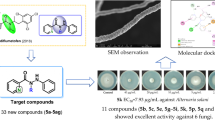
Abstract
To discover novel and effective potential agricultural antifungal agents, various kinds of imidazo[1,2-a]quinoxaline derivatives were designed, and synthesized from available and inexpensive reagents. Their antifungal activities were first evaluated against ten typical phytopathogenic fungi. The in vitro antifungal activity showed that some compounds exhibited more obvious broad-spectrum fungicidal activity than the two commercially-available fungicides chlorothalonil and hymexazol. Valsa mali and Botrytis cinerea strains exhibited the highest susceptibility with EC50 values of 1.4–27.0 μg/mL to more than ten compounds. Compounds 5c and 5f showed the most promising inhibitory effects against Valsa mali (EC50 = 5.6 μg/mL) and Fusarium solani (EC50 = 5.1 μg/mL), respectively. Preliminary studies on the mechanism of action indicated that the imidazo[1,2-a]quinoxaline skeleton likely exerted its antifungal effects by disrupting hyphal differentiation, spore germination, and germ tube growth. Moreover, the cell experiment results indicated that these target compounds possessed good safety to BV2 cells. Overall, compounds 5c and 5f can be considered candidate compounds against specific fungi for further detailed research. This study can provide a theoretical basis for the application of imidazo[1,2-a]quinoxaline scaffolds as novel fungicides in agriculture.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant diseases caused by phytopathogenic fungi are the primary factor causing global agricultural losses, which can seriously affect the normal growth, transportation, and storage of grains, vegetables, and fruits [1]. Taking the filamentous fungi Botrytis cinerea and Fusarium graminearum as examples, Botrytis cinerea is the major fungal species that triggers gray mold, which can infect over 200 plant species and cause considerable yield loss during agricultural production [2]. Fusarium graminearum is commonly found on wheat, barley, and corn, infected kernels appear shrunken and have white-to-pink colored mold, which leads to a reduction in grain yield and nutritional value by producing various mycotoxins [3]. Currently, the application of chemical fungicides has given a satisfactory effect on the prevention and control of these devastating diseases. Nevertheless, the long-term overuse and misuse of a single chemical fungicide are responsible for the disadvantages of resistance, residue, and resurgence (“3R” problems) [4]. Therefore, it is urgent to search for small molecules with high efficiency and low toxicity to expand the selection range of fungicides.
Nitrogen-containing heterocyclic compounds are currently considered a promising framework for the development of new pesticides [5, 6], especially compounds containing quinoxaline rings that exhibit various biological properties including antitumor, antimalarial, antiviral, antibacterial, and anti-inflammatory [7, 8]. Meanwhile, imidazoquinoxaline has attracted much attention owing to its striking antitumor [9,10,11,12], antiepileptic (LU 73068) [13], and antiallergic (Dazoquinast) activity [14], while there are rare reports on the utilization of the imidazoquinoxaline backbone as a pesticide. Therefore, to explore the application value of the imidazolequinoxaline skeleton in the prevention and control of plant diseases, this paper adopts homologous derivative and molecular hybridization strategy to design and synthesize various kinds of imidazo[1,2-a]quinoxaline derivatives via available and inexpensive reagents, and their antifungal properties against ten phytopathogenic fungi of agricultural relevance were evaluated firstly (Fig. 1). Finally, the antifungal mechanism of the compounds with excellent inhibitory activity was preliminarily explored by observing the mycelial morphology changes and spore germination via electron microscopy.
Results and discussion
Chemistry
Initially, the construction method of the imidazo[1,2-a]quinoxaline skeleton is shown in Scheme 1. 2,3-dichloroquinoxaline (1a) was reacted with 2,2-dimethoxyethanamine, prop-2-yn-1-amine or ammonium hydroxide in different solvents to obtain the respective 2-amino-3-chloroquinoxaline (2c) and its derivatives (2a and 2b), followed by treatment of compounds 2a and 2b with strong acids (HBr and H2SO4) to afford cyclization products 3a and 3b, which were converted to key intermediate compounds 4a, b via chlorination with POCl3 [15]. Meanwhile, compound 4d can be obtained directly by condensation of compound 2c with ethyl 3-bromo-2-oxopropanoate, but the resulting yield was relatively low (15.2%) [14]; the preparation procedure of compound 4c includes reduction, cyclization, hydrolysis, decarboxylation and chlorination five synthetic steps [16]. Chlorinated (4e) and brominated (4f) derivatives were synthesized by reacting intermediate 4a with N-chlorosuccinimide (NCS) and N-bromosuccinimide (NBS), respectively [17].
To investigate the effect of substituents on the antifungal activity, the B ring of imidazo[1,2-a]quinoxalines (4a–c) was modified by the introduction of the corresponding amine, ether, sulfonamide, and hydrazine functionalities as depicted in Schemes 2, 3. Compounds 5a–c and 6a–c (by-products) were obtained by ammonolysis of intermediate 4a–c with NH3–CH3OH at 100 °C, and methylamino (5d–f), dimethylamino (5g–i), and tryptamino (5j–l) substituted compounds were also formed under similar conditions (100/80 °C, tube sealing). Compounds 5m–o were prepared by replacing the chlorine atom of compounds 4a–c with morpholine. In addition, etherification of compounds 4a–c with various alcohols or phenols under basic conditions afforded ether derivatives 6d–i. Finally, as depicted in Scheme 3, direct coupling of compounds 4a, b with various sulfonamides in Pd(OAc)2/xantphos/Cs2CO3/1,4-dioxane system at 100 °C gave compounds 7a–d. Compound 4a was hydrolyzed with 85% hydrazine hydrate and subsequently acylated with acyl chloride to afford 8a, b. Spectroscopic data (HRMS, 1H NMR, and 13C NMR) of the target compounds were consistent with their structures.
Antifungal activity
Ten phytopathogenic fungi, including Fusarium solani (FS), Fusarium oxysporum (FO), Botryosphaeria dothidea (BD), Fusarium graminearum (FG), Sclerotinia sclerotiorum (SS), Valsa mali (VM), Alternaria alternata (AA), Pyricularia oryzae (PO), Alternaria brassicae (AB), and Botrytis cinerea (BC), that frequently occur in the Chinese agroecosystem were selected as the test strains. The in vitro antifungal activities of the target compounds were investigated through the classical mycelium linear growth rate method [18]. Hymexazol (Hym) and chlorothalonil (Chl), two commercial fungicides served as positive controls.
The results of the preliminary antifungal activity of all the target compounds are summarized in Table 1. The obtained data revealed that the synthesized compounds displayed good to excellent antifungal activity at 50 μg/mL. Among them, eight compounds (including compounds 4b, 4c, 4e, 5c, 5f, 6e, 6 h, and 7d) showed satisfactory antifungal effects against F. solani with inhibition rates of > 80%, which was superior to the positive controls hymexazol (63.9%) and chlorothalonil (76.6%); eleven compounds exhibited better antifungal activity (> 50%) against F. oxysporum than hymexazol (46.2%), especially compounds 4e (73.9%), 5c (84.1%), and 7d (72.1%), which gave higher activity than chlorothalonil (69.2%). Toward B. dothidea, compounds 4e (81.9%), 5a (98.4%), 6 g (91.9%), and 7d (89.6%) exerted more promising antifungal effects than the two commercial fungicides. For F. graminearum, seven compounds (4b, 4c, 4e, 5c, 5f, 5i, and 7c) displayed slightly higher antifungal activity (75.8–86.7%) than chlorothalonil (70.7%) and hymexazol (63.8%). Regretfully, only five compounds 4e, 5c, 5i, 7c, and 7d possessed comparable antifungal activities (67.9–74.5%) to hymexazol (65.6%) against A. alternata, and no noticeable inhibitory efficacies were observed for all compounds toward S. sclerotiorum, P. oryzae, and A. brassicae in comparison with the positive control hymexazol. However, it is worth mentioning that eighteen compounds demonstrated satisfactory inhibitory effects (> 60%) against V. mali, and thirteen compounds revealed better antifungal activity (> 80%) against B. cinerea than hymexazol (72.7%), especially the inhibition rates of compounds 4a, 4e, 5i, 6a, 6c, 6d, and 6f reached over 90%, which could almost completely suppress the growth of mycelium. Furthermore, the preliminary structure–activity relationship of these compounds is summarized in Fig. 2.
Inspired by the preliminary antifungal activity results, the median effective concentration (EC50) values of some selected compounds were further determined at six different concentrations (50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL). As displayed in Fig. 3, many compounds exhibited obvious inhibitory effects against the eight tested fungi. For instance, compound 5f (5.1 μg/mL) exhibited the best antifungal activity against F. solani; compounds 4c, 7c, and 7d had anti-A. brassicae EC50 values of 15.4, 18.9, and 19.7 μg/mL, respectively, superior to hymexazol (26.6 μg/mL) and chlorothalonil (> 50 μg/mL); the EC50 range values of compounds 4b, 4e, 5a, 6 g, 7c, and 7d against B. dothidea were 10.3–24.9 μg/mL, which was better than hymexazol (> 50 μg/mL) but lower than chlorothalonil (7.9 μg/mL); the inhibitory effects of 4e (28.8 μg/mL) and 7c (23.7 μg/mL) on P. oryzae were equivalent to that of hymexazol (24.9 μg/mL). For A. alternata, eight compounds possessed more pronounced antifungal activity than chlorothalonil, especially compound 7c (11.2 μg/mL), which was better than hymexazol (16.7 μg/mL). Regarding the F. graminearum strain, seven compounds exhibited higher activity than hymexazol, but failed to exceed chlorothalonil. Furthermore, it is fortunate that twelve compounds demonstrated more promising potential in controlling V. mali, especially compounds 5c and 5f with EC50 values lower than 6.0 μg/mL; thirteen compounds (1.4–15.2 μg/mL) displayed obvious antifungal activity against B. cinerea, particularly compounds 4a, 4e, 6a, 6c, and 6d exhibited 3.0–8.5 folds more potent activities than hymexazol. In addition, the concentration-dependent suppression of the mycelial growth of F.solani, V.mali, and B. cinerea by compounds 5f or 6a could also be clearly observed in Fig. 4.
Effects of compounds on mycelial growth and spore germination [19]
To elucidate the preliminary mechanism of the antifungal activity of these compounds, light microscopy was used to investigate the influence of compound 5c (6.25 μg/mL) on the hyphal growth and spore germination of V. mali (VM) fungi. As shown in Fig. 5, in the blank control, the mycelium had a smooth surface, much-branched and abundant attachment of spindle-shaped spores. In contrast, the mycelium of the compound 5c treatment group appeared obvious shrinkage and no spore formation. Furthermore, inhibiting spore germination is an important means to prevent fungal regeneration and infection in plants. Figure 6 shows that the spore germination and germ tube elongation were both significantly suppressed in the presence of compound 5c at different concentrations, and the inhibition rates at concentrations of 25, 12.5, and 6.25 μg/mL were 94.4%, 70.3%, and 54.1%, respectively. This phenomenon demonstrated that compound 5c likely exerted antifungal effects by disrupting hyphal differentiation, spore germination, and germ tube growth.
Cell cytotoxicity
Finally, the cell cytotoxicity of the six compounds (4a, 4e, 5c, 6a, 6c, and 6d) with promising antifungal activity against mouse microglia (BV2) cells was further investigated in vitro using the CCK-8 assay [20, 21]. From Fig. 7, we can see that the cell viability of the tested compounds was more than 82.7% on BV2 cells at the high concentration of 100 μg/mL, and the current results suggested that the tested compounds showed low toxicities.
Conclusions
In summary, thirty-six imidazo[1,2-a]quinoxaline derivatives were synthesized and evaluated for their fungicidal activity against ten common phytopathogenic fungi. The results showed that some compounds exhibited more excellent and broad-spectrum fungicidal activity in vitro than the positive controls chlorothalonil and hymexazol, particularly V. mali and B. cinerea strains exhibited the highest susceptibility with an EC50 values of 1.4–27.0 μg/mL for more than ten compounds. Among them, compounds 5c and 5f displayed the most promising antifungal activity against V. mali and F. solani, with an EC50 values of 5.6 and 5.1 μg/mL, respectively, which can be considered as the potential candidate compounds for controlling specific fungi. SAR analysis showed that the type of substituents on the imidazo[1,2-a]quinoxaline skeleton significantly effects the antifungal activity. Preliminary studies on the mechanism of action indicated that these compounds likely exerted their antifungal effects by disrupting hyphal differentiation, spore germination, and germ tube growth. Moreover, the cell experiment results indicated that the significantly bioactive compounds possessed good safety to BV2 cells. It is worth pointing out that this is the first report on the application of an imidazo[1,2-a]quinoxaline skeleton as agricultural antifungal agent, and further studies on the structural optimization and target exploration are still underway in our laboratory. Overall, our findings may provide a theoretical basis for the future utilization of imidazo[1,2-a]quinoxaline scaffolds as novel fungicides in agriculture.
Experimental
All starting materials were obtained from commercial sources and used without further purification. Melting points were determined by the X-4 digital display micro melting point apparatus (Beijing Tech Instrument Co., Ltd). 1H NMR and 13C NMR spectra were recorded on Bruker Avance NEO 600 MHz and 150 MHz instruments, respectively, using TMS as the internal standard and CDCl3 or DMSO-d6 as the solvent. High-resolution mass spectra (HRMS) were carried out with an APEX II Bruker 4.7 T AS instrument.
Synthesis
See the “Supporting information” section for the synthetic methods of the target compounds.
Antifungal activity and spore germination assay
Antifungal activity assay [18]
The target compounds were screened in vitro for their antifungal activity against ten phytopathogenic fungi (Fusarium solani, Fusarium oxysporum, Botryosphaeria dothidea, Fusarium graminearum, Sclerotinia sclerotiorum, Valsa mali, Alternaria alternata, Pyricularia oryzae, Alternaria brassicae, and Botrytis cinerea) by using the mycelial growth rate method. Potato dextrose agar (PDA) medium was prepared in the flasks and sterilized. The target compounds were dissolved in DMSO before mixing with PDA, and the concentration of test compounds in the medium was fixed at 50 μg/mL. The medium was then poured into sterilized Petri dishes. The mycelia disks (4 mm) were inoculated in the center of the Petri dishes (three replicates for each treatment) and incubated at 27 ± 1 °C for 4 days. DMSO without any compounds mixed with PDA served as a control (the final concentration of DMSO < 0.5%). Hymexazol and chlorothalonil were used as positive controls. The radial growth of the fungal colonies were measured and the data were statistically analyzed. The inhibitory rate was calculated by the following formula: inhibition rate (%) = (C–T) × 100/(C–4 mm), where C represents the diameter of fungi growth on untreated PDA, and T represents the diameter of fungi on treated PDA. Finally, the linear regressions of inhibition rates (%) versus seven concentrations of some selected compounds, were obtained, and the EC50 values were calculated. Statistical analyses of the data were performed with GraphPad Prism 5.0.
Spore germination assay [19]
The V. mali was retrieved from the storage tube and cultured for 2 weeks at 27.5 °C on potato dextrose agar (PDA, Difco). Plates were then flooded with sterile distilled water, and conidia were scraped with a glass stick. Mycelial debris was removed by filtration through double-layer cheesecloth, and the spores were harvested and suspended in sterile distilled water containing 0.1% (v/v) Tween 20. Spores were counted using a hemocytometer and adjusted to 1.0 × 106 spores/mL.
Three concentrations (6.25 µg/mL, 12.5 µg/mL, and 25 µg/mL) of compound 5c and the control (0.5% DMSO) were separately tested for spore germination of V. mali. The samples were inoculated with spore suspension of V. mali containing 1.0 × 106 spores/mL. Aliquots of 10 μL of prepared spore suspension were placed on 96-hole plate in six copies. 96-hole plate containing the spores was incubated in a moisture chamber at 25 °C for 48 h. Each hole was then observed under the microscope for spore germination. The spore-generated germ tubes were enumerated, and the percentage of spore germination was calculated.
Cytotoxicity activity [21]
The cytotoxicity of the target compounds was detected by Cell Counting CCK-8 kit (CCK-8 assay). CCK-8 was based on the water-soluble tetrazolium salt WST8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-dinitrophenyl)-2H-tetrazole monosodium salt). The BV2 cells were seeded at a density of 1.5 × 104 cells per well in the 96-hole plate and incubated at 37 °C in an atmosphere of 5% CO2 for 24 h. After incubation, different concentrations of the target compounds were added and incubated for 24 h. 10 μL of CCK-8 reagent was added to each well and incubated for 1 h in the dark. The absorbance at 450 nm was measured by microplate reader. The untreated group was considered as the control. The data were analyzed by GraphPad Prism 5.0.
Supporting information
Spectral images of 1H-NMR, 13C-NMR and HRMS are provided in the Supporting Information Section.
References
Salvatore MM, Andolfi A (2021) Phytopathogenic fungi and toxicity. Toxins 13(10):13689. https://doi.org/10.3390/toxins13100689
Williamson B, Tudzynski B, Tudzynski P, van Kan JA (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8(5):561–580. https://doi.org/10.1111/j.1364-3703.2007.00417.x
Imboden L, Afton D, Trail F (2018) Surface interactions of Fusarium graminearum on barley. Mol Plant Pathol 19(6):1332–1342. https://doi.org/10.1111/mpp.12616
Zhang XK, Li BQ, Zhang ZQ, Chen Y, Tian SP (2020) Antagonistic yeasts: a promising alternative to chemical fungicides for controlling postharvest decay of fruit. J Fungi 6(3):158. https://doi.org/10.3390/jof6030158
Fan LL, Luo ZF, Yang CF, Guo B, Miao J, Chen Y, Tang L, Li Y (2022) Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi. Mol Divers 26(2):981–992. https://doi.org/10.1007/s11030-021-10213-7
Fan LL, Luo ZF, Li Y, Liu XY, Fan JD, Xue W, Tang L, Li Y (2020) Synthesis and antifungal activity of imidazo[1,2-b]pyridazine derivatives against phytopathogenic fungi. Bioorg Med Chem Lett 30(14):127139. https://doi.org/10.1016/j.bmcl.2020.127139
Tariq S, Somakala K, Amir M (2018) Quinoxaline: an insight into the recent pharmacological advances. Eur J Med Chem 143:542–557. https://doi.org/10.1016/j.ejmech.2017.11.064
Suthar SK, Chundawat NS, Singh GP, Padrón JM, Jhala YK (2022) Quinoxaline: a comprehension of current pharmacological advancement in medicinal chemistry. Eur J Med Chem Rep 5:100040. https://doi.org/10.1016/j.ejmcr.2022.100040
Goel KK, Hussain A, Altamimi MA, Rajput SK, Sharma PP, Kharb R, Mahdi WA, Imam SS, Alshehri S, Alnemer OA, Chaudhary A (2023) Identification of potential antitubulin agents with anticancer assets from a series of imidazo[1,2-a]quinoxaline derivatives: in silico and in vitro approaches. Molecules 28(2):802. https://doi.org/10.3390/molecules28020802
Goel KK, Rajput SK, Kumar A, Nandi NK, Joshi G, Kharb R (2022) Imidazoquinoxaline as a privileged fused pharmacophore in anticancer drug development: a review of synthetic strategies and medicinal aspects. ChemistrySelect 7(37):e202200834. https://doi.org/10.1002/slct.202200834
Patinote C, Deleuze-Masquefa C, Kaddour KH, Vincent LA, Larive R, Zghaib Z, Guichou JF, Assaf MD, Cuq P, Bonnet PA (2021) Imidazo[1,2-a]quinoxalines for melanoma treatment with original mechanism of action. Eur J Med Chem 212:113031. https://doi.org/10.1016/j.ejmech.2020.113031
Skayneh H, Jishi B, Hleihel R, Hamie M, El Hajj R, Deleuze-Masquefa C, Bonnet PA, El Sabban M, El Hajj H (2022) EAPB0503, an imidazoquinoxaline derivative modulates SENP3/ARF mediated SUMOylation, and induces NPM1c degradation in NPM1 mutant AML. Int J Mol Sci 23(7):3421. https://doi.org/10.3390/ijms23073421
Potschka H, Löscher W, Wlaź P, Behl B, Hofmann HP, Treiber HJ, Szabo L (1998) LU 73068, a new non-NMDA and glycine/NMDA receptor antagonist: pharmacological characterization and comparison with NBQX and L-701, 324 in the kindling model of epilepsy. Br J Pharmacol 125(6):1258–1266. https://doi.org/10.1038/sj.bjp.0702172
Ager IR, Barnes AC, Danswan GW, Hairsine PW, Kay DP, Kennewell PD, Matharu SS, Miller P, Robson P, Westwood R (1988) Synthesis and oral antiallergic activity of carboxylic acids derived from imidazo[2,1-c][1,4]benzoxazines, imidazo[1,2-a]quinolines, imidazo[1,2-a]quinoxalines, imidazo[1,2-a]quinoxalinones, pyrrolo[1,2-a]quinoxalinones, pyrrolo[2,3-a]quinoxalinones, and imidazo[2,1-b]benzothiazoles. J Med Chem 31(6):1098–1115. https://doi.org/10.1021/jm00401a009
Ceccarelli S, D’Alessandro A, Prinzivalli M, Zanarella S (1998) Imidazo[1,2-a]quinoxalin-4-amines: a novel class of nonxanthine A1-adenosine receptor antagonists. Eur J Med Chem 33(12):943–955. https://doi.org/10.1016/S0223-5234(99)80019-1
Mamedov VA, Kalinin AA (2014) Advances in the synthesis of imidazo[1,5-a]-and imidazo[1,2-a]quinoxalines. Russ Chem Rev 83(9):820–847. https://doi.org/10.1070/RC2014v083n09ABEH004424
Chouchou A, Patinote C, Cuq P, Bonnet PA, Deleuze-Masquéfa C (2018) Imidazo[1,2-a]quinoxalines derivatives grafted with amino acids: synthesis and evaluation on A375 melanoma cells. Molecules 23(11):2987. https://doi.org/10.3390/molecules23112987
Li Y, Luo ZF, Luo BL, Lan Q, Fan JD, Xue W, Miao J, Li Y, Tang L, Fan LL (2020) Design, synthesis and antifungal activities of 6-substituted 3-butylphthalide derivatives against phytopathogenic fungi. Chem Biodivers 17:e2000435. https://doi.org/10.1002/cbdv.202000435
Li SK, Ji ZQ, Zhang JW, Guo ZY, Wu WJ (2010) Synthesis of 1-acyl-3-isopropenylbenzimidazolone derivatives and their activity against Botrytis cinerea. Eur J Med Chem 58(5):2668–2672. https://doi.org/10.1021/jf903855y
Nallathamby N, Phan CW, Sova M, Saso L, Sabaratnam V (2021) Synthesized 2-trifluoromethylquinazolines and quinazolinones protect BV2 and N2a cells against LPS- and H2O2-induced cytotoxicity. Med Chem 17(6):623–629. https://doi.org/10.2174/1573406416666191218095635
Cai L, Qin XJ, Xu ZH, Song YY, Jiang HJ, Wu Y, Ruan HJ, Chen J (2019) Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 4(7):12036–12042. https://doi.org/10.1021/acsomega.9b01142
Acknowledgements
This research was supported by The National Natural Science Foundation of China (No. 32060627 and No. 22207021), The State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Ministry of Education, Guizhou University (No. 2021GDP0101, QianjiaoheKYzi[2022]362, and QianjiaoheKYzi[2020]250), Special Research Project of Rural Economic Revitalization and Agricultural Industry Technology Project of Guizhou Medical University (No. 26222190618).
Author information
Authors and Affiliations
Contributions
Lingling Fan, Yong Li, Bing Guo and Lei Tang designed the experiments; Taigui Ma and Xu Zhong synthesized the target compounds and analyzed the data; Judi Fan, Ya Yang and Wenjing Liu are in charge of bioactivity and cytotoxicity tests; Lingling Fan and Yong Li wrote the paper. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, T., Zhong, X., Yang, Y. et al. Synthesis and evaluation of imidazo[1,2-a]quinoxaline derivatives as potential antifungal agents against phytopathogenic fungi. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10739-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10739-y