at pH 5.8. The microspores were squeezed out and then filtered through a 40-µm mesh into a 10-ml centrifuge tube. The microspore suspension was centrifuged at 1200 rpm for 3 min. The pellet was re-suspended in B5-13 medium and centrifuged at 1200 rpm for 3 min; this procedure was repeated twice.
After decanting supernatant, the microspores were re-suspended in NLN-13 medium (pH 5.8) with 13% sucrose supplemented with AC to achieve the final suspension density of microspore at a concentration of 1 ~ 2 × 105 microspore/ml. The microspore suspension was then poured into 35-mm Petri dishes (2 ml per Petri dish), and the cultures were incubated at 32.5 ℃ in the dark for 2 days, and then incubation continued at 25 ℃ in the dark for 20 days. The embryoid numbers were calculated and developmental stages of microspores were observed with an inverted microscope.
Temperature shock and AC treatment in radish genotypes
The selected buds of four genotypes (L-1, L-11, L-14, and L-18) were treated with cold treatment at 4 ℃ for 0, 12, 36, 48, 60, 72, and 96 h before the isolation of microspore. And four genotypes (L-1, L-2, L-3, and L-14) were also selected for investigating the effect of microspore suspension heat shock treated at 32.5 ℃ for 0, 24, 48, 72, and 96 h before culturing at 25 ℃. To investigate the effect of AC on microspore embryogenesis, four genotypes (L-2, L-14, L-15, and L-18) with different concentrations of AC were supplemented into the medium to maintain the concentration at 0.25, 0.50, 0.75, 1.0, 1.25, and 1.50 g/l, respectively.
Microspore embryoid germination and plant regeneration
Microspore embryoid germination was conducted based on the procedure of Takahata et al. (1996) with some modifications. When the embryoids were visible to the naked eyes, they were cultured at 25 ℃ under a 16-h photoperiod (4000 Lx) for 5 ~ 7 days. After the microspore embryoid turned green, the cotyledonary embryoids were transferred onto solid B5 medium with 3% sucrose, 0.8% agar, 0.2 mg/l 6-BA, 0.02 mg/l NAA, and 1 g/l AC (pH 5.8). The embryoid viability was evaluated after 1 week. After several subcultures, the plantlets were transferred onto solid B5 medium with 3% sucrose, 0.7% agar, and 0.2 mg/l NAA for rooting.
The rooted plantlets were transplanted into a pot with sterilized mixture of peat soil, nutrient soil, and vermiculite (2:1:1) and kept in a growth chamber for 1 week with a day temperature of 25 ℃ (14 h) and night temperature of 16 ℃ (10 h) under high relative humidity (85%) during the first 7 days, and then transplanted into an experimental plot in the greenhouse.
Comparison of microspore embryogenesis in different genotypes
Twenty-three genotypes were selected to compare the embryogenesis rate among different genotypes. Buds on inflorescences were selected at the uninucleate stage, with a ratio of petal length to anther length (P/A) in buds of about 3/4 ~ 1, and treated with 12-h cold treatment at 4 ℃. After microspore isolation, the microspore suspension was cultured in medium with the supplement of 0.75 g/l AC, and incubated at 32.5 ℃ for 48 h. Four genotypes were selected for each test. Three replications and each consisting of at least three Petri dishes were conducted in this study.
Ploidy level evaluation
Ploidy levels of the microspore-derived embryoid (MDE) plantlets were determined by chromosome counting and flow cytometry analysis. For chromosome counting, root tips were pretreated with ice water for 24 h and fixed in a mixture of absolute ethanol and glacial acetic acid (3:1, v/v) for 24 h at 4 ℃. Fixed root tips were hydrolyzed in 1 M hydrochloric acid in the water bath at 60 ℃ for 6 min and stained with a drop of 1% acetocarmine solution. The cells were then squashed and observed under a light microscope. The flow cytometry procedure was carried out as described by Zhang et al. (2007). The 100-mg young leaves were chopped with a sharp scalpel in 15 mM Tris–HCl (pH 7.5), 80 mM KCl, 20 mM NaCl, 20 mM EDTA-Na2, 15 mM mercaptoethanol, and 0.05% (v/v) Triton X-100. Nuclei isolated from young leaves of plants were then stained with RNase and PI (propidium iodide) for 2 h at 4 ℃ in the dark. The DNA contents were measured by flow cytometry (American Beckman Co.).
Homozygosity analysis of microspore-derived plantlets
The developed EST-SSR and genetic-SSR markers (Zhai et al. 2014) were employed to identify the homozygosity of microspore-derived plantlets. Genomic DNA was extracted from young leaves using a modified CTAB protocol (Luo et al. 2020). PCR amplification was performed on a thermal cycler (Senso Quest) with a 15-μl final reaction volume containing 2.0 mM MgCl2, 0.2 mM dNTPs, 0.75 U Taq DNA polymerase (TaKaRa Bio Inc.), 0.1 μM of each primer, and 10 ng of template DNA. The PCR conditions comprised initial denaturation at 94 ℃ for 2 min, followed by 35 cycles of 94 ℃ for 40 s, 55 ~ 60 ℃ (varying with the Tm of the different primers) for 45 s, 72 ℃ for 1 min, and a final extension of 72 ℃ for 7 min. PCR products were separated on 8% non-denaturing polyacrylamide gels at 120 V for 2 ~ 2.5 h and visualized with a silver staining method (Luo et al. 2017).
Reverse transcription quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from MDE using RNAsimple total RNA kit (Tiangen). Then, the RNA was reversely transcribed into cDNA using PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa) according to the manufacturer’s instructions. The RT-qPCR was conducted in a 20-μl reaction volume with 10 μl of 2 × SYBR Green PCR Master Mix (TaKaRa), 0.2 μM of each primer (Supplementary Table S1), and 2 μl diluted cDNA. RT-qPCR was performed on a LightCycler 480 System (Roche, Mannheim, Germany) with the following conditions: 95 ℃ for 3 min, and 45 cycles of 95 ℃ for 5 s, 58 ℃ for 30 s, and 72 ℃ for 10 s, and the RsActin was used as the reference gene for normalization (Fan et al. 2020). The relative expression level of each gene was calculated by the 2−△△CT method (Livak and Schmittgen. 2001).
Statistical analysis
The number of embryoids per Petri dish was counted 40 days after microspore isolation. The data were analyzed and compared using SPSS 16.0 software by Duncan’s multiple range test (p < 0.05) to determine the significance of differences among treatments.
Results
The effect of genotype on microspore embryogenesis
To explore the effect of genotype on microspore embryogenesis, 23 radish genotypes were used for IMC. The results showed that 16 of 23 radish genotypes could produce embryoids, while embryoid yield was significantly different. The maximum microspore embryogenesis rate of 20.00 (embryoids per dish) occurred in genotype L-22, while minimum rate of 0.12 was found in genotype L-8 (Table 1). However, MDE could not be obtained in seven genotypes through IMC, indicating that in microspore embryogenesis the difference in the responsiveness of genotypes was significant in radish.
The effect of developmental stage on microspore embryogenesis
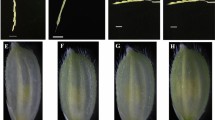
The optimal bud size for microspore embryogenesis in radish is different according to the genotypes (Table 2). Microspores from buds of 2.5 ~ 3.5 mm in length produced the highest ratio of embryoid per dish in genotype L-1, while buds 1.5 ~ 2.5 mm long were optimal for IMC in genotype L-4, indicating that the developmental stage of microspores was critical for microspore embryogenesis. Cytological observation indicated that microspores in the corresponding buds were in the late uninucleate to early binucleate stage, and the ratio of petal/anther length (P/A) was 0.75 ~ 1.0 (Supplementary Fig. S1). As compared with the bud length, the ratio of P/A was relatively reliable for optimal anther selection due to the variation of environmental factors and genotypes of donor plants.
Temperature treatment and microspore embryogenesis
Four genotypes (L-1, L-11, L-14, and L-18) were used to investigate the effect of cold treatment on microspore embryogenesis. The results showed that suitable cold pretreatment duration was different among these four genotypes (Supplementary Fig. S2a). In genotype L-18, the highest yield of embryoid was observed in the control without cold treatment, and the embryoid rate decreased with the pretreatment duration. However, genotypes L-1 and L-14 produced maximum embryoid when the buds were treated at 4 ℃ for 36 h, and then the rate of MDE decreased significantly, indicating that cold pretreatment was genotype specific. Therefore, it is important to screen the effective cold pretreatment for different genotypes. Microspore embryogenesis was not observed in the cultures of four genotypes (L-1, L-2, L-3, and L-14) treated with heat shock (32.5 ℃) for 96 h or maintained continuously at 25 ℃, indicating that the isolated microspores could not be developed without heat shock pretreatments (Table 3). The appropriate heat shock pretreatment would be beneficial to improve the embryoid yield. The embryoid yield of genotypes L-3 and L-14 with 48-h heat treatment was higher than that for 24 h (Table 3), while there was no significant difference between these two treatments. Briefly, temperature shock (32.5 ℃) within 48 h is optimal for those four genotypes.
The effect of activated charcoal on microspore embryogenesis
The number of embryoid per dish was significantly increased with the increasing AC concentration and reached the maximum at 0.75 g/l of AC concentration (Supplementary Fig. S2b). With the supplement of AC, the embryoid yield increased 4.0-, 4.66-, 4.0-, and 10.0-fold in the L-2, L-14, L-15, and L-18 genotypes, respectively. Embryoid yields were significantly increased in all genotypes with the addition of a 0.75 g/l AC to the microspore culture media. The results indicated that a certain concentration of AC could absorb toxic substances and increase the rate of microspore embryogenesis in radish.
Expression analysis of RsSERK genes
To explore the function of RsSERK genes, the six SERK gene expression patterns of the MDE at main stages including globular, heart-shaped, torpedo-shaped, and cotyledonary embryoids in the process of MDE development, and plantlet regeneration of radish (Fig. 1), were investigated with RT-qPCR analysis (Fig. 2 and Fig. 3). Several genes including RsSERK1A, RsSERK1B, and RsSERK1C were upregulated during the development of MDE, while low expression level was observed in the RsSERK3A. A stable expression level in RsSERK3B was detected in MDE development and lower expression was found in the browning MDEs. Among RsSERKs except for RsSERK4, the expressions in shoots were downregulated during plant regeneration, and root and shoot development, while RsSERK4 was highly expressed in torpedo-shaped embryoid and developing roots.
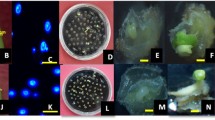
Microspore embryogenesis and plant regeneration of radish genotype L-18. a Isolated microspores from buds. b Growing microspores at 7 ~ 10 days after culture. c Globular embryoid. d Heart-shaped embryoid. e Torpedo-shaped embryoid. f Cotyledonary embryoid. g Germinated microspore embryoids (35 days after culture). h Cotyledonary embryoid transferred to subculture. i Plantlet regeneration on root-inducing medium. j Plantlets developed through microspore embryogenesis
Expression profiles of RsSERKs during the microspore embryogenesis and plantlet regeneration in Raphanus sativus L. G-H, Globular-heart staged MDEs. T, torpedo-staged MDEs. EC, early cotyledonary MDEs. LC, late cotyledonary MDEs. B, browning MDEs. PS, primary shoots. PR, primary roots. DS, developing shoots. DR, developing roots. The error bar represents mean ± SD of three biological replicates
Chromosome counting and ploidy level of microspore-derived plantlets
The chromosome number of microspore-derived plantlets was analyzed. It could be found that the regenerated plantlets from microspore embryoids consisted of haploid (n = x = 9), diploid (2n = 2x = 18), and tetraploid plants (2n = 4x = 36) as compared with the diploid donor plant (2n = 2x = 18) (Fig. 4). At the same time, ploidy level of regenerated plants was also determined by flow cytometric analysis, and the results were in accordance with those from chromosome analysis of root tip cell (Fig. 5).
Analysis of ploidy level of plantlets by flow cytometric determination of leaf nuclear DNA contents. a Haploid plantlet. b Diploid plantlet. c Tetraploid plantlet. The abscissa represents DNA content, the ordinate represents relative cell numbers, and D and E refer to the G1 and G2 phases, respectively
Homozygosity identification of microspore-derived plantlets with molecular markers
A total of ten pairs of SSR primers that could generate co-dominant markers were selected for PCR amplification in microspore-derived plantlets and 11 accessions (Supplementary Table S2, Supplementary Table S3). Microspore-derived plantlets (no. 1–13) showed a single band at a specific allele, while other materials show co-dominant bands (no. 14–24) (Supplementary Fig. S3), indicating that these microspore-derived plantlets were homozygous plants and could be further used for DH population construction.
Discussion
Radish is a typical cross-pollinated crop. Due to self-incompatibility, it is difficult to obtain inbred lines with complete homozygosity by conventional breeding methods. The IMC technology is considered to be an effective method to obtain homozygous DH lines for successful hybrid breeding programs (Corra-Martinez and Segui-Simarro 2012). However, the application of IMC technology is very limited due to unavailability of highly efficient protocols for the development of DHs. The ability of microspores to convent its gametophyte development pathway which leads to mature pollen grains into sporophyte pathway determines the success of IMC. This critical transition results in the cell formation of embryoids (Bhatia et al. 2018). Many factors such as donor plant genotype, microspore developmental stage, temperature treatment, and supplements of vancomycin, putrescine, abscisic acid, etc. were critical for IMC (Ahmadi et al. 2014a, b; Bhatia et al. 2018). In this study, a total of 23 genotypes were used to optimize the protocol of microspore embryogenesis in radish and critical factors including genotype, bud developmental stage, temperature stress, and AC treatment were investigated to optimize the technology of IMC in radish. Furthermore, the potential role of RsSERKs in the process of the development of MDE was explored.
The effectiveness of microspore embryogenesis is determined by a complex network of internal and environmental factors (Żur et al. 2021). Embryogenesis ability of microspore and embryoid yield might be genetically controlled (Zhang et al. 2020). It was found that genotypes play an important role in the microspore embryogenesis of Chinese cabbage (Shumilina et al. 2015). Different genotypes of the same species have various responses to microspore embryogenesis (Bhatia et al. 2017). Recently, it was reported that genotype was one of the most crucial factors for microspore embryogenesis, which significantly affected the embryoid yield among radish cultivars (Shumilina et al. 2020). In most cases, microspore embryogenesis tends to be with high genotype specificity (Tuncer et al. 2016). The response of MDE formation is different according to the genotype of donor plants (Han et al. 2014). In this study, the embryoid yield of different genotypes ranged from 0 to 20.0 per dish, and the embryogenesis rate of various genotypes was quite different, which was in good accordance with the finding of genotypic dependence. Therefore, it can be concluded that genotypes play a critical role in radish microspore embryogenesis.
Floral bud length is consumed as a simple parameter in selecting buds for microspore culture. Chun et al. (2011) observed that the highest MDE yield was obtained in radish when the microspore was isolated from buds that were 2.5 ~ 4.5 mm in length; Han et al. (2014) found that the flower bud of 4.0 mm with high contents of late-uninucleate-stage microspores could result in the maximum MDE yield in radish. However, characterization of bud size and P/A ratio could reveal development process of microspores (Mao et al. 2012). The optimum bud size varied considerably among genotypes for microspore embryogenesis (Bhatia et al. 2018). In cabbage, bud size of 3.2 ~ 3.5 mm was most effective for microspore culture; at this time, most microspores were found to be at the uninucleate stage (Cristea, 2013). However, bud size between 4.0 and 5.0 mm was found to be most suitable for isolated microspore culture in cabbage (Bhatia et al. 2018). For a synchronous development within a single bud, the bud length could not be regarded as the appropriate index for bud selection. The length of the optimum bud for microspore culture was 2.5 ~ 4.0 mm, and the ratio of petal to anther length (P/A) was 2/3 ~ 7/6 in B. oleracea (Wang et al. 2013a). In this study, the optional developmental stages of microspores could be estimated by the P/A ratio of 0.75 ~ 1.0 since microspores with this ratio were at the stages of uninucleate and early binucleate. The anther selection with this P/A ratio is of great practical significance for microspore culture.
The success of IMC depends on the ability of microspores to transfer their gametophytic developmental pathway to sporophytic pathway, resulting in cell division at a haploid ploidy level and formation of embryoid (Bhatia et al. 2018). The yield of MDE production has several bottlenecks at various stages of the process, such as induction and initiation of embryogenesis. The induction of microspore embryogenesis is carried out by specific stress treatments (cold/heat) to the isolated microspores (Testillano, 2019). Heat treatment can trigger auxin polarization and induce microspore embryogenesis of B. napus (Dubas et al. 2014). It was found that the exine of heat-stressed microspores can be broken at one or more pollen germination furrows (Tang et al. 2013). For Brassicaceae crops, heat shock treatment at the beginning of the culture was essential to promote microspore embryogenesis and enhance the embryoid quality (Testillano, 2019). Temperature exhibited a significant effect on embryogenesis, and treatment at 30.5 ℃ for 48 h is the best treatment to induce embryogenesis of microspores of all sizes (Winarto et al. 2011). It was found that heat shock at 32 °C for 48 h is the most suitable for most genotypes in radish (Kozar et al. 2020). Cold pretreatment has an effect on microspore embryogenesis in radish (Chun et al. 2011). In cauliflower, the efficiency of microspore embryogenesis in each group could be optimized by cold treatment (Bhatia et al. 2017). In this study, it was evident that the effect is different among various genotypes. Genotype L-18 responded positively to cold pretreatment, but genotype L-11 was not sensitive, indicating that the effect of cold treatment is genotype-dependent (Gu et al. 2014; Bhatia et al. 2017). In oilseed rape and other cabbage crops, cold pretreatment of floral buds has been used to improve embryoid yield (Sato et al. 2002; Gu et al. 2004; Bhatia et al. 2017). The effect of bud cold pretreatment on the development of embryoids in microspore culture was confirmed with an increase in the number of embryoids in the microspore culture and a simultaneous decrease in callus formation in Brassica (Shumilina et al. 2020). This study has proved that cold pretreatment has a positive effect on some genotypes, but further investigations are needed to confirm the relationship between cold pretreatment and callus formation in radish.
AC has strong adsorption properties and is usually used to absorb gases and dissolved solids (Cheng et al. 2013). The supplement of AC to the medium was reported to modify medium composition in vitro. It was found that the in vitro androgenesis rate of pepper in 10 g/L AC medium was the highest, regardless of the low-response or high-response genotypes of microspore culture (Supena et al. 2006). Among the tested genotypes, the yield of embryoid-like structures increased significantly after adding 0.5 g/l AC to microspore medium (Cheng et al. 2013). The addition of 10 g/l AC promoted the development of a large number of double haploid plants (Shumilina et al. 2015). In this study, embryoid yield and quality were significantly increased in genotypes L-14, L-15, and L-18 with the supplement of 0.75 g/l AC, resulting in up to 4.66-, 4.00-, and 10.0-fold of embryoid yield, respectively. While the concentration of AC was above 0.75 g/l, embryoid yield began to decrease. It was found that 20 g/l of active charcoal impaired embryonic shoot development (Supena et al. 2006). Therefore, the suitable concentration should be ascertained for certain species. It could be found that the supplement of AC at 0.75 g/l had a great effect on embryogenesis, which played a certain role in the optimization of effective microspore embryogenesis in radish.
The SERK family of receptor kinases is involved in cell-to-embryo transition and controlling a number of other fundamental aspects of plant development (Ahmadi et al. 2016). Over-expression of AtSERK1 was proved to enhance embryogenesis (Hecht et al. 2001). The TnSERK was upregulated during the development of embryoids (Pilarska et al. 2016). Besides, a steady increase in BnSERK2 expression was detected during MDE development, indicating that these genes were involved in the process of MDE development in B. napus. Our results showed that the expression of RsSERK1A, RsSERK1B, and RsSERK1C increased steadily during MDE development, implying that these upregulated genes might play roles in this procedure. Intriguingly, the expression level of RsSERK genes except for RsSERK4 in primary shoots and roots was higher than that in developed shoots and roots, indicating their role(s) in the early stage of organ formation, rather than being specific to the embryogenesis. Furthermore, the expression of RsSERKs was distinct. For example, the RsSERK3A had a low expression level only at the beginning of MDE development, and its expression was not detected during the later MDE development, but the high expression of the RsSERK3A was detected in primary shoots and roots. Meanwhile, a high expression level of RsSERK4 was found in torpedo-shaped embryoid and developed roots. Overall, RsSERK genes might be involved in the process of MDE development and plantlet regeneration in radish.
Conclusions
In this study, the significant influence of important factors including genotype, bud size, heat shock treatment, and AC is very critical for the success of embryogenesis in radish. Furthermore, the expression of RsSERKs in MDE development and plantlet regeneration would benefit the process of microspore embryogenesis, which may facilitate overcoming the challenges related to MDE production and plant regeneration in recalcitrant species. The haploid plants could be obtained successfully as well as the double haploids and tetraploid from IMC. Plants produced by microspore culture were identified as homozygous through the developed EST-SSR and genetic SSR markers. Homozygous plants obtained from IMC can be used directly for hybrid breeding based on the specific breeding objectives, and their offspring were highly uniform. This newly established IMC system could be employed for highly efficient haploid induction and further application in radish heterosis breeding.
Data availability
All data generated or analyzed in this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Ahmadi B, Masoomi-Aladizgeh F, Shariatpanahi ME (2016) Molecular characterization and expression analysis of SERK1 and SERK2 in Brassica napus L.: implication for microspore embryogenesis and plant regeneration. Plant Cell Rep 35(1):1–9. https://doi.org/10.1007/s00299-015-1878-6
Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA (2014a) Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tiss Org 116:343–351. https://doi.org/10.1007/s11240-013-0408-x
Ahmadi B, Shariatpanahi ME, Ojaghkandi MA (2014b) Plant Cell Tiss Org 118:497–505. https://doi.org/10.1007/s11240-014-0501-9
Bhatia R, Dey SS, Parkash C, Sharma K, Sood S, Kumar R (2018) Modification of important factors for efficient microspore embryogenesis and doubled haploid production in field grown white cabbage (Brassica oleracea var. capitata L.) genotypes in India. Sci Hortic 233:178–187. https://doi.org/10.1016/j.scienta.2018.01.017
Bhatia R, Dey SS, Sood S, Sharma K, Sharma VK, Parkash C, Kumar R (2016) Optimizing protocol for efficient microspore embryogenesis and doubled haploid development in different maturity groups of cauliflower (B. oleracea var. botrytis L.) in India. Euphytica 212:439–454. https://doi.org/10.1007/s10681-016-1775-2
Bhatia R, Dey SS, Sood S, Sharma K, Parkash C, Kumar R (2017) Efficient microspore embryogenesis in cauliflower (Brassica oleracea var. botrytis L.) for development of plants with different ploidy level and their use in breeding programme. Sci Hortic 216:83–92. https://doi.org/10.1016/j.scienta.2016.12.020
Brew-Appiah Rhoda AT, Ankrah N, Liu W, Konzak CF, von Wettstein D, Rustgi S (2013) Generation of doubled haploid transgenic wheat lines by microspore transformation. PLoS ONE 8(11):e80155. https://doi.org/10.1371/journal.pone.0080155
Chen WS, Zhang Y, Ren J, Ma YY, Liu Z, Hui F (2019) Effects of methylene blue on microspore embryogenesis and plant regeneration in ornamental kale (Brassica oleracea var. acephala). Sci Hortic 248:1–7. https://doi.org/10.1016/j.scienta.2018.12.048
Cheng Y, Ma RL, Jiao YS, Qiao N, Li TT (2013) Impact of genotype, plant growth regulators and activated charcoal on embryogenesis induction in microspore culture of pepper (Capsicum annuum L.). S Afr J Bot 88:306–309. https://doi.org/10.1016/j.sajb.2013.08.012
Chun C, Park H, Na H (2011) Microspore-derived embryo formation in radish (Raphanus sativus L.) according to nutritional and environmental conditions. Hortic Environ Biotechnol 52, 530
Corral-Martínez and Seguí-Simarro, 2012 Corral-Martínez P, Seguí-Simarro JM (2012) Efficient production of callus-derived doubled haploids through isolated microspore culture in eggplant (Solanum melongena L.). Euphytica 187(1):47–61 https://doi.org/10.1007/s10681-012-0715-z
Cristea TO (2013) The influence of pH on microspore embryogenesis of white cabbage (Brassica oleracea L). Notulae Scientia Biologicae 54:485–489. https://doi.org/10.15835/nsb549122
Dias JS (1999) Effect of activated charcoal on Brassica oleracea microspore culture embryogenesis. Euphytica 108:65–69. https://doi.org/10.1023/A:1003634030835
Dubas E, MoravčíÍková J, Libantová J, Matušíková I, Benková E, Zur I, Krzewska M (2014) The influence of heat stress on auxin distribution in transgenic B. napus microspores and microspore-derived embryos. Protoplasma 251(5):1077–1087. https://doi.org/10.1007/s00709-014-0616-1
Fan LX, Wang Y, Xu L, Tang MJ, Zhang XL, Ying JL, Li C, Dong JH, Liu LW(2020) A genome-wide association study uncovers a critical role of the RsPAP2 gene in red-skinned Raphanus sativus L. Hortic Res 7:164. https://doi.org/10.1038/s41438-020-00385-y
Gu HH, Zhao ZQ, Sheng XG, Yu HF, Wang JS (2014) Efficient doubled haploid production in microspore culture of loose-curd cauliflower (Brassica oleracea var. botrytis). Euphytica 195:467–475. https://doi.org/10.1007/s10681-013-1008-x
Han N, Kim SU, Park HY, Na H (2014) Microspore-derived embryo formation and morphological changes during the isolated microspore culture of radish (Raphanus sativus L.). Kor J Hort Sci Technol 32(3):382–389. https://doi.org/10.7235/hort.2014.13170
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816. https://doi.org/10.1104/pp.010324
Hosp J, Ribarits A, Jin Y, Tashpulatov A, Resch T, Friedmann C, Ankele E, Voronin V, Palme K, Heberle-Bors E, Touraev E (2014) A tobacco homolog of DCN1 is involved in pollen development and embryogenesis. Plant Cell Rep 33(7):1187–1202
Kozar EV, Domblides EA, Soldatenko AV (2020) Factors affecting DH plants in vitro production from microspores of European radish. Vavilovskii Zhurnal Genet Sel 24:31–39
Kozar EV, Domblides EA, Soldatenko AV (2021) Embryogenesis of European radish (Raphanus sativus L. subsp. sativus Convar. Radicula) in culture of isolated microspores in vitro. Plants 10(10):2117. https://doi.org/10.3390/plants10102117
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus L. Z Pflanzenphysiol 105:427–434. https://doi.org/10.1016/S0044-328X(82)80040-8
Lionneton E, Beuret W, Delaitre C, Ochatt S, Rancillac M (2001) Improved microspore culture and doubled-haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rep 20:126–130. https://doi.org/10.1007/s002990000292
Luo XB, Xu L, Liang DY, Wang Y, Zhang W, Zhu XW, Zhu YL, Jiang HY, Tang MJ, Liu LW (2017) Comparative transcriptomics uncovers alternative splicing and molecular marker development in radish (Raphanus sativus L.). BMC genomics 18:505. https://doi.org/10.1186/s12864-017-3874-4
Luo XB, Xu L, Wang Y, Dong JH, Chen YL, Tang MJ, Fan LX, Zhu YL, Liu LW (2020) An ultra-high-density genetic map provides insights into genome synteny, recombination landscape and taproot skin colour in radish (Raphanus sativus L.). Plant Biotechnol J 18:274–286. https://doi.org/10.1111/pbi.13195
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Makowska K, Oleszczuk S, Zimny A, Czaplicki A, Zimny J (2015) Androgenic capability among genotypes of winter and spring barley. Plant Breed 134:668–674. https://doi.org/10.1111/pbr.12312
Mao ZL, Zhang ZC, Yao YM, Dai ZL, QIN WF, Pan YP, (2012) Observation of microspore embryogenesis and ploidy identification of regenerated plants. Acta Botanica Sinica 32(10):2016–2022
Munoz-Amatriain M, Svensson JT, Castillo AM, Close TJ, Valle’s MP (2009) Microspore embryogenesis: assignment of genes to embryo formation and green vs. albino plant production. Funct Integr Genomic 9:311–323. https://doi.org/10.1007/s10142-009-0113-3
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Oleszczuk S, Sowa S, Zimny J (2004) Direct embryogenesis and green plant regeneration from isolated microspores of hexaploid triticale (×Triticosecale Wittmack) cv. Bogo Plant Cell Rep 22:885–893. https://doi.org/10.1007/s00299-004-0796-9
Pilarska M, Malec P, Salaj J, Bartnicki F, Konieczny R (2016) High expression of Somatic Embryogenesis Receptor-like Kinase coincides with initiation of various developmental pathways in vitro culture of Trifolium nigrescens. Protoplasma 253:345–355. https://doi.org/10.1007/s00709-015-0814-5
Podio M, Felitti SA, Siena LA, Delgado L, Mancini M, Seijo JG, Gonza ´lez AM, Pessino SC, Ortiz JP, (2014) Characterization and expression analysis of somatic embryogenesis receptor kinase (SERK) genes in sexual and apomictic Paspalum notatum. Plant Mol Biol 84:479–495. https://doi.org/10.1007/s11103-013-0146-9
Sato S, Katoh N, Iwai S, Hagimori M (2002) Effect of low temperature pretreatment of buds or inflorescence on isolated microspore culture in Brassica rapa (syn. B. campestris). Breed Sci 52:23–26
Segui-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore derived embryogenesis. Physiol Plant 134:1–12
Shariatpanahi ME, Ahmadi B (2016) Isolated microspore culture and its applications in plant breeding and genetics. In: Anis M, Ahmad N (eds) Plant tissue culture: propagation, conservation and crop improvement. Springer, Singapore, pp 487–507. https://doi.org/10.1007/978-981-10-1917-3_21
Shumilina D, Kornyukhin D, Domblides E, Soldatenko A, Artemyeva A (2020) Effects of genotype and culture conditions on microspore embryogenesis and plant regeneration in Brassica Rapa ssp. Rapa l Plants (basel) 9(2):278. https://doi.org/10.3390/plants9020278
Shumilina DV, Shmykova NA, Bondareva LL, Suprunova TP (2015) Effect of genotype and medium culture content on microspore derived embryo formation in Chinese cabbage (Brassica rapa ssp. Chinensis cv. Lastochka). Biol. Bull 42(4):302–309. https://doi.org/10.1134/S1062359015040135
Solís MT, Berenguer E, Risueño MC, Testillano PS (2016) BnPME is progressively induced after microspore reprogramming to embryogenesis, correlating with pectin de-esterifcation and cell differentiation in Brassica napus. BMC Plant Biol 16:176. https://doi.org/10.1186/s12870-016-0863-8
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot peppers (Capsicum annuum L.). Plant Cell Rep 25:1–10. https://doi.org/10.1007/s00299-005-0028-y
Takahata Y, Komatsu H, Kaizuma N (1996) Microspore culture of radish (Raphanus sativus.L): influence of genotype and culture conditions on embryogenesis. Plant Cell Report 16:163–166. https://doi.org/10.1007/BF01890859
Tang X, Liu Y, He Y, Ma L, Sun MX (2013) Exine dehiscing induces rape microspore polarity, which results in different daughter cell fate and fixes the apical-basal axis of the embryo. Exp Bot 64:215–228. https://doi.org/10.1093/jxb/ers327
Testillano PS (2019) Microspore embryogenesis: targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J Exp Bot 70(11):2965–2978. https://doi.org/10.1093/jxb/ery464
Tsuwamoto R, Fukuoka H, Takahata Y (2007) Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta 225(3):641–652. https://doi.org/10.1007/s00425-006-0388-8
Tuncer B, Cig A, Yanmaz R, Yasar F (2016) Effect of heat shock treatment on microspore embryogenesis in Brassica oleracea Species. Tarim Bilimleri Dergisi 22:548–554. https://doi.org/10.1501/Tarimbil_0000001413
Wang C, Yao Y, Peng L (2013) Study of isolated microspore culture and plant regeneration from embryoid of radish (Raphanus sativus L.). J Anhui Agri Sci 41(27):19–22
Wang WH, Ye GR, Li BY, Yue ZC, Zhong XM (2013b) Isolated microspore culture and plant regeneration in cabbage. Acta Agriculturae Nucleatae Sinica 27(006):715–722
Winarto B, Teixeira DS, Jaime A (2011) Microspore culture protocol for Indonesian Brassica oleracea. Plant Cell Tissue Organ Cult 107:305–315. https://doi.org/10.1007/s11240-011-9981-z
Xiao J, Chen Y (2002) Study on the cytology and morpholgy of pollen development of Brassica vegetable crops. Journal of Changjiang Vegetables S1:107–108
Yu FQ, Liu HL (1995) The effects of donor plant and medium component on embryo yield in Brassica napus. J HuaZhong Agri University 14(4):327–331
Zhai L, Xu L, Wang Y, Cheng H, Chen Y, Gong Y, Liu L (2014) Novel and useful genic-SSR markers from de novo transcriptome sequencing of radish (Raphanus sativus L.). Mol Breeding 33:611–624. https://doi.org/10.1007/s11032-013-9978-x
Zhang L, Wang Q, Wang Y (2020) Development of inbred lines of Raphanus sativus L. var. ‘Xinlimei’ by isolated microspore culture technology. China Vegetables 1(8):53–56
Zhang L, Zheng P (2013) Isolated microspore culture of spring-white radish (Raphanus sativus L. var. longpinnatus Bailey). Northern Horticulture 000(023):31–33
Zhang Z, Zhang S, Zhang W, Zhang H (2007) Induction of tetraploidy of non-heading Chinese cabbage with late-bolting and identification of chromosome configuration. Acta Botan Boreali-Occiden Sin 27(1):0028–0032
Zhou Y, Feng H, Wang CN, Liu RE (2006) Isolated microspore culture and plantlet formation in Chinese cabbage (Brassica campestris ssp.pekinensis). Journal of Shenyang Agricultural University 37(6):816–820
Zhou Z, Gong Y, Wang X, Liu L, Zhang Y (2007) Induction of isolated microspore and optimization of their culture system of different radish varieties. Acta Bot Boreal-Occident Sin 27(1):0033–0038
Żur I, Dubas E, Krzewska M, Kope P, Malage S (2021) Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. Plant Cell Tiss ORG 145:347–366. https://doi.org/10.1007/s11240-021-02012-7
Acknowledgements
The authors thank the anonymous reviewers and the editors for their helpful comments and suggestions on this manuscript.
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (32172579), the Jiangsu Agricultural S&T Innovation Fund [CX(21)2020, CX(18)3067], the earmarked fund for Jiangsu Agricultural Industry Technology System [JATS(2022)], and the Central Agricultural Major Technology Collaborative Extension Plan-Root and Stem Vegetable Project (2020-SJ-047–01-3).
Author information
Authors and Affiliations
Contributions
Wrote first draft: YC, LL. Designed experimental work: YC, LL, YW, LX, and XS. Provided experimental materials: LL, YZ, LZ, and CZ. Analyzed data: YC. Wrote original manuscript: YC, LL. Wrote and edit review: YC, LL. Supervised the whole work: LL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Chen, Y., Wang, Y., Xu, L. et al. Effects of genotype and culture conditions on microspore embryogenesis in radish (Raphanus sativus L.). Mol Breeding 42, 43 (2022). https://doi.org/10.1007/s11032-022-01312-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-022-01312-w