Abstract
Phytoene synthase-1 (Psy-1) homoeologs are associated with yellow pigment content (YPC) in endosperm of durum and bread wheat. In the present study, microsatellite variation in promoter region of Psy-A1 was identified in durum wheat and marker Psy-1SSR, targeting the microsatellite variation was developed which amplifies variation in Psy-A1 and Psy-B1 loci simultaneously. Psy-A1SSR was mapped within QYp.macs-7A, a major QTL for YPC identified earlier in PDW 233/Bhalegaon 4 population. Marker Psy-A1SSR was further validated in two different RIL populations and a set of 222 tetraploid wheat accessions including less cultivated tetraploid wheat species. Eight alleles of Psy-A1SSR were identified in 222 wheat accessions, while seven alleles were observed for Psy-B1SSR. Variation at Psy-A1SSR showed significant association with YPC, whereas no association was observed with Psy-B1SSR. Marker-assisted introgression of Psy-A1SSRe allele from PDW 233, to durum wheat cultivars MACS 3125 and HI 8498 resulted in improvement of YPC. Backcrossed BC3F2:4 and BC2F2:3 lines selected using Psy-A1SSR showed 89 to 98% gain in YPC over recurrent parents indicating robustness of marker. The marker can thus be utilized in marker-assisted improvement of YPC in durum wheat cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yellow pigments (carotenoids) present in endosperm impart bright yellow color to semolina and pasta. Higher the yellow pigment content (YPC), better the appearance and acceptability of the pasta in the market. This makes the color of the pasta one of the important quality parameters for the pasta industry (Troccoli et al. 2000) and hence, it has been included in several durum wheat quality improvement programs (Clarke et al. 1998; Dick and Youngs 1988; Di Fonzo et al. 2005; Pfeiffer and Payne 2005). Considering their significance, chemical composition of yellow pigments has been studied extensively with the help of high-performance liquid chromatography (HPLC) by several researchers. Hentschel et al. (2002) found that all-trans- lutein and zeaxanthin accounted around 30 to 50% of the yellow pigment quantity and hence concluded that there are still some more compounds in durum wheat not yet identified that contribute considerably to the yellow color of the grain extracts. With an improved normal-phase HPLC procedure, lutein was detected as a major component along with α+β-carotene, β-cryptoxanthin, and zeaxanthin in trace quantity in durum wheat (Fratianni et al. 2005; Panfili et al. 2004).
Since YPC shows complex inheritance, several efforts have been made to identify the genetic components controlling the trait. Major QTL controlling YPC were reported on chromosomes 7AL and 7BL in durum as well as bread wheat (Blanco et al. 2011; Colasuonno et al. 2014; Elouafi et al. 2001; Mares and Campbell 2001; Parker et al. 1998; Patil et al. 2008; Pozniak et al. 2007; Roncallo et al. 2012; Zhai et al. 2016a; Zhang et al. 2008). In further studies on these QTL, Psy-A1 and Psy-B1 encoding Phytoene synthase involved in carotenoid biosynthesis were identified to be linked with QTL for YPC on chromosome 7A and 7B, respectively (He et al. 2008; Pozniak et al. 2007). Moreover, allelic variation at Psy-A1 and Psy-B1 was also studied to test the effect of various alleles on the trait. In durum wheat, eight and seven alleles of Psy-A1 and Psy-B1, respectively, have been identified (He et al. 2009; Singh et al. 2009). In studies on bread wheat, 11 alleles of Psy-A1, five of Psy-B1, and four of Psy-D1 have been reported (Crawford et al. 2011; Ficco et al. 2014; He et al. 2008, 2009; Howitt et al. 2009; Wang et al. 2009). These reports provided considerable evidence that allelic variation at Psy-A1, Psy-B1, and Psy-D1 is associated with variation in yellow pigment content in wheat. Genome-wide association studies also supported involvement of Psy-A1 and Psy-B1 in determination of YPC in bread wheat (Zhai et al. 2018). Studies on transcription of Psy1 homoeologs and their functional characterization using reverse genetic tools provided in-depth insight into function and transcriptional regulation of Psy1 in bread and durum wheat (Qin et al. 2016; Zhai et al. 2016b). In recent studies, two paralogs of Psy1, namely Psy2 and Psy3, were also identified and mapped on short and long arm, respectively, of group 5 chromosome of wheat. Psy2 was associated with yellow index in tetraploid wheat (Colasuonno et al. 2017), whereas, Psy3 showed elevated expression in leaves and roots when plants were exposed to abiotic stresses (Dibari et al. 2012; Flowerika et al. 2016; Li et al. 2008).
Marker-assisted breeding (MAB) has become popular among wheat breeders across the world during the last 20 years, mainly because it improves precision for selection even at early generation stage and also enables breeders to pyramid desirable genes in a single genetic background. MAB has been convincingly utilized to introgress genes for disease resistance, plant height, and quality traits in elite wheat background (Kumar et al. 2011; Randhawa et al. 2013; Tyagi et al. 2014; Vasistha et al. 2016; Vishwakarma et al. 2016; William et al. 2007). Genes for grain protein content (Gpc-B1), gluten strength (x7 of Glu-B1; x1, xNull and x2* of Glu-A1; x2, x5, y10 and y12 of Glu-D1) and low cadmium uptake (Cdu1) have been targeted in MAB for wheat quality improvement (de Bustos et al. 2001; Randhawa et al. 2013; Tyagi et al. 2014; Vishwakarma et al. 2016; William et al. 2007). Although several gene/allele-specific markers have been reported with their potential use in improvement of YPC in durum wheat, reports on marker-assisted development of advanced wheat breeding lines with improved YPC are lacking (Zhai et al. 2016c). Since majority of the Indian durum wheat cultivars have low YPC with an average of 4.5 ppm (Santra et al. 2006), deployment of DNA-based markers in breeding program can lead to overall improvement of YPC in Indian durum cultivars.
In our earlier study, we identified a major QTL, QYp.macs-7A for YPC in PDW 233/Bhalegaon 4 population (Patil et al. 2008). Psy-A1 in PDW 233 and Bhalegaon 4 was also characterized and investigated for its association with QYp.macs-7A and variation in YPC; however, its linkage with QYp.macs-7A could not be established due to lack of polymorphism in coding regions and introns of Psy-A1 between PDW 233 and Bhalegaon 4. Moreover, the dominant SCAR markers reported in the study may not be useful in selection in early generations due to chances of false positives. The objectives of the present study were (a) to analyze flanking regions of Psy-A1 and Psy-B1 in PDW 233 and Bhalegaon 4 for polymorphism to develop Psy-A1- and Psy-B1-specific co-dominant markers for selection of high YPC, (b) to test association between identified markers and variation in YPC in diverse germplasm and RIL populations, and (c) to test the utility of identified marker in marker-assisted backcross breeding for improvement of YPC in Indian durum wheat cultivars.
Material and methods
Plant material
PDW 233/Bhalegaon 4 RIL population reported in our earlier study (Patil et al. 2008) was used for identification and mapping of marker for YPC. The population was evaluated for YPC across five environments (Table 1) and same phenotype data was used to estimate marker-trait association. The marker was further validated on two sets of F2:6 RILs populations developed from durum wheat crosses MACS 3125/UC 1114 and Bijaga Yellow/Castelporziano. MACS 3125 and Bijaga Yellow are durum wheat cultivars released for cultivation in the states of Maharashtra and Karnataka, respectively, while Castelporziano (PI347331) is a mutant derived from Triticum durum cv. Cappelli. UC 1114 is durum wheat selection containing dicoccoides Gpc-B1 functional allele, obtained from Prof. J. Dubcovsky, University of California, Davis, CA. In addition to these populations, a set of 222 tetraploid wheat genotypes comprising 189 durum breeding lines, 14 Indian local durums, and 12 durum cultivars as well as 7 accessions of less cultivated species (T. carthlicum and T. polonicum) was also used to test association between identified marker and yellow pigment content. The durum wheat breeding lines included entries from the 37th and 45th IDYN (International Durum Yield Nursery) as well as IDSN (International Durum Screening Nursery), from CIMMYT, Mexico. Detailed parentage and selection history of cultivars and breeding lines is given in Supplementary Table S1.
Field trials
PDW 233/Bhalegaon 4 RIL population was planted in randomized block design (RBD) with two replications across five different environments as given in Table 1. Details of field trials and year × location combinations are reported in Patil et al. (2008). Other two RIL populations MACS 3125/UC 1114 and Bijaga Yellow/Castelporziano were planted in augmented design during 2012–2013 and 2017–2018, respectively, at the Pune location. Out of 222 tetraploid wheat accessions, durum cultivars, local durums, and less cultivated tetraploid species were planted in RBD with two replications at Pune location during 2005–2006, while, breeding lines from CIMMYT were sown in RBD with two replications during 2012–2013. Similarly, selected BC3F2:3 and BC2F2:3 lines carrying Psy-A1SSRe allele developed through marker-assisted breeding were sown in replicated trial with RBD during 2013–2014, 2014–2015, and 2015–2016 along with recurrent parent, donor, and best checks. All the field trials were conducted under irrigated high fertility conditions.
Evaluation of yellow pigment content
Yellow pigment content in the grains was evaluated as described in Santra et al. (2003). Briefly, 125 mg of flour was suspended in 625 μL of n-butanol saturated with water. The samples were mixed thoroughly and allowed to stand for 16 h in dark for pigment extraction. The samples were centrifuged at 10000 g for 5 min and the absorbance of supernatant was measured at 440 nm. In marker-assisted selection experiments, yellow index (YI) of whole meal was measured in terms of b* values using CR 410 Chroma Meter, (Konica Minolta; Symons and Dexter 1991).
Development of marker
Earlier, we had sequenced 2336 bp region of Psy-A1 in parental genotypes PDW 233 and Bhalegaon 4 (Patil et al. 2008). The primers for this sequencing analysis were designed using GenBank accessions (DQ642439, `DQ642440, DQ642443, DQ642444, EU096090) of Phytoene synthase gene sequences reported in durum wheat (Pozniak et al. 2007; Zhang and Dubcovsky 2008). Continuing the work further, flanking 5′ upstream (942 bp) and 3′ downstream regions (399 bp) in PDW 233 and Bhalegaon 4 were sequenced. A 1488 bp fragment spanning promoter region and exon 1 was amplified using primers Psy-7A5′F1 (5′CTACTCCTACAGATGAGGAGC3′) and Psy-7ARg (5′GCTCAAGAAAAACAGAGTATCCAC3′). Similarly, 3′ downstream region was amplified using primers Psy-7AFd (5′AAAATGATGCTACGTGTAGTTCG3′) and Psy-7A3′R (5′CATCTTCTCGCATCCATTCCTC3′). Amplicons were sequenced on ABI PRISM® 3100-Avant Genetic Analyzer. Sanger sequencing reads were aligned using ClustalW multiple alignment application available in BioEdit version 7.2.5 (Hall 1999) to obtain consensus sequences of Psy-A1 for PDW 233 and Bhalegaon 4. The consensus sequences were aligned to find out differences, if any, using multiple sequence alignment program Multalin (Corpet 1988) available at http://multalin.toulouse.inra.fr/multalin/. To target observed microsatellite variation in the promoter region of Psy-A1, a pair of primers (Psy-1SSRF: 5′GTCCATCCATCCCTTTCCAGG3′; Psy-1SSRR: 5′ATGCGAGGACAAAGTCCAGTG3′) was designed. PCR reaction contained 250 nM of each primer, 0.2 mM of each deoxynucleotide, 10 mM Tris–HCl pH 9.5, 1.5 mM MgCl2, 50 mM KCl, 0.5 U Taq polymerase, and 40–60 ng of template DNA. The thermal cycling conditions were 94 °C for 4 min followed by 35 cycles of 30 s at 94 °C, 30 s at 56 °C, and 30 s at 72 °C followed by 72 °C for 5 min. PCR products were separated on 6% denaturing polyacrylamide gel and visualized by silver stain. Since the primer pair Psy-1SSRF/R also amplified microsatellite variation in promoter of Psy-B1, it was also investigated for sequence variation using primers Psy-1SSRF: 5′GTCCATCCATCCCTTTCCAGG3′ and Psy-7BR 5′GCTCCATGATCATCAGAACATG3′. Previously reported markers Psy1-A1_STS (Singh et al. 2009) and YP7A-2 (He et al. 2009) for Psy-A1 were also tested on 66 accessions to correlate their allelic frequencies with Psy-1SSR marker. PCR amplification conditions for Psy1-A1_STS and YP7A-2 were as described in the original references.
Marker-assisted selection using Psy-A1SSR
To test utility of the Psy-A1SSR marker in improvement of YPC content using marker-assisted selection (MAS), Psy-A1SSRe allele from PDW233, a cultivar with high YPC, was transferred to two popular Indian durum cultivars with low YPC viz. MACS 3125 (released for Maharashtra state) and HI 8498 (released for Central Zone of India) using backcross breeding approach. BC1F1, BC2F1, BC3F1, and BC3F2 plants were screened for the presence of Psy-A1SSRe allele using Psy-A1SSR marker. Detail marker-assisted breeding protocol is given in Supplementary Fig. 1. Selected BC3F2:3 lines in MACS 3125 background and BC2F2:3 lines in HI 8498 background were evaluated for YI, YPC, grain yield, grain characteristics, flowering time, and spike traits in replicated trial with randomized block design conducted for 3 and 2 years, respectively.
Data analysis
Arithmetic means, standard deviation, and coefficient of variation were calculated using SPSS 11.0. Single marker regression analysis for YPC was carried out using linear regression model in SPSS package version 11.0. Rare alleles with frequency < 3% in wheat accessions were not considered for single marker regression analysis. In MAS trials, the one-way ANOVA was carried out using MS excel office 10 (Microsoft Office 10). The improved lines in the background MACS 3125 and HI 8498 were compared with recipient genotypes applying LSD test at α = 0.05.
Results
Yellow pigment content in durum wheat
Total 222 durum wheat accessions including durum wheat cultivars, Indian local durums, breeding lines, and less cultivated tetraploid relatives showed a wide range in YPC from 2.95 to 9.91 ppm. Average YPC was 5.69, 4.91, and 5.77 ppm in cultivars, local durums and breeding lines from CIMMYT, respectively (Table 1). Less cultivated tetraploid wheat showed comparatively higher average YPC (7.04 ppm) than the other classes of durum wheat studied. In MACS 3125/UC 1114 and Bijaga Yellow/Castelporziano, RIL populations YPC varied from 2 to 7.15 ppm and 1.48 to 7.30 ppm with the average of 4.34 ppm and 3.21 ppm, respectively. YPC in PDW 233/Bhalegaon 4 population ranged from 1.62 to 12.97 ppm with an average of 5.44 ppm as reported earlier (Patil et al. 2008). PDW 233, showing highest average YPC 8.36 ppm among durum cultivars, was further used as a donor in marker-assisted introgression of Psy-A1 in MACS 3125 and HI 8498.
Marker development and mapping of Psy-A1
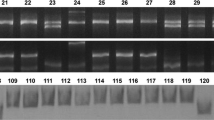
Primer pair Psy-7A5′F1/Psy-7ARg amplified a 1488 bp DNA segment spanning part of exon-1 and 942 bp region upstream to start codon in PDW 233 and Bhalegaon 4. Similarly, Psy-7AFd/Psy-7A3′R amplified 878 bp fragment covering part of exon-6 and 584 bp downstream to stop codon. These fragments were sequenced and complete sequence of Psy-A1 including coding regions (sequenced earlier, Patil et al. 2008) and flanking regions was derived in PDW 233 (4424 bp; GenBank accession MG733357) and Bhalegaon 4 (4312 bp; GenBank accession MG733358). While no sequence variation in 3′ downstream region (399 bp) was observed between PDW 233 and Bhaleagon 4, promoter region (942 bp) of Psy-A1 showed presence of a microsatellite at 560 bp upstream to start codon, with variation in number of TC repeats between both the parents. PDW 233 has di-nucleotide repeat (TC)21 as compared to (TC)25 in Bhalegaon 4 (Fig. 1a). The primer pair Psy-1SSRF/Psy-1SSRR targeting variation in this microsatellite region was used to genotype and map Psy-A1 in the PDW 233/Bhalegaon 4 RIL population. This primer pair amplified 236 bp and 244 bp microsatellite fragments linked to Psy-A1 in PDW 233 and Bhalegaon 4, respectively (Fig. 1b). This microsatellite variation present in promoter region of Psy-A1 was named as Psy-A1SSR, which was mapped on long arm of chromosome 7A within the QTL for YPC, QYp.macs-7A (Supplementary Fig. 2). Same primer pair also amplified another 201 bp monomorphic band in PDW 233 and Bhalegaon 4 (Fig. 1b). Using nullisomic/tetrasomic lines in cv. Chinese Spring, 201 bp amplicon was assigned to chromosome 7B. In sequence analysis, the amplicon showed a complex microsatellite- (TC)5(AG)9 at 545 bp upstream to the start codon of Psy-B1 in PDW 233 and Bhalegaon 4 (GenBank accessions MG733359 and MG733360).
Association of Psy-1SSR with yellow pigment content
Psy-A1SSR showed significant association with YPC in PDW 233/Bhalegaon 4 population across all the five environments (R2 24.70% to 62.70%, P < 0.001). The marker was mapped within the major QTL for YPC QYp.macs-7A in PDW 233/Bhalegaon 4 population. Psy-B1SSR was monomorphic in the population. Since the primer pair Psy-1SSRF/Psy-1SSRR targeted microsatellite variation at Psy-A1 and Psy-B1 in single PCR reaction, effect of both the homoeologs on YPC was tested in two different RIL populations segregating for both the homoeologs of the microsatellite. In single marker analysis, Psy-A1SSR showed significant association with YPC in MACS 3125/UC 1114 (R2 23.76%, P < 0.001) and Bijaga Yellow/Castelporziano (R2 36.90%, P < 0.001) RIL populations (Table 2). However, in both the populations, variation in YPC explained by microsatellite linked to Psy-B1 was not significant. When tested in a set of 222 durum wheat accessions, Psy-1SSR showed eight and seven alleles of microsatellite homoeologs linked to Psy-A1 and Psy-B1, respectively. Psy-A1SSRe was the most predominant allele among 222 accessions (frequency 86.9%); however, its frequency was still higher (97.4%) in advanced breeding lines in IDSN and IDYN from CIMMYT. In contrast, its frequency was only 24.2% in cultivars and local durums. Psy-A1SSRe was present in very few cultivars such as PDW 233, WH 896, DWR 1006, and HI 8627 that are selected or derived for durum lines from CIMMYT. Psy-A1SSRa, c, and d were observed in durum cultivars and local durums with frequencies 9.1%, 18.2%, and 27.3% respectively. Psy-A1SSRf, g, and h were observed in less cultivated tetraploid wheat. Among Psy-B1SSR alleles, d allele showed the highest occurrence (frequency 67.7%) followed by c allele (frequency 23.1%) in 222 accessions. However, since the overall frequency of Psy-A1SSRa, b, f, g, and h alleles was < 3%, these were not considered for regression analysis. Results from regression analysis with Psy-A1SSRc, d, and e alleles showed significant association (R2 8.75%, P < 0.001) between Psy-A1SSR and YPC in durum wheat accessions. Association of Psy-B1SSR with YPC was not significant (Table 2; Supplementary Fig. 3). The results were also confirmed by analysis of variance, where allelic variation at Psy-A1SSR showed significant association with YPC (Supplementary Table S2). Further, a post hoc multiple comparison test was applied to compare mean YPC values for alleles Psy-A1SSRc, d, and e. The significance of the difference between two alleles was tested by least significant difference test and also validated by applying Bonferroni adjustment method to eliminate significance by chance. The accessions with a predominant allele Psy-A1SSRe, showed significantly higher (P < 0.01) YPC (5.85 ppm) than the accessions with Psy-A1SSRc and d alleles (Table 3 and Supplementary Table S3). Mean YPC difference was not significant for allele c and d. Psy-A1SSRa, b, f, g, and h were observed as rare alleles with very low frequencies (< 3%) which do not permit a valid comparison for mean YPC of these alleles, hence could not be compared with other alleles for their association with the trait.
Allelic frequencies of Psy-A1SSR were compared with co-dominant markers for Psy-A1 reported earlier. Majority of the accessions with Psy-A1SSRa, d, and e allele showed Psy1-A1l allele with Psy1-A1_STS (Singh et al. 2009) and Psy1-A1c allele with YP7A-2 marker (He et al. 2009). Most of the accessions carrying Psy-A1SSRc and Psy-A1SSRf alleles showed Psy1-A1a and Psy1-A1o alleles, respectively, with Psy1-A1_STS and YP7A-2 markers. The least frequent alleles Psy-A1SSRg and h did not show any association with reported alleles of Psy-A1 gene. Linkage disequilibrium (LD) between Psy-A1SSR, Psy1-A1_STS, YP7A-2, and nearby SSR was estimated. It was observed that LD estimated as r2 was significant for pair-wise comparison among Psy-A1SSR, Psy1-A1_STS, YP7A-2, and gpw4050 (Supplementary Fig. 4).
Improvement of yellow pigment content by marker-assisted breeding
To test the utility of the marker for improvement in YPC content, Psy-A1SSRe allele from PDW 233 was introgressed in the background of two durum cultivars with low YPC, MACS 3125 (YPC 3.57 ppm; b* 9.5) and HI 8498 (YPC 3.26 ppm; b* 9.9) using backcross breeding approach and only foreground screening with Psy-A1SSR marker (Supplementary Fig. 1). MACS 3125 is a high yielding durum wheat cultivar while HI 8498 is a popular durum cultivar notified for Central Zone in India. All the BC1F1 plants carrying Psy-A1SSRe were compared for morphological traits with recipient parent. Since PDW233 flowers later than recipient parents, healthy plants with flowering time similar to recurrent parent were identified and back crossed with recurrent parent. Same procedure was followed up to BC3F1 (for MACS 3125 background) and BC2F1 (for HI 8498 background). In case of MACS 3125, out of 230 BC3F2 plants, 57 homozygous for Psy-A1SSRe allele were selected and 12 families were selected based on early flowering time. BC3F2:4 and BC3F2:5 families were grown in replicated trials in 2013–2014 and 2014–2015 along with recurrent parent and donor. Grains were harvested and YI (b* value) of these lines were measured using whole meal. These were compared with MACS 3125 for b* value using LSD at α = 0.05. All these lines were also evaluated for agronomic traits such as grain yield, thousand grain weight, spike length, spikelets per spike, grains per spike, and grain weight per spike. Based on 2-year data for agronomical traits and elevated YI, five best lines were identified (Table 4). YI (b* value) in these improved lines ranged from 11.5 to 11.8 with an average of 11.65 which was 22.6% higher than b* value of recurrent parent MACS 3125, whereas, grain yield and other agronomic traits were at par with MACS 3125. YPC in marker-assisted introgressed lines was confirmed by microestimation method as described in Santra et al. (2003). YPC in these lines increased significantly (6.16–7.7 ppm) and showed an average 88.9% increase over recurrent parent MACS 3125 (3.57 ppm).
In case of HI 8498, 30 BC2F2 plants homozygous for Psy-A1SSRe allele were selected out of 125 individuals and advanced into BC2F2:3 families. Out of these 30 families, nine healthy BC2F2:3 families with flowering time similar to recurrent parent were selected. These selected families were advanced up to BC2F2:6 stage and simultaneously tested for 3 years in a replicated trial in 2013–2014 (BC2F2:4), 2014–2015 (BC2F2:5), and 2015–2016 (BC2F2:6) along with recurrent parent and donor. Based on 3-year data for agronomical traits, elevated YI, best four lines were selected (Table 4). YI (b* value) in these selected lines ranged from 11.8 to 12.3 with an average of 11.96 which was 20.27% higher than HI 8498. YPC in marker-assisted introgressed lines was confirmed by microestimation method as described in Santra et al. (2003). YPC in these lines increased significantly (range 5.00 to 7.46 ppm) and showed an average 98% increase over recurrent parent HI 8498 (3.26 ppm). Grain yield and agronomic traits in these lines were at par with HI 8498. Selected lines in both the backgrounds showed improved YPC and comparable agronomic traits and grain yield, thus demonstrating utility of the marker for durum wheat end-use quality improvement.
Discussion
Variation at Psy-A1SSR is associated with yellow pigment content in tetraploid wheat
Several studies have reported involvement of Psy-A1 and Psy-B1 loci in determination of YPC and development of PCR-based markers for their selection in bread and durum wheat (Crawford et al. 2011; Ficco et al. 2014; He et al. 2008, 2009; Howitt et al. 2009; Pozniak et al. 2007; Ravel et al. 2013; Singh et al. 2009; Wang et al. 2009). However, most of the reported markers are specific to certain alleles or haplotypes of A, B, and D homoeologs of Psy-1, that results into increase in number of analyses for screening of breeding lines. Therefore, development of a robust marker that can be tagged to multiple homoeologs and multiple alleles, at the same time, is warranted to accelerate selection for desired homoeologs/alleles of Psy-1 in breeding program and also to reduce cost involved in marker analyses. In our earlier study, we had identified QTL QYp.macs-7A in RIL population developed from PDW 233/Bhalegaon 4 cross. Since the QTL falls in same region where Psy-A1 was reported in previous study (Pozniak et al. 2007), DNA sequences of exon and intron regions of Psy-A1 were analyzed in parents (Patil et al. 2008). Although PDW 233 and Bhalegaon 4 showed contrasting YPC, no sequence variation at Psy-A1 was observed between them and hence association of QTL QYp.macs-7A and Psy-A1 could not be established. Due to the sequence similarity between PDW 233 and Bhalegaon 4, Psy-A1-specific reported markers Psy1-A1_STS (Singh et al. 2009) and YP7A-2 (He et al. 2009) showed monomorphic Psy1-A1l and Psy1-A1c alleles, respectively, in both the parents; and could not be used to map Psy-A1 in the population. Monomorphic alleles for markers Psy1-A1_STS and YP7A-2 were also observed in parental genotypes of other two populations namely Castelporziano, Bijaga Yellow, MACS 3125, and UC 1114, although they showed contrasting YPC. Thus, same allele of Psy-A1 identified by Psy1-A1_STS and YP7A-2 seems to be contributing differently to YPC in PDW 233, Bhalegaon 4, Castelporziano, Bijaga Yellow, MACS 3125, and UC 1114. These observations also highlighted the need of robust marker that can be useful in diverse genetic backgrounds.
Here, we report microsatellite variation in the promoter region of both the homoeologs of Psy-1. Considering its tight linkage with Psy-1, this variation was used to develop functional marker which could be used for selection of various alleles of A as well as B homoeolog of Psy-1. The marker was mapped within QTL QYp.macs-7A, confirming its association with YPC in PDW 233/Bhalegaon 4 population (Supplementary Fig. 2). The marker was also validated for its association with variation in YPC in two more RIL populations (Bijaga Yellow/Castelporziano; MACS 3125/UC 1114) and 222 tetraploid wheat accessions using single marker regression analysis. Moreover, the PCR primer pair targeting the functional marker amplified both the homoeologs of microsatellite linked to Psy-A1 and Psy-B1 in single PCR reaction. Using this marker, eight alleles of Psy-A1SSR and seven alleles of Psy-B1SSR in tetraploid wheat accessions could be differentiated suggesting its usefulness in wide range of genetic backgrounds for selection of either or both the homoeologs of Psy-1 simultaneously. Recent reports suggest that transcript levels of Psy-1 homoeologs are associated with variation in YPC in wheat (Qin et al. 2016) and maize (Fu et al. 2013). Putative light response element (LRE), gibberellin response element (P Box), MeJA response element (MeJA), and abscisic acid response element (ABRE) were identified as potential cis-regulatory elements in the promoter region of Psy-A1 in durum wheat. The sequence variation in the promotor region was predicted to be associated with differential expression of Psy-1 homoeologs and thereby variation in YPC in the endosperm. Sequence alignment showed that the Psy-A1SSR identified in present study does not fall in any of these elements identified by Qin et al. (2016), though it is flanked by two LRE. Microsatellite variation at UTR is known to be associated with change in transcription level of many genes in human (Bagshaw 2017) and Arabidopsis (Zhang et al. 2006). Recently, repeat length variation in 5’ UTR of myo-inositol monophosphatase gene was found to be associated with seed size (Dwivedi et al. 2017) as well as phytic acid content and drought tolerance in chickpea (Joshi-Saha and Reddy 2015). Similarly, microsatellite-mediated enhanced expression of Tryptophan decarboxylase gene due to polymorphic (GA/CT)n repeat present in 5′UTR was reported in Catharanthus roseus (Kumar and Bhatia 2016). The microsatellite variation identified in the promoter of Psy-1 homoeologs in present study has provided a unique site for functional marker development, based on the several earlier reports its role as potential key regulatory element in modulation of the gene expression could be speculated. However, further experimentation would be necessary to prove the role of microsatellite variation in modulating Psy-1 expression.
In the present study, Psy-A1 showed association with YPC in three RIL populations as well as in 222 tetraploid wheat accessions. The accessions of less cultivated species of tetraploid wheat, viz. T. carthlicum and T. polonicum, with Psy-A1SSRf, g, and h alleles showed high YPC and can be used as donors for improvement of YPC in durum wheat. Singh et al. (2014) has reported that gene for YPC derived from wild wheat Lophopyrum ponticum improved YPC by 24%, but lowered thousand grain weight, test weight, and grain hardness in durum wheat. Therefore, one should be cautious about undesirable linkage drag, if any, associated with these alleles present in the less cultivated tetraploid species while using them in breeding program. Although Psy-A1 was associated with YPC, Psy-B1 did not explain variation in YPC in any of the plant material used in present study including CIMMYT durum wheat lines. Similar results were reported by He et al. (2009) in CIMMYT spring wheat lines, where no significant difference in YPC was observed among the CIMMYT lines carrying four different alleles of Psy-B1. In a recent study, Campos et al. (2016) also reported that variation in Psy-A1 alone exhibit significant difference in yellowness in Mediterranean landraces and modern cultivars. These results suggested that selection of desirable allele of Psy-A1 alone is sufficient to improve YPC in durum wheat. However, the other homoeolog Psy-B1 is also important when the donor is from different genetic background such as Kofa (Pozniak et al. 2007; Zhang and Dubcovsky 2008), Chinese winter wheat (He et al. 2009) and bread wheat Apache (Ravel et al. 2013), where Psy-B1 has been associated with variation in YPC.
Psy-A1SSR marker tags a major QTL for YPC having Psy-A1 as the candidate gene. Besides Psy-A1, another QTL for YPC was mapped at about 40 cM proximal to Psy-A1 on chromosome 7A in durum wheat (Blanco et al. 2011; Elouafi et al. 2001; Zhang and Dubcovsky 2008). Similarly, a marker-trait association (MTA) affecting YPC was identified at 181 Mbp away from Psy-A1 in a genome-wide association study (Zhai et al. 2018). Short arm of chromosome 7A also showed presence of QTL as well as MTA affecting YPC in bread and durum wheat (N’Diaye et al. 2017; Roncallo et al. 2012; Zhai et al. 2016a). All these reports suggest existence of three candidate genes affecting YPC on chromosome 7A. Selection for these QTL and MTA may lead to further additive improvement in YPC in durum wheat breeding lines.
Deployment of Psy-A1SSR in marker-assisted breeding for improved YPC
Marker-assisted breeding has contributed significantly in improvement of traits governed by single major gene (Randhawa et al. 2013; William et al. 2007) such as plant height, resistance to wheat rusts caused by Puccinia species, and resistance to insects, particularly, Russian wheat aphid, Hessian fly, and wheat midge. Similarly, some complex polygenic traits like grain protein content and gluten strength that determine end-use quality of wheat as well as resistance to Fusarium head blight and spot blotch have also been successfully improved in wheat by MAB for major gene/QTL contributing significantly to the target trait (de Bustos et al. 2001; Tyagi et al. 2014; Vasistha et al. 2016; Vishwakarma et al. 2016; William et al. 2007). In the present study, improvement in a polygenic trait YPC was demonstrated by Psy-A1SSR-assisted introgression of Psy-A1 in two cultivars MACS 3125 and HI 8498 using PDW 233 as a donor. Marker-assisted foreground selection for Psy-A1SSRe allele coupled with selection based on morphological and agronomic traits at the later stages resulted into development of elite durum lines with YPC significantly higher than recurrent parents MACS 3125 and HI 8498.
In many durum wheat breeding programs, selection for elevated yellow pigment content is practiced in advanced breeding lines in later generations by estimation of whole meal color and semolina color. However, the use of PCR markers will be useful for precise selection in early generations and can be used instead of trait evaluation, thus saving time and resources required for analyses. Both MACS 3125 and HI 8498 are popular durum wheat cultivars but low YPC limits their pasta-making potential. Marker-assisted introgression of superior Psy-A1SSRe allele from PDW 233 resulted into improvement of YPC in both the backgrounds. The improved lines showed significantly higher YPC and b* values without altering other agronomic traits and grain yield potential in both the backgrounds.
Conclusion
We have developed a robust DNA-based marker that can simultaneously tag A and B homoeologs of Psy-1 gene associated with YPC in durum wheat. The marker was also validated for its association with YPC in two RIL populations and a set of 222 tetraploid wheat accessions. The utility of newly developed marker in marker-assisted breeding program to improve YPC in two durum wheat cultivars MACS 3125 and HI 8498 was also demonstrated. The results show that with MAB, value addition of elite cultivars for specific traits such as end use quality is possible. Use of marker Psy-A1SSR in breeding will be useful to enhance YPC in durum wheat cultivars and can result in the release of better quality durum wheat from future breeding programs.
References
Bagshaw ATM (2017) Functional mechanisms of microsatellite DNA in eukaryotic genomes. Genome Biol Evol 9:2428–2443
Blanco A, Colasuonno P, Gadaleta A, Mangini G, Schiavulli A, Simeone R, Digesù AM, De Vita P, Mastrangelo AM, Cattivelli L (2011) Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. J Cereal Sci 54:255–264
Campos KM, Royo C, Schulthess A, Villegas D, Matus I, Ammar K, Schwember AR (2016) Association of phytoene synthase Psy1-A1 and Psy1-B1 allelic variants with semolina yellowness in durum wheat (Triticumturgidum L. var. durum). Euphytica 207:109–117
Clarke JM, Marchylo BA, Kovacs MIP, Noll JS, McCaig TN, Howes NK (1998) Breeding durum wheat for pasta quality in Canada. Euphytica 100:163–170
Colasuonno P, Gadaleta A, Giancaspro A, Nigro D, Giove S, Incerti O, Mangini G, Signorile A, Simeone R, Blanco A (2014) Development of a high-density SNP-based linkage map and detection of yellow pigment content QTLs in durum wheat. Mol Breed 34:1563–1578
Colasuonno P, Lozito ML, Marcotuli I, Nigro D, Giancaspro A, Mangini G, De Vita P, Mastrangelo AM, Pecchioni N, Houston K, Simeone R, Gadaleta A, Blanco A (2017) The carotenoid biosynthetic and catabolic genes in wheat and their association with yellow pigments. BMC Genomics 18:122
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Crawford AC, Stefanova K, Lambe W, McLean R, Wilson R, Barclay I, Francki MG (2011) Functional relationships of phytoene synthase 1 alleles on chromosome 7A controlling flour colour variation in selected Australian wheat genotypes. Theor Appl Genet 123:95–108
de Bustos A, Rubio P, Soler C, García P, Jouve N (2001) Marker assisted selection to improve HMW-Glutenins in wheat. In: Bedö Z, Láng L (eds) Wheat in a global environment. Developments in plant breeding, vol 9. Springer, Dordrecht, pp 171–176
Di Fonzo N, Ravaglia S, DeAmbrogio E, Blanco A, Troccoli A (2005) Durum wheat improvement in Italy. In: Royo C, Nachit MM, Di Fonzo N, Araus JL, Pfeiffer WH, Slafer GA (eds) Durum Wheat Breeding: current approaches and future strategies, vol 1 and 2. The Haworth Press Inc, Binghamton, pp 825–881
Dibari B, Murat F, Chosson A, Gautier V, Poncet C, Lecomte P, Mercier I, Bergès H, Pont C, Blanco A, Salse J (2012) Deciphering the genomic structure, function and evolution of carotenogenesis related phytoene synthases in grasses. BMC Genomics 13:221
Dick JW, Youngs VL (1988) Evaluation of durum wheat, semolina, and pasta in the United States. In: Fabriani G, Lintas C (eds) Durum: Chemistry and Technology. American Association of Cereal Chemists, St. Paul, pp 237–248
Dwivedi V, Parida SK, Chattopadhyay D (2017) A repeat length variation in myo-inositol monophosphatase gene contributes to seed size trait in chickpea. Sci Rep 7:4764
Elouafi I, Nachit MM, Martin LM (2001) Identification of a microsatellite on chromosome 7B showing a strong linkage with yellow pigment in durum wheat (Triticum turgidum L. var. durum). Hereditas 135:255–261
Ficco DBM, Mastrangelo AM, Trono D, Borrelli GM, Vita PD, Fares C, Beleggia R, Platani C, Papa R (2014) The colours of durum wheat: a review. Crop Pasture Sci 65:1–15
Flowerika AA, Kumar J, Thakur N, Pandey A, Pandey AK, Upadhyay SK, Tiwari S (2016) Characterization and expression analysis of Phytoene Synthase from bread wheat (Triticum aestivum L.). PLoS One 11(10):e0162443
Fratianni A, Irano M, Panfili G, Acquistucci R (2005) Estimation of color of durum wheat, comparison of WSB, HPLC, and reflectance colorimeter measurements. J Agric Food Chem 53:2373–2378
Fu Z, Chai Y, Zhou Y, Yang X, Warburton ML, Xu S, Cai Y, Zhang D, Li J, Yan J (2013) Natural variation in the sequence of PSY1 and frequency of favorable polymorphisms among tropical and temperate maize germplasm. Theor Appl Genet 126:923–935
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
He XY, Zhang YL, He ZH, Wu YP, Xiao YG, Ma CX, Xia XC (2008) Characterization of Phytoene synthase 1 gene (Psy1) located on common wheat chromosome 7A and development of a functional marker. Theor Appl Genet 116:213–221
He XY, He ZH, Ma W, Appels R, Xia XC (2009) Allelic variants of phytoene synthase 1 (Psy1) genes in Chinese and CIMMYT wheat cultivars and development of functional markers for flour colour. Mol Breed 23:553–563
Hentschel V, Kranl K, Hollmann J, Lindhauer MG, Böhm V, Bitsch R (2002) Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. J Agric Food Chem 50:6663–6668
Howitt C, Cavanagh C, Bowerman A, Cazzonelli C, Rampling L, Mimica J, Pogson B (2009) Alternative splicing, activation of cryptic exons and amino acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm. Funct Integr Genomics 9:363–376
Joshi-Saha A, Reddy KS (2015) Repeat length variation in the 5′UTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.). J Exp Bot 66:5683–5690
Kumar S, Bhatia S (2016) A polymorphic (GA/CT)n - SSR influences promoter activity of Tryptophan decarboxylase gene in Catharanthus roseus L. Don. Sci Rep 6:33280
Kumar J, Jaiswal V, Kumar A, Kumar N, Mir RR, Kumar S, Dhaliwal R, Tyagi S, Khandelwal M, Prabhu KV, Prasad R, Balyan HS, Gupta PK (2011) Introgression of a major gene for high grain protein content in some Indian bread wheat cultivars. Field Crop Res 123:226–233
Li F, Vallabhaneni R, Wurtzel ET (2008) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress – induced root carotenogenesis. Plant Physiol 146:1333–1345
Mares DJ, Campbell AW (2001) Mapping components of flour and noodle colour in Australian wheat. Aust J Agric Res 52:1297–1309
N’Diaye A, Haile JK, Cory AT, Clarke FR, Clarke JM, Knox RE, Pozniak CJ (2017) Single marker and haplotype-based association analysis of semolina and pasta colour in elite durum wheat breeding lines using a high-density consensus map. PLoS One 12(1):e0170941
Panfili G, Fratianni A, Irano M (2004) Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J Agric Food Chem 52:6373–6377
Parker GD, Chalmers KJ, Rathjen AJ, Langridge P (1998) Mapping loci associated with flour colour in wheat (Triticum aestivum L.). Theor Appl Genet 97:238–245
Patil RM, Oak MD, Tamhankar SA, Sourdille P, Rao VS (2008) Mapping and validation of a major QTL for yellow pigment content on 7AL in durum wheat (Triticum turgidum L. ssp. durum). Mol Breed 21:485–496
Pfeiffer WH, Payne TS (2005) CIMMYT durum wheat improvement program. In: Royo C, Nachit MM, Di Fonzo N, Araus JL, Pfeiffer WH, Slafer GA (eds) Durum Wheat Breeding: current approaches and future strategies, vol 1 and 2. The Haworth Press Inc, Binghamton, pp 1031–1048
Pozniak CJ, Knox RE, Clarke FR, Clarke JM (2007) Identification of QTL and association of a phytoene synthase gene with endosperm colour in durum wheat. Theor Appl Genet 114:525–537
Qin X, Fischer K, Dubcovsky J, Tian L (2016) Endosperm carotenoid concentrations in wheat are better correlated with PSY1 transcript levels than enzyme activities. Crop Sci 56:3173–3184
Randhawa HS, Asif M, Pozniak C, Clarke JM, Graf RJ, Fox SL, Humphreys DG, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Hucl P, Spaner D (2013) Application of molecular markers to wheat breeding in Canada. Plant Breed 132:458–471
Ravel C, Dardevet M, Leenhardt F, Bordes J, Joseph JL, Perretant MR, Exbrayat F, Poncet C, Balfourier F, Chanliaud E, Charmet G (2013) Improving the yellow pigment content of bread wheat flour by selecting the three homoeologous copies of Psy1. Mol Breed 31:87–99
Roncallo PF, Cervigni GL, Jensen C, Miranda R, Carrera AD, Helguera M, Echenique V (2012) QTL analysis of main and epistatic effects for flour color traits in durum wheat. Euphytica 185:77–92
Santra M, Rao VS, Tamhankar SA (2003) Modification of AACC procedure for measuring β-carotene in early generation durum wheat. Cereal Chem 80:130–131
Santra M, Rao VS, Tamhankar SA (2006) Biochemical estimation of beta carotene in Indian durum varieties. Wheat Inf Serv 102:1–4 http://www.shigen.nig.ac.jp/ewis
Singh A, Reimer S, Pozniak CJ, Clarke FR, Clarke JM, Knox RE, Singh K (2009) Allelic variation at Psy1-A1 and association with yellow pigment in durum wheat grain. Theor Appl Genet 118:1539–1548
Singh G, Saini JS, Bains NS, Singh RP (2014) Positive influence of Lophopyrum ponticum derived Y gene on yellow pigment content - a major durum wheat quality trait. Indian J Genet Plant Breed 74:651–655
Symons SJ, Dexter JE (1991) Computer analysis of fluorescence for the measurement of flour refinement as determined by flour ash content, flour grade colour, and tristimulus colour measurements. Cereal Chem 68:454–460
Troccoli A, Borrelli GM, De Vita P, Fares C, Di Fonzo N (2000) Durum wheat quality: a multidisciplinary concept. J Cereal Sci 32:99–113
Tyagi S, Mir RR, Kaur H, Chhuneja P, Ramesh B, Balyan HS, Gupta PK (2014) Marker-assisted pyramiding of eight QTLs/genes for seven different traits in common wheat (Triticum aestivum L.). Mol Breed 34:167–175
Vasistha NK, Balasubramaniam A, Mishra VK, Chand R, Srinivasa J, Yadav PS, Joshi AK (2016) Enhancing spot blotch resistance in wheat by marker-aided backcross breeding. Euphytica 207:119–133
Vishwakarma MK, Arun B, Mishra VK, Yadav PS, Kumar H, Joshi AK (2016) Marker-assisted improvement of grain protein content and grain weight in Indian bread wheat. Euphytica 208:313–321
Wang JW, He XY, He ZH, Wang H, Xia XC (2009) Cloning and phylogenetic analysis of phytoene synthase 1 (Psy1) genes in common wheat and related species. Hereditas 146:208–256
William HM, Trethowan R, Crosby-Galvan EM (2007) Wheat breeding assisted by markers: CIMMYT’s experience. Euphytica 157:307–319
Zhai S, He Z, Wen W, Jin H, Liu J, Zhang Y, Liu Z, Xia X (2016a) Genome-wide linkage mapping of flour color-related traits and polyphenol oxidase activity in common wheat. Theor Appl Genet 129:377–394
Zhai S, Li G, Sun Y, Song J, Li J, Song G, Li Y, Ling H, He Z, Xia X (2016b) Genetic analysis of phytoene synthase 1 (Psy1) gene function and regulation in common wheat. BMC Plant Biol 16:228
Zhai SN, Xia XC, He ZH (2016c) Carotenoids in staple cereals: metabolism, regulation, and genetic manipulation. Front Plant Sci 7:1197
Zhai S, Liu J, Xu D, Wen W, Yan J, Zhang P, Wan Y, Cao S, Hao Y, Xia X, Ma W, He Z (2018) A genome-wide association study reveals a rich genetic architecture of flour color-related traits in bread wheat. Front Plant Sci 9:1136
Zhang W, Dubcovsky J (2008) Association between allelic variation at the Phytoene synthase 1 gene and yellow pigment content in the wheat grain. Theor Appl Genet 116:635–645
Zhang L, Zuo K, Zhang F, Cao Y, Wang J, Zhang Y, Sun X, Tang K (2006) Conservation of noncoding microsatellites in plants: implication for gene regulation. BMC Genomics 7:323
Zhang W, Chao S, Manthey F, Chicaiza O, Brevis JC, Echenique V, Dubcovsky J (2008) QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theor Appl Genet 117:1361–1377
Funding
This study was financially supported by the Department of Biotechnology, Ministry of Science & Technology, Govt. of India, vide Grant No. BT/PR11695/AGR/02/643/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, R., Oak, M., Deshpande, A. et al. Development of a robust marker for Psy-1 homoeologs and its application in improvement of yellow pigment content in durum wheat. Mol Breeding 38, 136 (2018). https://doi.org/10.1007/s11032-018-0895-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-018-0895-x