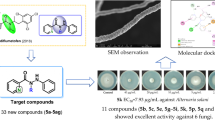
Abstract
A series of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives were designed, synthesized and evaluated for their antifungal activities against Fusarium graminearum (Fg), Rhizoctonia solani (Rs), Botrytis cinerea (Bc) and Colletotrichum capsici (Cc). The bioassay results in vitro showed that most of the title compounds exhibited impressive antifungal activities against the above plant fungi. Particularly, the compounds 5c, 5f, 5g, 5i, 5m and 5p displayed desirable anti-Rs activities, with the corresponding EC50 values of 0.37, 0.32, 0.49, 0.50, 0.46 and 0.45 µg/mL, respectively, which are superior to the positive control carbendazim (0.55 µg/mL). Further in vivo bioassay results showed that the anti-Rs activity of title compound 5f at 200 µg/mL reached 95.84% on detached rice leaves and 93.96% on rice plants. Featuring convenient synthesis, novel structures and desirable antifungal activity, these 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives could be further studied as the potential candidates of novel agricultural fungicides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant pathogenic fungi are destructive parasitic organisms that easily generate numerous spores to infect various economic crops and are extremely difficult to control in agriculture [1]. In addition, fungal infections tend to produce various toxins in infected plants, thereby reducing the quality and output of commercial crops [2]. Nowadays, the rational utilization of agricultural fungicides is still a realistic and principal measure to effectively control the fungal infections in agriculture [3]. Unfortunately, some problems related the long-term application of traditional fungicides in agriculture, such as negative impacts on the environment [4], pernicious effects against non-target species [5] and ceaseless evolutions of fungal resistances [6], gradually attract great attentions and vigilances. Therefore, developing novel and highly efficient fungicides is still an urgent demand in agriculture [7].
1,3,5-Thiadiazine-2-thione derivatives have attracted enormous attentions from chemists and biologists due to their various bioactivities, such as anticancer [8], anti-bacterial [9], anti-epileptic [10], antifungal [11], anti-leishmanial [12], antimalarial [13], antioxidant [14], anti-tubercular [15], trypanocidal [16] and herbicidal [17] properties. Recently, pharmacokinetic studies demonstrated that 1,3,5-thiadiazine-2-thione derivatives feature high lipid solubility and desirable enzymatic hydrolyzation that facilitate their absorption and bioavailability within organisms [18, 19]. Besides its extensive applications in pharmaceutical developments, these nitrogenous heterocycles also have important development values in agricultural chemistry. As important examples of 1,3,5-thiadiazine-2-thione derivatives, milneb (Fig. 1a) and dazoment (Fig. 1b) have been developed as the agricultural fumigants that are widely used to protect fruit trees, vegetables and ornamental plants [9, 20, 21].
Hydrazide is widely researched as the nitrogenous configuration that exists in many impressive molecules with anticancer [22], anticoagulant [23], anti-inflammatory [24], antimalarial [25], anti-bacterial [26], antifungal [27], anti-viral [28], insecticidal [29] and herbicidal [30] properties. As representative compounds bearing a hydrazide group, tebufenozide (Fig. 1c) and chromafenozide (Fig. 1d) were launched as commercial insecticides that target the nonsteroidal ecdysone of agricultural pests [31]. Subsequently, the studies on structural optimizations of hydrazide derivatives documented that introducing an arylhydrazide fragment into a heterocycle nucleus (e.g., pyrazole [32], 1,2,3-triazole [33] and coumarin [34]) could greatly enhance and broaden their inhibition effects against plant fungi. Inspired by the excellent biochemical characteristics of hydrazide substructure, our previous work investigated the antifungal effects of quinazolin-4(3H)-one derivatives bearing a hydrazide moiety and found that a N′-phenylacethydrazide fragment in molecular structures played a key role in maintaining their biological activity [35].
Aiming to continue our previous works on searching for hydrazide derivatives as novel agricultural fungicides, we theorized that introducing a 1,3,5-thiadiazine-2-thione group into N′-phenylacethydrazide scaffolds might generate novel lead molecules with better physicochemical properties. Thus, a series of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives were designed (Fig. 2), synthesized (Scheme 1) and evaluated for their in vitro and in vivo antifungal activities against Fusarium graminearum (Fg), Rhizoctonia solani (Rs), Botrytis cinerea (Bc) and Colletotrichum capsici (Cc) in this work. To the best of our knowledge, it is the first report on the synthesis and antifungal activity of 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives.
Results and discussion
Chemistry
The synthetic route to title compounds was shown in Scheme 1. Methylamine 1 was firstly reacted with carbon disulfide in sodium hydroxide solution at room temperature for 4 h to produce a turbid liquid. After filtration by filter paper, the filtrate containing the intermediate 2 was directly reacted with formaldehyde at room temperature for 2 h to obtain a clear liquid. Then, the obtained solution containing the intermediate 3 was reacted with glycine or (S)-alanine in phosphate buffer (pH 7.8) at room temperature for 2 h and was subsequently acidified with hydrochloric acid until the pH value reached 2.0 to generate a substituted 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)acetic acid 4. An intermediate 4 was reacted with substituted phenylhydrazine to produce a corresponding 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivative 5 (Scheme 1).
Spectral characteristic of title compounds
The structures of obtained title compounds were confirmed by FTIR, 19F NMR, 1H NMR, 13C NMR and HRMS spectra. In IR spectra, the obvious signals at 3368–3200 cm−1 and 1692–1655 cm−1 are, respectively, attributed to the stretching vibrations of the NH and C=O fragments. In 19F NMR spectra, the obvious singlets at − 133.24 ppm, (− 126.35)–(− 126.45) ppm and − 59.30 ppm confirm, respectively, the presence of a 2-F atom, a 4-F atom and a 4-CF3 group at the benzene ring of phenylhydrazine configurations. In 1H NMR spectra, two signal peaks at 10.18–9.83 ppm and 8.40–7.36 ppm confirm the presence of CONHNH fragments. The other two signal peaks at 4.71–4.45 ppm are assigned to the CH2 protons at the 4- and 6-positions of thiadiazine ring. In 13C NMR spectra, two singlets at 191.15–190.41 ppm and 172.06–168.09 ppm are, respectively, assigned to the characteristic peaks of C=O and C=S groups. The 13C NMR spectra also show multiple signal peaks at 71.94–51.57 ppm, which confirm the existence of CH2 fragments in the molecular structures of title compounds. Furthermore, in HRMS spectra of title compounds, the obvious absorption signals of [M + H]+ or [M + Na]+ ions are consistent with the corresponding molecular weights.
X-ray crystal structure of compound 5k
Aiming to further understand the structural characteristics of title compounds, the structure of compound 5k was studied as a representative example by single-crystal X-ray analysis. The evaporation of dimethyl formamide containing the compound 5k took place slowly at room temperature to crystallize a yellow single crystal that was suitable for X-ray diffraction analyses. The crystal diffraction data collected by the reported methods in our previous works [36, 37] are presented in Table 1. The corresponding crystal structure diagram and crystal packing diagram are shown in Figs. 3 and 4, respectively. Figure 3 shows that the intramolecular hydrogen bond N2–H2···N3 combines with the C9 and C10 atoms to form a latent penta-heterocycle. The above latent penta-heterocycle unites a benzene ring and a 1,3,5-thiadiazine configuration to construct the structural skeleton of 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives. As shown in Fig. 4, the three-dimensional structure of title compound 5k was constructed by three intermolecular hydrogen bonds C13–H13B···S2, C13–H13A···O1 and N1–H1D···O1.
In vitro antifungal activities of title compounds
The antifungal effects of title compounds against plant fungi Fg, Bc, Rs and Cc were tested by a mycelial growth rate method [3, 32,33,34,35,36,37,38,39,40,41], and the obtained EC50 values are summarized in Table 2. Under the same conditions, the agricultural fungicides carbendazim, penthiopyrad and azoxystrobin were used as the positive controls for evaluating the antifungal effects of title compounds. As shown in Table 2, the title compounds 5a, 5c, 5f, 5o, 5p, 5q and 5r exhibited desirable anti-Fg activities, with the corresponding EC50 values of 3.63, 2.30, 3.77, 1.63, 2.43, 3.47 and 3.23 µg/mL, respectively. Meanwhile, Table 2 also exhibits that the title compounds 5a, 5c, 5e, 5f, 5i, 5j, 5o, 5p and 5q obviously inhibited the mycelian growth of Bc in vitro, with the EC50 values of 3.88, 1.37, 2.13, 1.02, 1.59, 3.69, 1.72, 1.50 and 2.08 µg/mL, respectively. In addition, Table 2 shows that the title compounds 5b, 5c, 5d, 5e, 5g, 5h, 5m, 5o and 5p had obvious anti-Cc effects, with the corresponding EC50 values of 1.74, 3.92, 1.73, 3.05, 1.21, 0.80, 0.76, 2.72 and 2.58 µg/mL, respectively. Strikingly, the EC50 values of title compounds 5c, 5f, 5g, 5i, 5m and 5p against Rs reached 0.37, 0.32, 0.49, 0.50, 0.46 and 0.45 µg/mL, respectively, which are better than that of carbendazim (0.55 µg/mL).
In vivo anti-Rs activity of title compound 5f
Table 2 indicates that 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives show impressive antifungal activities against Fg, Bc, Rs and Cc. Strikingly, the EC50 value of compound 5f reached 0.32 µg/mL against Rs, which is obviously superior to that of carbendazim (0.55 µg/mL). Aiming to further understand the antifungal activities of title compounds, the in vivo anti-Rs effects of title compound 5f on detached rice leaves and on rice plants were tested by the reported methods [42]. Five days after inoculating a mycelian cake on detached leaves, Table 3 and Fig. 5 show that the title compound 5f obviously inhibited the mycelian growth of Rs with the inhibition rates of 31.81% at 100 µg/mL, 61.34% at 150 µg/mL and 95.84% at 200 µg/mL, respectively. As shown in Table 4 and Fig. 6, the title compound 5f also has significant anti-Rs effects on rice plants at 100 µg/mL and 200 µg/mL, with control effects of 56.32% and 93.96%, respectively. The above research results indicated that 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives can serve as the potential lead structures in searching for novel agricultural fungicides.
Structure–activity relationships
The results of antifungal assays in Table 2 showed that the structural variations of 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivative have greatly affected their biological activity against agricultural fungi. Based on the antifungal effects of title compounds in Table 2, some structure–activity relationships were analyzed and concluded as below. First, the anti-Rs effects of all title compounds are better than their antifungal effects against Fg, Bc and Cc. Second, when the R1 substituent group was substituted with a methyl, the corresponding target compounds overall exhibited better anti-Fg and anti-Cc effects than those compounds bearing a H atom at the R1 position. For instance, the antifungal effects of title compounds 5o, 5p and 5q (R1 = Me; R2 = 4-F, 4-Cl and 4-Br) were 1.63, 2.43 and 3.47 µg/mL against Fg and 2.72, 2.58 and 4.24 µg/mL against Cc, which are better than that of title compounds 5c, 5f and 5i (R1 = H; R2 = 4-F, 4-Cl and 4-Br) against Fg (2.30, 3.77 and 4.22 µg/mL) and Cc (3.92, 5.14 and 4.51 µg/mL). Third, introducing a methyl at the R1 position is adverse for the antifungal activity of title compounds against Rs and Bc. For example, title compounds bearing a H atom (5a, 5c, 5f, 5i and 5j) have better EC50 values against Bc and Rs than those compounds containing a Me group (5n, 5o, 5p, 5q and 5r) at the R1 position. Fourth, a presence of 4-F, 4-Cl and 4-Br groups at the R2 position plays a pivotal role in improving the antifungal activities against Fg, Bc and Rs. Table 2 shows that the EC50 values against Fg, Bc and Rs of title compounds bearing a 4-F (5c and 5o), 4-Cl (5f and 5p) or 4-Br (5i and 5q) group at R2 position are obviously better than that of compounds containing a H (5a and 5n), 2-F (5b), 2-Cl (5d), 3-Cl (5e), 2,4-di-Cl (5g), 2,4,6-tri-Cl (5h), 4-Me (5j and 5r), 3,4-di-Me (5k), 4-OMe (5 l) or 4-CF3 (5m) group.
Conclusions
A series of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives were designed, synthesized and well confirmed by FTIR, 19F NMR, 1H NMR, 13C NMR, HRMS and single-crystal X-ray diffraction analyses. The in vitro bioassay results showed that most of the title compounds exhibited impressive antifungal activities against Fg, Rs, Bc and Cc. Strikingly, the EC50 values of compounds 5c, 5f, 5g, 5i, 5m and 5p against Rs, respectively, reached 0.37, 0.32, 0.49, 0.50, 0.46 and 0.45 µg/mL, which are superior to that of carbendazim (0.55 µg/mL). Meanwhile, the in vivo bioassay studies on the anti-Rs activity of title compound 5f indicated that these 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazides could be served as potential candidates of novel agricultural fungicides. The present works will lay a significant foundation for the development of novel agricultural fungicides bearing a 1,3,5-thiadiazine or acethydrazide fragment. Further antifungal mechanism and structural modification of 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazides are currently underway.
Methods and materials
General methods
Melting points (m.p.) of synthetic compounds were determined by an uncorrected SMP50 Automatic Melting Point Apparatus (Bibby Scientific LTD, Staffordshire, UK). Infrared spectra (IR) were recorded on a Thermo Nicolet 380 FTIR spectrometer (Thermo Nicolet Corporation, America) in a KBr disk. Using DMSO-d6 as the solvent, 1H, 13C and 19F nuclear magnetic resonance spectra (NMR) were acquired by a BRUKER 400 NMR spectrometer (Bruker Corporation, Germany) at room temperature. Multiplicities of NMR signals were expressed by the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet. Mass spectral studies were performed on a JMS-AX505HA high-resolution mass spectrometer (JEOL, Japan). The thin-layer chromatography (TLC) on silica gel GF254 was used to monitor reactions under ultraviolet light at 254 nm. All reagents and reactants were purchased from commercial suppliers and were analytical reagent grade or chemically pure.
General synthetic procedure for key intermediates 4a and 4b
The intermediates 4a and 4b were synthesized according to the synthetic method reported in the literature [43] with small modification. A mixture containing carbon disulfide (10 mmol), methylamine 1 (10 mmol) and potassium hydroxide (10 mmol) in distilled water (40 mmol) was stirred at room temperature for 4 h, and the resulting reaction mixture was then filtered through filter paper. The filtrate containing an intermediate 2 was added into formaldehyde solution (37%, 20 mmol) and subsequently stirred at room temperature for 2 h. After that, the obtained mixture containing an intermediate 3 was slowly added into a stirred solution of glycine or (S)-alanine (10 mmol) in phosphate buffer (pH 7.8, 20 mL). After stirring for 6 h at room temperature, the reaction mixture was acidified with 10% hydrochloric acid until the pH value reached 2.0 to generate a white precipitate. The precipitate was filtered and washed with cool diethyl ether to obtain an intermediate 4. The structures of intermediates 4a and 4b were confirmed by 1H NMR and HRMS spectra.
2-(5-Methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acetic acid (4a). White solid; m.p. 129.1–130.4 °C; yield 67.2%; 1H NMR (400 MHz, DMSO-d6) δ: 12.71 (s, 1H, COOH), 4.54 (s, 2H, thiadiazine-4-2H), 4.52 (s, 2H, thiadiazine-6-2H), 3.57 (s, 2H, CH2), 3.36 (d, J = 6.5 Hz, 3H, CH3); HRMS calcd for C6H10N2NaO2S2 [M + Na]+ 229.0076, found 229.0054.
(S)-2-(5-Methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanoic acid (4b). White solid; m.p. 125.1–125.9 °C; yield 68.9%; 1H NMR (400 MHz, DMSO-d6) δ: 12.81 (s, 1H, COOH), 4.64 (dd, J = 18.6, 13.3 Hz, 2H, thiadiazine-4-2H), 4.55–4.43 (m, 2H, thiadiazine-6-2H), 3.62 (q, J = 7.0 Hz, 1H, CH), 3.36 (d, J = 3.9 Hz, 3H, CH3), 1.33 (d, J = 7.0 Hz, 3H, CH3); HRMS calcd for C7H12N2NaO2S2 [M + Na]+ 243.0232, found 243.0219.
General synthetic procedure for title compounds
A mixture of an intermediate 4 (2.00 mmol), O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluroniumtetrafluoroborate (TBTU, 2.40 mmol) and NEt3 (6.00 mmol) in dichloromethane (30 mL) was stirred at room temperature for 15 min. Then, a substituted phenylhydrazine (3.00 mmol) dissolved in dichloromethane (10 mL) was added in the resulting mixture, and the obtained solution was stirred for 2 h under room temperature. After that, the white precipitate was filtered and washed with dichloromethane to produce a target compound 5 with a good yield. The structures of obtained title compounds 5a–5r were confirmed by FTIR, 19F NMR, 1H NMR, 13C NMR and HRMS spectra.
N′-Phenyl-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5a). White solid; m.p. 176.2–177.5 °C; yield 64.5%; IR (KBr, cm−1) ν: 3360, 3279, 1672, 1597, 1494, 1473, 1352, 1324, 1198, 1100, 976, 890; 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CONH), 7.75 (d, J = 2.5 Hz, 1H, NH), 7.14 (t, J = 7.7 Hz, 2H, Ar-3,5-2H), 6.72 (q, J = 6.4 Hz, 3H, Ar-1,4,6-3H), 4.55 (s, 4H, thiadiazine-4,6-4H), 3.56 (s, 2H, CH2), 3.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.57, 168.61, 149.59, 129.20, 119.06, 112.64, 71.89, 58.90, 52.27, 40.41; HRMS calcd for C12H16N4NaOS2 [M + Na]+ 319.0658, found 319.0656.
N′-(2-Fluorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5b). White solid; m.p. 165.0–166.5 °C; yield 73.1%; IR (KBr, cm−1) ν: 3362, 3277, 2971, 1676, 1506, 1487, 1470, 1447, 1321, 1194, 1101, 745; 1H NMR (400 MHz, DMSO-d6) δ: 9.95 (s, 1H, CONH), 7.66 (s, 1H, NH), 7.11–7.03 (m, 1H, Ar-3-H), 6.99 (t, J = 7.7 Hz, 1H, Ar-5-H), 6.82–6.69 (m, 2H, Ar-4,6-2H), 4.54 (s, 4H, thiadiazine-4,6-4H), 3.57 (s, 2H, CH2), 3.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.54, 168.72, 151.78, 149.41, 137.19, 137.08, 125.02, 119.27, 119.20, 115.41, 115.23, 114.07, 114.04, 71.82, 58.87, 52.26, 40.38; 19F NMR (376 MHz, DMSO-d6) δ: − 133.24; HRMS calcd for C12H16FN4OS2 [M + H]+ 315.0744, found 315.0714.
N′-(4-Fluorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5c). White solid; m.p. 165.8–166.7 °C; yield 85.6%; IR (KBr, cm−1) ν: 3364, 3283, 1674, 1502, 1466, 1349, 1322, 1196, 1094, 980, 831; 1H NMR (400 MHz, DMSO-d6) δ: 9.91 (d, J = 2.1 Hz, 1H, CONH), 7.73 (d, J = 2.8 Hz, 1H, NH), 6.98 (t, J = 8.8 Hz, 2H, Ar-3,5-2H), 6.76–6.68 (m, 2H, Ar-2,6-2H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.55 (s, 2H, CH2), 3.39 (d, J = 7.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.61, 168.70, 157.51, 155.20, 146.19, 115.77, 115.55, 113.94, 113.86, 71.94, 58.94, 52.33, 40.42; 19F NMR (376 MHz, DMSO-d6) δ: − 126.45; HRMS calcd for C12H16FN4OS2 [M + H]+ 315.0744, found 315.0744.
N′-(2-Chlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5d). White solid; m.p. 170.1–171.4 °C; yield 80.3%; IR (KBr, cm−1) ν: 3280, 2992, 2901, 1667, 1593, 1515, 1476, 1341, 1314, 1199, 1104, 958, 893, 780; 1H NMR (400 MHz, DMSO-d6) δ: 10.04 (s, 1H, CONH), 7.44 (s, 1H, NH), 7.29 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.16 (t, J = 7.7 Hz, 1H, Ar-5-H), 6.76 (dd, J = 13.4, 7.6 Hz, 2H, Ar-4,6-2H), 4.55 (s, 4H, thiadiazine-4,6-4H), 3.59 (s, 2H, CH2), 3.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.51, 168.65, 144.94, 129.61, 128.23, 120.05, 117.64, 113.40, 71.80, 58.89, 52.35, 40.44; HRMS calcd for C12H16ClN4OS2 [M + H]+ 331.0449, found 331.0451.
N′-(3-Chlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5e). White solid; m.p. 161.1–162.4 °C; yield 75.5%; IR (KBr, cm−1) ν: 3368, 3262, 2987, 2903, 1681, 1601, 1511, 1476, 1353, 1324, 1200, 1100, 893, 842, 776; 1H NMR (400 MHz, DMSO-d6) δ: 9.95 (s, 1H, CONH), 8.06 (d, J = 1.9 Hz, 1H, NH), 7.15 (t, J = 8.0 Hz, 1H, Ar-5-H), 6.75–6.63 (m, 3H, Ar-2,4,6-3H), 4.55 (s, 4H, thiadiazine-4,6-4H), 3.58 (s, 2H, CH2), 3.39 (d, J = 6.3 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.55, 168.73, 151.18, 133.98, 130.86, 118.49, 111.88, 111.28, 71.88, 58.90, 52.23, 40.42; HRMS calcd for C12H16ClN4OS2 [M + H]+ 331.0449, found 331.0450.
N′-(4-Chlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5f). White solid; m.p. 166.3–167.6 °C; yield 65.4%; IR (KBr, cm−1) ν: 3366, 3275, 2977, 1909, 1674, 1487, 1465, 1350, 1321, 1095, 893, 827, 811, 703; 1H NMR (400 MHz, DMSO-d6) δ: 9.93 (d, J = 2.4 Hz, 1H, CONH), 7.94 (d, J = 2.2 Hz, 1H, NH), 7.17 (d, J = 8.8 Hz, 2H, Ar-3,5-2H), 6.71 (d, J = 8.8 Hz, 2H, Ar-2,6-2H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.56 (s, 2H, CH2), 3.39 (d, J = 4.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.56, 168.69, 148.55, 128.96, 122.34, 114.14, 71.90, 58.92, 52.29, 40.42; HRMS calcd for C12H16ClN4OS2 [M + H]+ 331.0449, found 331.0437.
N′-(2,4-Dichlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5g). White solid; m.p. 168.2–169.1 °C; yield 93.2%; IR (KBr, cm−1) ν: 3274, 2978, 2900, 1678, 1518, 1476, 1417, 1198, 1097, 889, 801, 743; 1H NMR (400 MHz, DMSO-d6) δ: 10.07 (s, 1H, CONH), 7.65 (s, 1H, NH), 7.43 (d, J = 2.1 Hz, 1H, Ar-3-H), 7.22 (dd, J = 8.8, 2.2 Hz, 1H, Ar-5-H), 6.77 (d, J = 8.8 Hz, 1H, Ar-6-H), 4.54 (d, J = 9.2 Hz, 4H, thiadiazine-4,6-4H), 3.58 (s, 2H, CH2), 3.40 (d, J = 5.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.49, 168.73, 144.20, 128.89, 128.17, 122.44, 118.03, 114.44, 71.79, 58.85, 52.31, 40.42; HRMS calcd for C12H14Cl2N4NaOS2 [M + Na]+ 386.9878, found 386.9939.
N′-(2,4,6-Trichlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5h). White solid; m.p. 159.5–160.8 °C; yield 52.9%; IR (KBr, cm−1) ν: 3336, 3266, 2978, 2897, 1663, 1505, 1478, 1324, 1315, 1201, 1099, 877, 769; 1H NMR (400 MHz, DMSO-d6) δ: 10.18 (d, J = 1.9 Hz, 1H, CONH), 7.50 (s, 2H, Ar-3,5-2H), 7.36 (d, J = 1.8 Hz, 1H, NH), 4.49 (d, J = 3.1 Hz, 4H, thiadiazine-4,6-4H), 3.48 (s, 2H, CH2), 3.35 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.41, 168.09, 141.16, 128.97, 125.39, 124.43, 71.74, 58.77, 51.57, 40.36; HRMS calcd for C12H13Cl3N4NaOS2 [M + Na]+ 420.9489, found 420.9486.
N′-(4-Bromophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5i). White solid; m.p. 161.9–162.8 °C; yield 74.3%; IR (KBr, cm−1) ν: 3360, 3272, 2970, 2908, 1672, 1506, 1465, 1350, 1316, 1199, 1099, 894, 806; 1H NMR (400 MHz, DMSO-d6) δ: 9.94 (s, 1H, CONH), 7.96 (d, J = 1.9 Hz, 1H, NH), 7.29 (d, J = 8.6 Hz, 2H, Ar-3,5-2H), 6.67 (d, J = 8.7 Hz, 2H, Ar-2,6-2H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.56 (s, 2H, CH2), 3.38 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.55, 168.68, 148.95, 131.80, 114.65, 109.86, 71.90, 58.92, 52.28, 40.43; HRMS calcd for C12H15BrN4NaOS2 [M + Na]+ 396.9763, found 396.9798.
N′-(4-Methylphenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5j). White solid; m.p. 164.1–165.4 °C; yield 90.5%; IR (KBr, cm−1) ν: 3362, 3274, 2970, 2901, 1672, 1509, 1465, 1350, 1319, 1200, 1097, 979, 953, 892, 813; 1H NMR (400 MHz, DMSO-d6) δ: 9.85 (d, J = 2.2 Hz, 1H, CONH), 7.57 (d, J = 2.6 Hz, 1H, NH), 6.95 (d, J = 8.1 Hz, 2H, Ar-3,5-2H), 6.62 (d, J = 8.1 Hz, 2H, Ar-2,6-2H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.54 (s, 2H, CH2), 3.38 (s, 3H, CH3), 2.17 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.57, 168.52, 147.30, 129.60, 127.70, 112.90, 71.86, 58.91, 52.27, 40.41, 20.65; HRMS calcd for C13H18N4NaOS2 [M + Na]+ 333.0814, found 333.0812.
N′-(3,4-Dimethylphenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5k). White solid; m.p. 176.5–177.6 °C; yield 58.2%; IR (KBr, cm−1) ν: 3343, 3286, 2970, 1901, 1671, 1512, 1350, 1317, 1200, 1102, 974, 949, 888, 814; 1H NMR (400 MHz, DMSO-d6) δ: 9.83 (d, J = 3.0 Hz, 1H, CONH), 7.48 (d, J = 3.0 Hz, 1H, NH), 6.89 (d, J = 8.1 Hz, 1H, Ar-5-H), 6.52 (s, 1H, Ar-2-H), 6.45 (d, J = 8.0 Hz, 1H, Ar-6-H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.54 (s, 2H, CH2), 3.38 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.09 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.56, 168.48, 147.64, 136.64, 130.13, 126.52, 114.31, 110.31, 71.83, 58.86, 52.21, 20.21, 18.97; HRMS calcd for C14H20N4NaOS2 [M + Na]+ 347.0971, found 347.0968.
N′-(4-Methoxyphenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5 l). White solid; m.p. 167.3–168.4 °C; yield 57.1%; IR (KBr, cm−1) ν: 3356, 3275, 2979, 2903, 1672, 1505, 1475, 1320, 1239, 1097, 891, 827, 725; 1H NMR (400 MHz, DMSO-d6) δ: 9.86 (d, J = 3.3 Hz, 1H, CONH), 7.42 (d, J = 3.4 Hz, 1H, NH), 6.76 (d, J = 8.9 Hz, 2H, Ar-2,6-2H), 6.68 (d, J = 8.9 Hz, 2H, Ar-3,5-2H), 4.53 (s, 4H, thiadiazine-4,6-4H), 3.66 (s, 3H, CH3), 3.53 (s, 2H, CH2), 3.38 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.56, 168.51, 153.14, 143.44, 114.67, 114.15, 71.87, 58.89, 55.73, 52.29, 40.40; HRMS calcd for C13H19N4O2S2 [M + Na]+ 327.0944, found 327.0940.
N′-(4-(Trifluoromethyl)phenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acethydrazide (5m). White solid; m.p. 177.2–178.6 °C; yield 53.6%; IR (KBr, cm−1) ν: 3356, 3275, 2979, 1672, 1505, 1475, 1350, 1320, 1237, 1193, 1097, 827; 1H NMR (400 MHz, DMSO-d6) δ: 10.05 (s, 1H, CONH), 8.40 (s, 1H, NH), 7.47 (d, J = 8.6 Hz, 2H, Ar-3,5-2H), 6.81 (d, J = 8.5 Hz, 2H, Ar-2,6-2H), 4.55 (s, 4H, thiadiazine-4,6-4H), 3.58 (s, 2H, CH2), 3.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 190.56, 168.78, 152.72, 129.51, 126.82, 126.69, 126.65, 126.62, 126.58, 124.13, 121.45, 119.19, 118.87, 118.56, 118.24, 111.95, 71.91, 58.89, 52.26, 40.41; 19F NMR (376 MHz, DMSO-d6) δ: − 59.30; HRMS calcd for C13H16F3N4OS2 [M + H]+ 365.0712, found 365.0707.
(S)-N′-Phenyl-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanohydrazide (5n). White solid; m.p. 151.2–152.6 °C; yield 47.6%; IR (KBr, cm−1) ν: 3246, 3224, 2986, 2892, 1692, 1505, 1329, 1206, 1094, 943, 893, 832, 696; 1H NMR (400 MHz, DMSO-d6) δ: 10.04 (s, 1H, CONH), 7.75 (s, 1H, NH), 7.14 (t, J = 7.6 Hz, 2H, Ar-3,5-2H), 6.71 (t, J = 9.0 Hz, 3H, Ar-2,4,6-3H), 4.71–4.61 (m, 2H, thiadiazine-4-2H), 4.46 (dd, J = 13.2, 5.6 Hz, 2H, thiadiazine-6-2H), 3.68 (q, J = 6.6 Hz, 1H, CH), 3.39 (s, 3H, CH3), 1.34 (d, J = 6.7 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 191.15, 172.00, 149.70, 129.26, 119.12, 112.60, 69.00, 56.81, 56.18, 40.33, 16.86; HRMS calcd for C13H18N4NaOS2 [M + H]+ 333.0814, found 333.0820.
(S)-N′-(4-Fluorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanohydrazide (5o). White solid; m.p. 184.3–185.6 °C; yield 45.9%; IR (KBr, cm−1) ν: 3329, 3200, 2978, 1678, 1536, 1483, 1325, 1317, 1229, 1134, 1100, 1076, 867; 1H NMR (400 MHz, DMSO-d6) δ: 10.05 (d, J = 3.1 Hz, 1H, CONH), 7.74 (d, J = 3.1 Hz, 1H, NH), 7.03–6.95 (m, 2H, Ar-3,5-2H), 6.72–6.67 (m, 2H, Ar-2,6-2H), 4.70–4.60 (m, 2H, thiadiazine-4-2H), 4.45 (dd, J = 13.3, 3.9 Hz, 2H, thiadiazine-6-2H), 3.66 (q, J = 6.7 Hz, 1H, CH), 3.38 (s, 3H, CH3), 1.33 (d, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 191.14, 172.02, 157.50, 155.18, 146.25, 146.24, 115.82, 115.60, 113.85, 113.77, 68.97, 56.82, 56.20, 40.32, 16.81; 19F NMR (376 MHz, DMSO-d6) δ: − 126.35; HRMS calcd for C13H17FN4NaOS2 [M + Na]+ 351.0720, found 351.0723.
(S)-N′-(4-Chlorophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanohydrazide (5p). White solid; m.p. 186.5–187.8 °C; yield 94.5%; IR (KBr, cm−1) ν: 3224, 2967, 2898, 1658, 1485, 1328, 1312, 1211, 1098, 1079, 961, 891, 826, 701; 1H NMR (400 MHz, DMSO-d6) δ: 10.08 (d, J = 2.7 Hz, 1H, CONH), 7.95 (d, J = 2.6 Hz, 1H, NH), 7.18 (d, J = 8.8 Hz, 2H, Ar-3,5-2H), 6.69 (d, J = 8.8 Hz, 2H, Ar-1,6-2H), 4.66 (t, J = 12.4 Hz, 2H, thiadiazine-4-2H), 4.50–4.42 (m, 2H, thiadiazine-6-2H), 3.67 (q, J = 6.7 Hz, 1H, CH), 3.39 (s, 3H, CH3), 1.33 (d, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 191.14, 172.06, 148.68, 129.05, 122.39, 114.09, 68.97, 56.84, 56.23, 40.32, 16.81; HRMS calcd for C13H17ClN4NaOS2 [M + Na]+ 367.0425, found 367.0420.
(S)-N′-(4-Bromophenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanohydrazide (5q). White solid; m.p. 182.4–183.9 °C; yield 95.4%; IR (KBr, cm−1) ν: 3241, 2985, 2897, 1655, 1510, 1484, 1321, 1209, 1099, 1086, 890, 814; 1H NMR (400 MHz, DMSO-d6) δ: 10.08 (d, J = 2.7 Hz, 1H, CONH), 7.96 (d, J = 3.6 Hz, 1H, NH), 7.29 (t, J = 5.9 Hz, 2H, Ar-3,5-2H), 6.65 (t, J = 5.9 Hz, 2H, Ar-2,6-2H), 4.64 (q, J = 12.2 Hz, 2H, thiadiazine-4-2H), 4.50–4.41 (m, 2H, thiadiazine-6-2H), 3.70–3.63 (m, 1H, CH), 3.39 (s, 3H, CH3), 1.33 (d, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 191.14, 172.05, 149.08, 131.89, 114.60, 109.90, 68.97, 56.83, 56.22, 40.33, 16.82; HRMS calcd for C13H17BrN4NaOS2 [M + Na]+ 410.9919, found 410.9917.
(S)-N′-(4-Methylphenyl)-2-(5-methyl-6-thioxo-1,3,5-thiadiazinan-3-yl)propanohydrazide (5r). White solid; m.p. 186.5–187.8 °C; yield 94.5%; IR (KBr, cm−1) ν: 3240, 2972, 2895, 1691, 1660, 1596, 1519, 1494, 1318, 1208, 1100, 937, 891, 757, 690; 1H NMR (400 MHz, DMSO-d6) δ: 10.00 (d, J = 3.2 Hz, 1H, CONH), 7.57 (d, J = 3.2 Hz, 1H, NH), 6.95 (d, J = 8.2 Hz, 2H, Ar-3,5-2H), 6.61 (d, J = 8.4 Hz, 2H, Ar-2,6-2H), 4.65 (dd, J = 13.2, 7.9 Hz, 2H, thiadiazine-4-2H), 4.45 (dd, J = 13.2, 8.7 Hz, 2H, thiadiazine-6-2H), 3.66 (q, J = 6.7 Hz, 1H, CH), 3.38 (s, 3H, CH3), 2.18 (d, J = 6.9 Hz, 3H, CH3), 1.32 (d, J = 6.8 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 191.14, 171.90, 147.45, 129.66, 127.74, 112.85, 69.00, 56.77, 56.12, 40.32, 20.63, 16.85; HRMS calcd for C14H20N4NaOS2 [M + Na]+ 347.0971, found 347.0969.
Antifungal bioassay in vitro of title compounds
The tested plant pathogenic fungi strains Fg, Bc, Rs and Cc, which were, respectively, isolated from infected wheat, strawberries, rice and peppers from disease outbreak regions in Jiangsu Province, were provided by the Laboratory of Plant Disease Control at Nanjing Agricultural University. The antifungal effects of title compounds 5a–5r against the above fungi were tested by a mycelial growth rate method [3, 32,33,34,35,36,37,38,39,40,41]. Under same conditions, the agricultural fungicides carbendazim, penthiopyrad and azoxystrobin were used as positive controls to evaluate the antifungal effects of title compounds. The antifungal effects of all title compounds against the four tested fungi at five double-declining concentrations were tested to calculate the corresponding EC50 values.
Anti-Rs activity of title compound 5f on detached rice leaf
Using the agricultural fungicide carbendazim as the positive control, the in vivo anti-Rs activity of title compound 5f was carried out on rice leaves by detached leaf assay [42] with some minor modifications. Appropriate amounts of the title compound 5f and carbendazim in 200 uL of DMSO were mixed with a solution containing distilled water (50 mL) and Tween 20 (500 uL). At tillering stages of rice plants, fresh leaf pieces with a length of approximate 10 cm were collected, washed by 75% aqueous ethyl alcohol and dipped for 10 min in the above solution. After evaporation under room temperature, the cuticles on the center of rice leaves were punctured with a sterilized needle and inoculated via a mycelia cake with a diameter of approximate 5 mm. Then, all inoculated leaves were placed in an illumination incubator (25 °C and 90% relative humidity) in an environment with a 12-h light/12-h dark photoperiod. Five days after inoculation, the lesion lengths of all treatments were timely measured, and then, the control effects of all treatments were statistically calculated.
Anti-Rs activity of title compound 5f on rice plant
Using carbendazim as the positive control and a rice variety (yiyou 186) as the tested plants, the in vivo protective activity of title compound 5f against Rs was carried out on potted rice plants by the reported method [42] with some minor modifications. The rice seeds (yiyou 186) were sown and grown in pots (18 cm in diameter and 16 cm in height) for approximate 6 weeks under greenhouse conditions. Appropriate amounts of the title compound 5f and carbendazim in 200 uL of DMSO were mixed with a solution containing distilled water (50 mL) and Tween 20 (500 uL). At tillering stages of rice plants, the obtained solution was uniformly sprayed on rice plants until the plants were completely wetted. One day later, a mycelia dish with a diameter of approximate 5 mm was placed in the leaf sheaves of rice plant by a sterilized needle. Then, all inoculated plants were placed into an illumination incubator (25 °C and 90% relative humidity) in an environment with a 12-h light/12-h dark photoperiod. Six days after inoculation, the lesion lengths of rice plants were timely measured and statistically calculated to obtain the control effects of all treatments.
References
Ray M, Ray A, Dash S, Mishra A, Achary KG, Nayak S, Singh S (2017) Fungal disease detection in plants: traditional assays, novel diagnostic techniques and biosensors. Biosens Bioelectron 87:708–723. https://doi.org/10.1016/j.bios.2016.09.032
McMullen M, Bergstrom G, Wolf ED, Dill-Macky R, Hershman D, Shaner G, Sanford DV (2012) A unified effort to fight an enemy of wheat and baeley: fusarium heat blight. Plant Dis 96:1712–1728. https://doi.org/10.1094/PDIS-03-12-0291-FE
Wang L, Li C, Zhang Y, Qiao C, Ye Y (2013) Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J Agric Food Chem 61:8632–8640. https://doi.org/10.1021/jf402388x
Jackson LS (2009) Chemical food safety issues in the United States: past, present, and future. J Agric Food Chem 57:8161–8170. https://doi.org/10.1021/jf900628u
Seiber JN, Kleinschmidt LA (2011) Contributions of pesticide residue chemiatry to improving food and environmental safety: past and present accomplishments and future challenges. J Agric Food Chem 59:7536–7543. https://doi.org/10.1021/jf103902t
Sparks TC, Lorsbach BA (2017) Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag Sci 73:672–677. https://doi.org/10.1002/ps.4457
Qian X, Lee PW, Cao S (2010) China: forward to the green pesticides via a basic research program. J Agric Food Chem 58:2613–2623. https://doi.org/10.1021/jf904098w
El-Shorbagi AN, El-Naggar M, Tarazi H, Chaudhary S, Abdu-Allan H, Hersi F, Omar H (2018) Bis-(5-substituted-2-thiono-1,3,5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multicomponent process. Med Chem Res 27:1103–1110. https://doi.org/10.1007/s00044-018-2133-9
Mao L, Jiang H, Wang Q, Yan D, Cao A (2017) Efficacy of soil fumigation with dazoment for controlling ginger bacterial wilt (Ralstonia solanacearum) in China. Crop Prot 100:111–116. https://doi.org/10.1016/j.cropro.2017.06.013
Semreen MH, El-Shorbagi AN, Al-Tel TH, Alsalahat IMM (2010) Targeting γ-aminobutyric acid (GABA) carriers to the brain: potential relevance as antiepileptic pro-drugs. Med Chem 6:144–149. https://doi.org/10.2174/1573406411006030144
Vicentini CB, Forlani G, Manfrini M, Romagnoli C, Mares D (2002) Development of new fungicides against Magnaporthe grisea: synthesis and biological activity of pyrazolo[3,4-d][1,3]thiazine, pyrazolo[1,5-c][1,3,5]thiadiazine, and pyrazolo[3,4-d]pyrimidine derivatives. J Agric Food Chem 50:4839–4845. https://doi.org/10.1021/jf0202436
Arshad N, Hashim J, Irfanullah Minhas MA, Aslam J, Ashraf T, Hamid SZ, Iqbal T, Javed S (2018) New series of 3,5-disubstituted tetrahydro-2H-1,3,5-thiadiazine thione (THTT) derivatives: synthesis and potent antileishmanial activity. Bioorg Med Chem Lett 28:3251–3254. https://doi.org/10.1016/j.bmcl.2018.07.045
Coro J, Atherton R, Little S, Wharton H, Yardley V, Alvarez A Jr., Suarez M, Perez R, Rodriguez H (2006) Alkyl-linked bis-THTT derivatives as potent in vitro trypanocidal agent. Bioorg Med Chem Lett 16:1312–1315. https://doi.org/10.1016/j.bmcl.2005.11.060
Ji X, Zhong Z, Chen X, Xing R, Liu S, Wang L, Li P (2007) Preparation of 1,3,5-thiadiazine-2-thione derivatives of chitosan and their potential antioxidant activity in vitro. Bioorg Med Chem Lett 17:4275–4279. https://doi.org/10.1016/j.bmcl.2007.05.020
Katiyar D, Tiwari VK, Tripathi RP, Srivastava A, Chaturvedi V, Srivastava R, Srivastava BS (2003) Synthesis and antimycrobacterial activity of 3,5-disubstituted thiadiazine thiones. Bioorg Med Chem 11:4369–4375. https://doi.org/10.1016/S0968-0896(03)00480-2
Coro J, Perez R, Rodriguez H, Suarez M, Vega C, Rolon M, Montero D, Nogal JJ, Gomez-Barrio A (2005) Synthesis and antiprotozoan evaluation of new alkyl-linked bis(2-thioxo-[1,3,5]thiadiazinan-3-yl) carboxylic acids. Bioorg Med Chem 13:3413–3421. https://doi.org/10.1016/j.bmc.2005.03.009
Vicentini CB, Guccione S, Giurato L, Ciaccio R, Mares D, Forlani G (2005) Pyrazole derivatives as photosynthetic electron transport inhibitors: new leads and structure-activity relationship. J Agric Food Chem 53:3848–3855. https://doi.org/10.1021/jf0500029
El-Shorbagi AN (1994) Model for delivery of amines through incorporation into a tetrahydro-2H-1,3,5-thiadiazine-2-thione structure. Eur J Med Chem 29:11–15. https://doi.org/10.1016/0223-5234(94)90120-1
Aboul-Fadl T, El-Shorbagi A (1996) New prodrug approach for amino acids and amino-acid-like drugs. Eur J Med Chem 31:165–169. https://doi.org/10.1016/0223-5234(96)80450-8
Nakamura M, Noda S, Kosugi M, Ishiduka N, Mizukoshi K, Taniguchi M, Nemoto S (2010) Determination of dithiocarbamates and milneb residues in foods by gas chromatography-mass spectrometry. Food Hyg Saf Sci 51:213–219. https://doi.org/10.3358/shokueishi.51.213
Lam WW, Kim JH, Sparks SE, Quistad GB, Casida JE (1993) Metabolism in rats and mice of the soil fumigants metham, methyl isothiocyanate, and dazoment. J Agric Food Chem 41:1497–1502. https://doi.org/10.1021/jf00033a027
Meyer F, Ueberschaar N, Dahse HM, Hertweck C (2013) Synthesis and biological evaluation of hydradomycin analogues. Bioorg Med Chem Lett 23:6043–6045. https://doi.org/10.1016/j.bmcl.2013.09.033
Khalid W, Badshah A, Khan A, Nadeem H, Ahmed S (2018) Synthesis, characterization, molecular docking evaluation, antiplatelet and anticoagulant actions of 1,2,4-triazole hydrazone and sulphonamide novel derivatives. Chem Cent J 11:1–16. https://doi.org/10.1186/s13065-018-0378-5
Park EB, Kim KJ, Jeong HR, Lee JK, Kim HJ, Lee HH, Lim JW, Shin JS, Koeberle A, Werz O, Lee KT, Lee JY (2016) Synthesis, structure determination, and biological evaluation of phenylsulfonyl hydrazide derivatives as potential anti-inflammatory agents. Bioorg Med Chem Lett 26:5193–5197. https://doi.org/10.1016/j.bmcl.2016.09.070
Soares RR, Silva JMF, Carlos BC, Fonseca CC, Souza LSA, Lopes FV, Dias RMP, Moreira POL, Abramo C, Viana GHR, Varotti FP, Silva AD, Scopel KKG (2015) New quinoline derivatives demonstrate a promising antimalarial activity against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg Med Chem Lett 25:2308–2313. https://doi.org/10.1016/j.bmcl.2015.04.014
Carvalho SA, Silva EF, Souza MVN, Lourenco MCS, Vicente FR (2008) Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg Med Chem Lett 18:538–541. https://doi.org/10.1016/j.bmcl.2007.11.091
Reheim MAMA, Baker SM (2017) Synthesis, characterization and in vitro antimicrobial activity of novel fused pyrazolo[3,4-c]pyridazine, pyrazolo[3,4-d]pyrimidine, thieno[3,2-c]pyrazole and pyrazolo[3′,4′:4,5]thieno[2,3-d]pyrimidine derivatives. Chem Cent J 112:1–14. https://doi.org/10.1186/s13065-017-0339-4
Yang L, Wang P, Wu JF, Yang LM, Wang RR, Pang W, Li YG, Shen YM, Zheng YT, Li X (2016) Design, synthesis and anti-HIV-1 evaluation of hydrazide-based peptidomimetics as selective gelatinase inhibitors. Bioorg Med Chem 24:2125–2136. https://doi.org/10.1016/j.bmcl.2016.03.043
Yu G, Luo L, Chen S, He F, Xie Y, Luo D, Xue W, Wu J (2018) Synthesis and insecticidal activity of novel diacyhydrazines derivatives containing a N-pyrazolepyrazole moiety. ChemistrySelect 3:10991–10995. https://doi.org/10.1002/slct.201802434
Huffman CW, Godar EM, Ohki K, Torgeson DC (1968) Synthesis of hydrazine derivatives as plant growth inhibitors. J Agric Food Chem 16:1041–1046. https://doi.org/10.1021/jf60160a035
Zhao Q, Shang J, Huang Z, Wang K, Bi F, Huang R, Wang Q (2008) Synthesis and insecticidal activities of novel N-sulfenyl-N′-tert-butyl-N, N′-diacylhydrazines. 2. N-substituted phenoxysulfenate derivatives. J Agric Food Chem 56:5254–5259. https://doi.org/10.1021/jf800740z
Yan T, Yu S, Liu P, Liu Z, Wang B, Xiong L, Li Z (2012) Design, synthesis and biological activities of novel benzoyl hydrazines containing pyrazole. Chin J Chem 30:919–923. https://doi.org/10.1002/cjoc.201100347
Wang X, Dai ZC, Chen YF, Cao LL, Yan W, Li SK, Wang JX, Zhang ZG, Ye YH (2017) Synthesis of 1,2,3-triazole hydrazide derivatives exhibiting anti-phytopathogenic activity. Eur J Med Chem 126:171–182. https://doi.org/10.1016/j.ejmech.2016.10.006
Yu X, Teng P, Zhang YL, Xu ZJ, Zhang MZ, Zhang WH (2018) Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia 127:387–395. https://doi.org/10.1016/j.fitote.2018.03.013
Wang X, Wang M, Yan J, Chen M, Wang A, Mei Y, Si W, Yang C (2018) Design, synthesis and 3D-QSAR of new quinazolin-4(3H)-one derivatives containing a hydrazide moiety as potential fungicides. ChemistrySelect 3:10663–10669. https://doi.org/10.1002/slct.201801575
Chen M, Wang XF, Wang SS, Feng YX, Chen F, Yang CL (2012) Synthesis, characterization and fungicidal activities of novel fluorinated 3,5-disubstituted-4H-1,2,4-triazol-4-amines. J Fluorine Chem 135:323–329. https://doi.org/10.1016/j.jfluchem.2011.12.015
Li LX, Jiao J, Wang XB, Chen M, Fu XC, Si WJ, Yang CL (2018) Synthesis, characterization, and antifungal activity of novel benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives. Molecules 23:746. https://doi.org/10.3390/molecules23040746
Du H, Fan Z, Yang L, Bao X (2018) Synthesis of novel quinazolin-4(3H)-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety as effective agricultural bactericides against the pathogen Xanthomonas oryzae pv. oryzae. Mol Divers 22:1–10. https://doi.org/10.1007/s11030-017-9782-3
Yang L, Ge S, Huang J, Bao X (2018) Synthesis of novel (E)-2-(4-(1H-1,2,4-triazol-1-yl)styryl-4-(alkyl/arylmethyleneoxy)quinazoline derivatives as antimicrobial agents. Mol Divers 22:71–82. https://doi.org/10.1007/s11030-017-9792-1
Fan Z, Shi J, Bao X (2018) Synthesis and antimicrobial evaluation of novel 1,2,4-triazole thioether derivatives bearing a quinazoline moiety. Mol Divers 22:657–667. https://doi.org/10.1007/s11030-018-9821-8
Wang X, Ren Z, Wang M, Chen M, Lu A, Si W, Yang C (2018) Design and synthesis of novel 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone moiety as potential fungicides. Chem Cent J 12:83. https://doi.org/10.1186/s13065-018-0452-z
Zhang ZJ, Zeng Y, Jiang ZY, Shu BS, Sethuraman V, Zhong GH (2018) Design, synthesis, fungicidal property and QSAR studies of novel β-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rice sheath blight. Pest Manag Sci 74:1736–1746. https://doi.org/10.1002/ps.4873
Echemendia R, Fernandez O, Coro J, Suarez M, Rivera DG (2017) A versatile approach to hybrid thiadiazine-based molecules by the Ugi four-component reaction. Tetrahedron Lett 58:1784–1787. https://doi.org/10.1016/j.tetlet.2017.03.075
Acknowledgements
The authors gratefully acknowledge the grants from the National Natural Science Foundation of China (No. 31772209) and the Fundamental Research Funds for the Central Universities of China (No. KYTZ201604).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Fu, X., Yan, J. et al. Design and synthesis of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives as potential fungicides. Mol Divers 23, 573–583 (2019). https://doi.org/10.1007/s11030-018-9891-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9891-7